Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism
Abstract
:1. Introduction
2. Chemical Kinetics Mechanism
3. Modelling Approach
3.1. Ignition Delay Time
3.2. Laminar Flame Speed
3.3. Species Concentration Profiles
3.4. Sensitivity Analysis
4. Fuel Mixtures Used in This Study
5. Results and Discussion
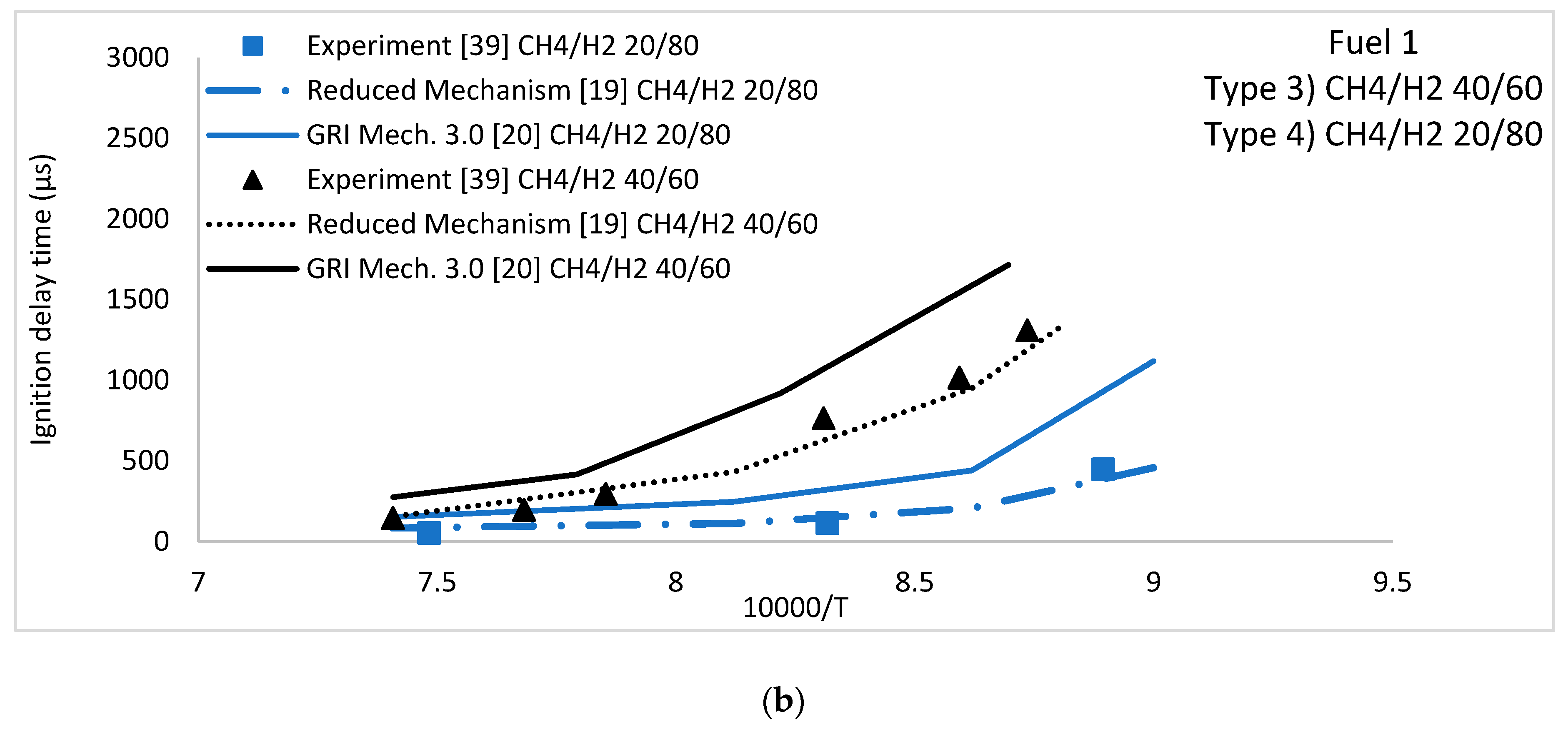
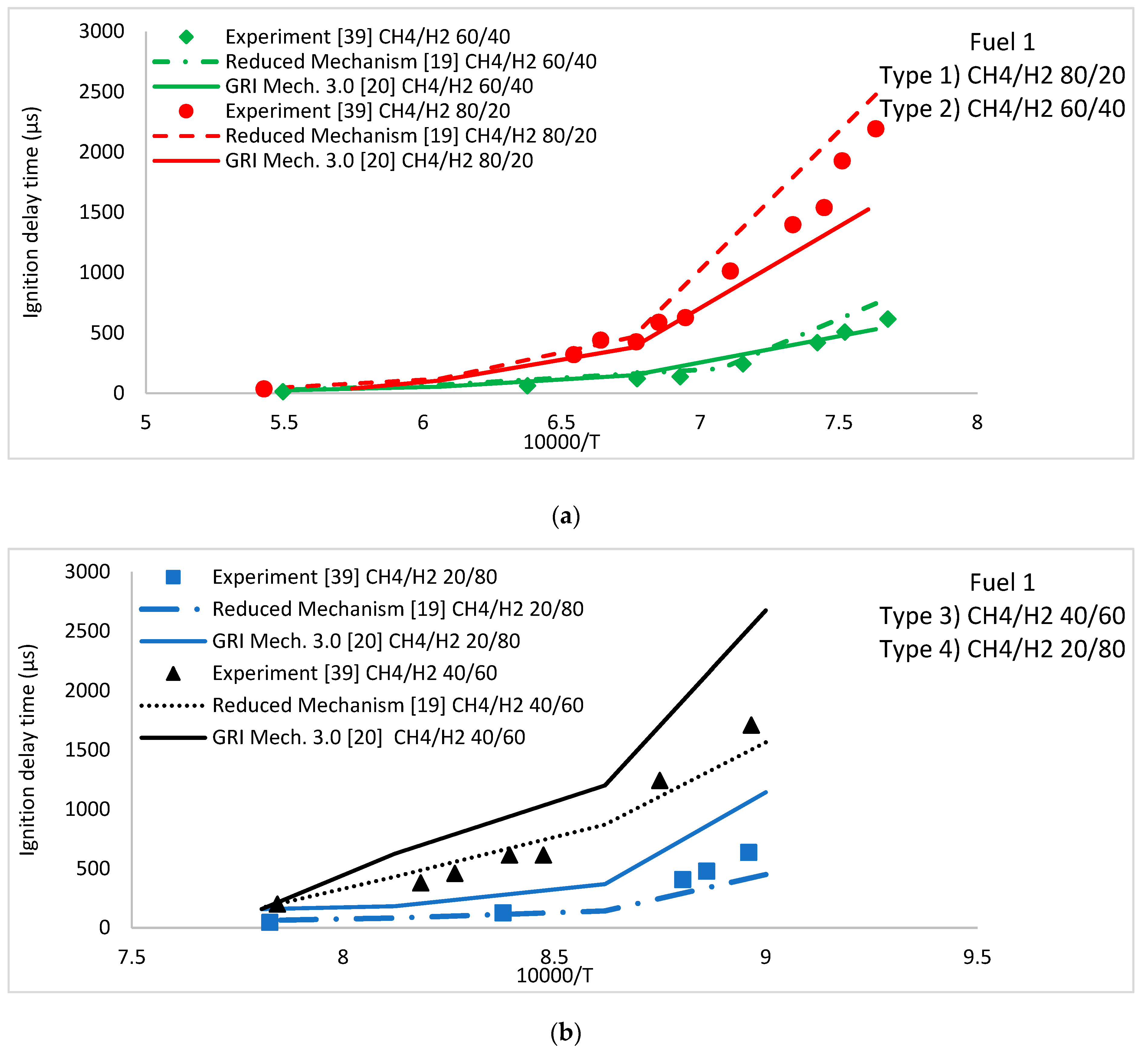
5.1. Ignition Delay Time
CH4/H2 Fuel Mixture
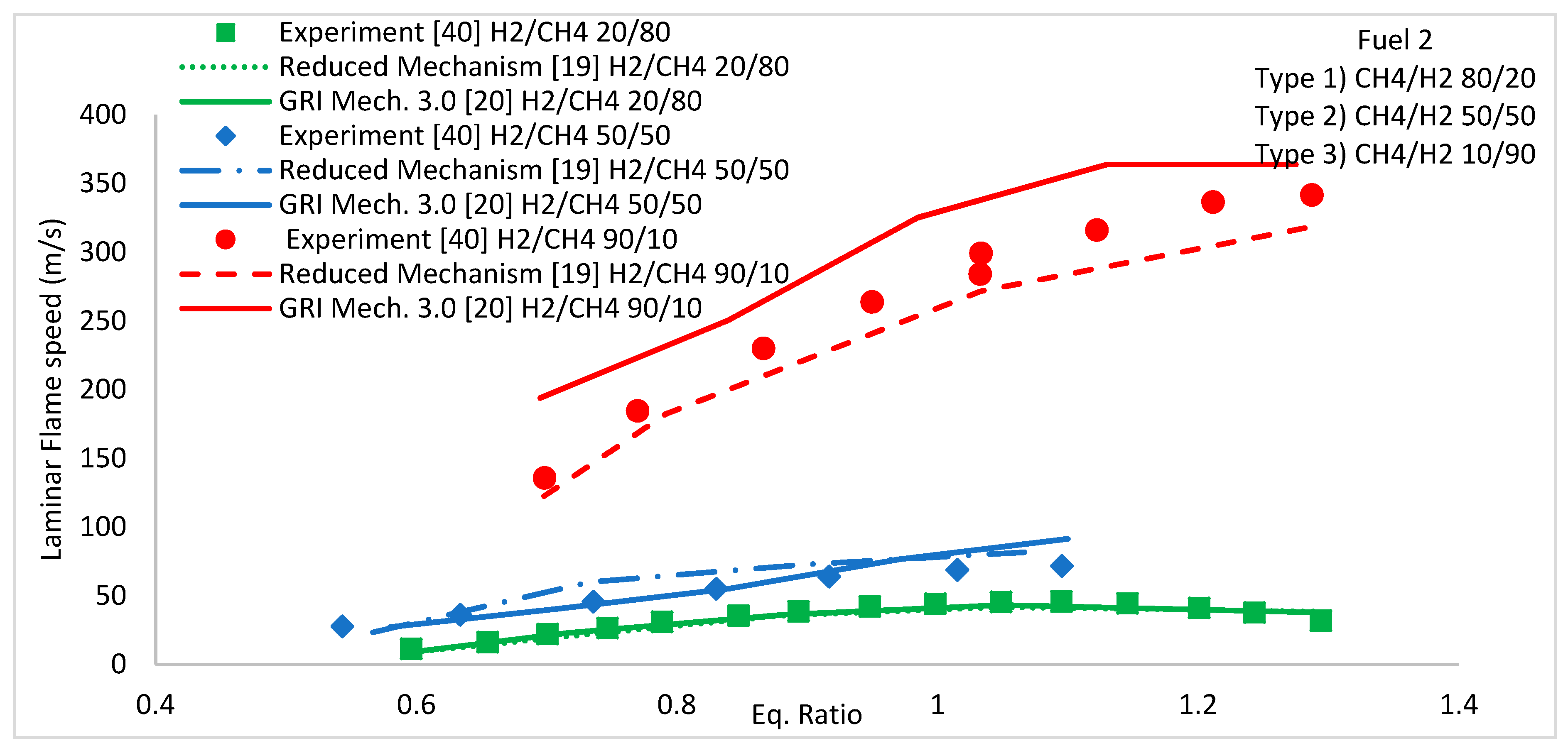
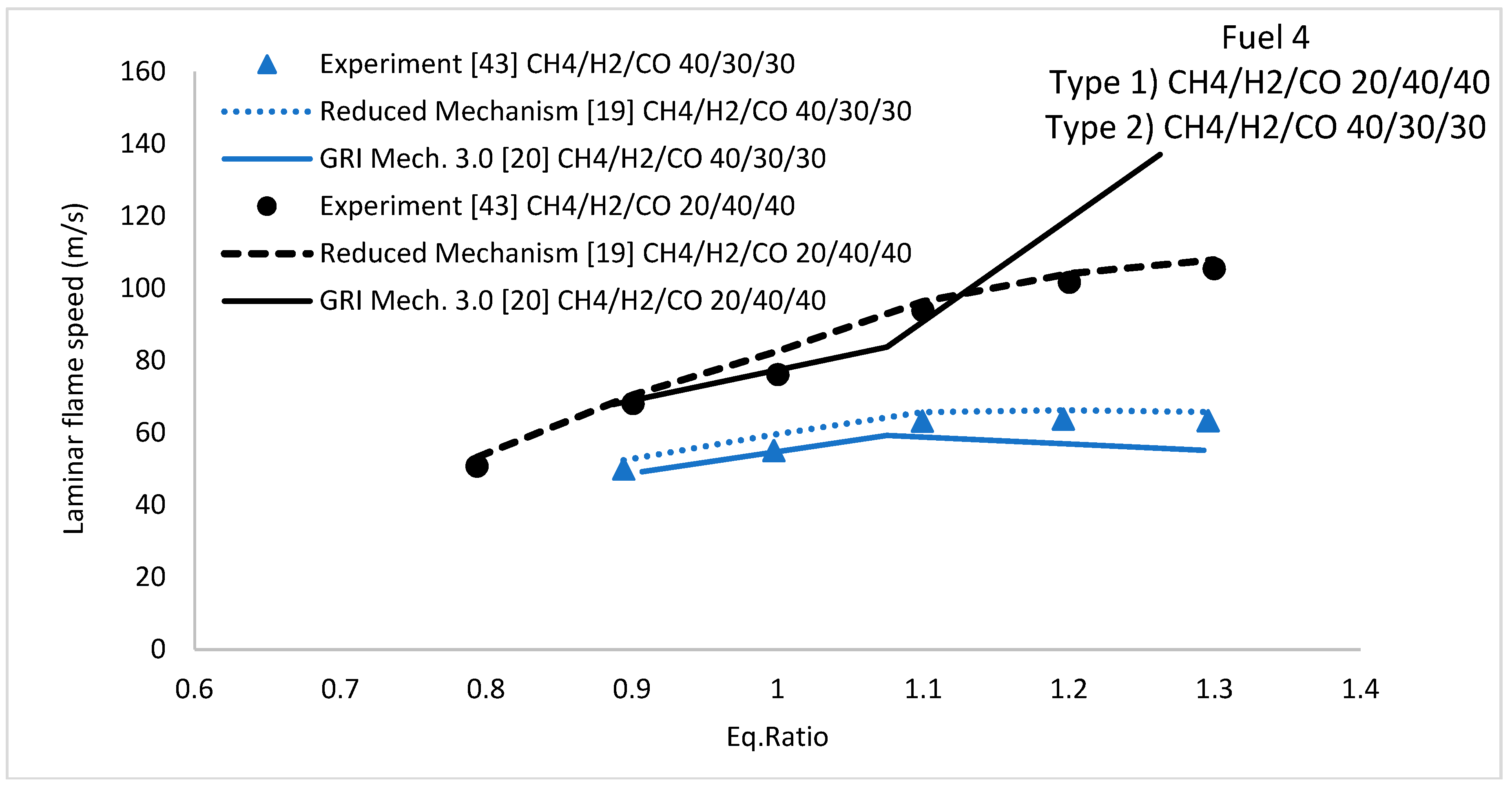
5.2. Laminar Flame Speed
5.2.1. H2/CH4 Fuel Mixture
5.2.2. H2/CO/CH4 Fuel Mixture
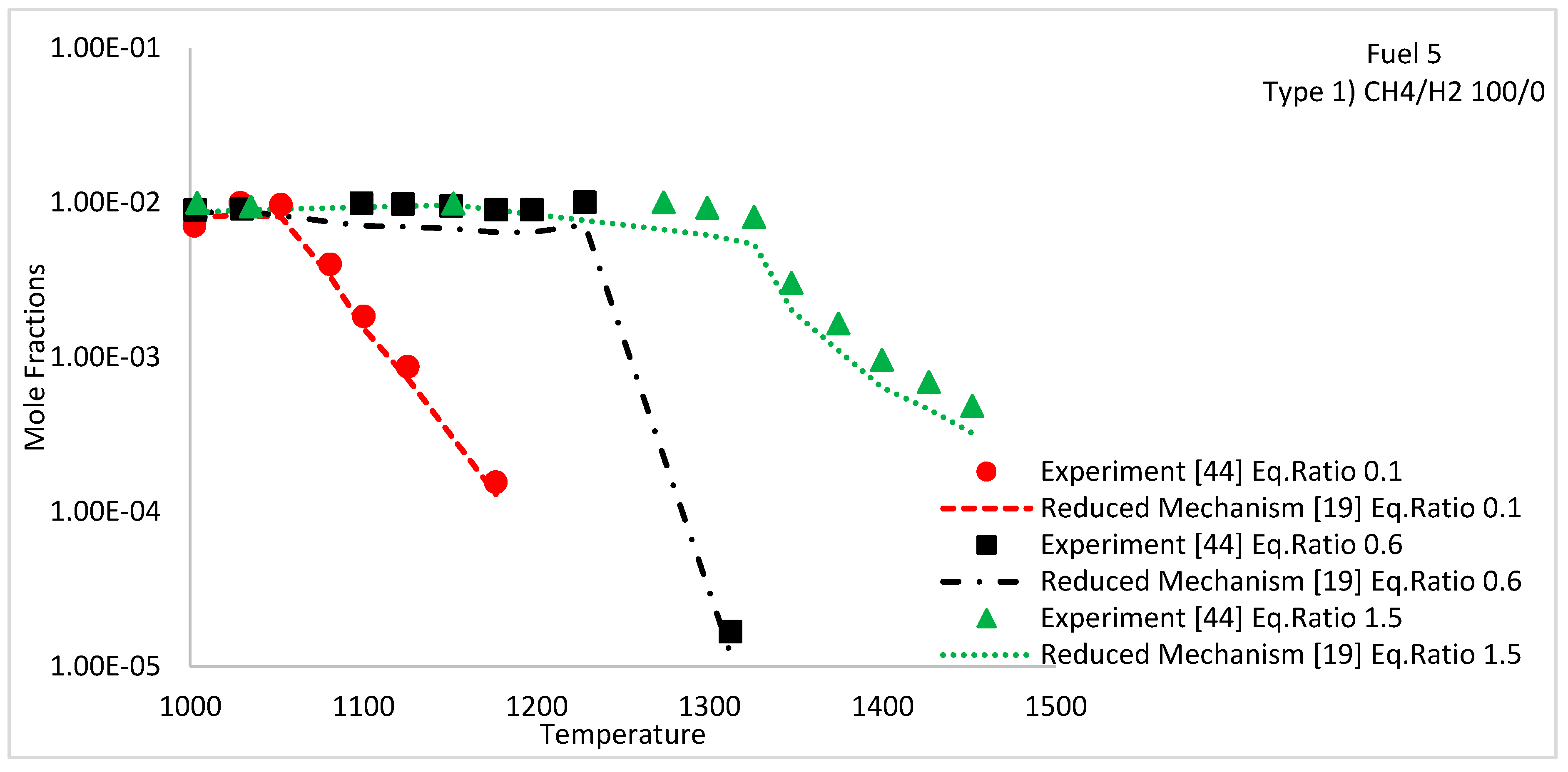
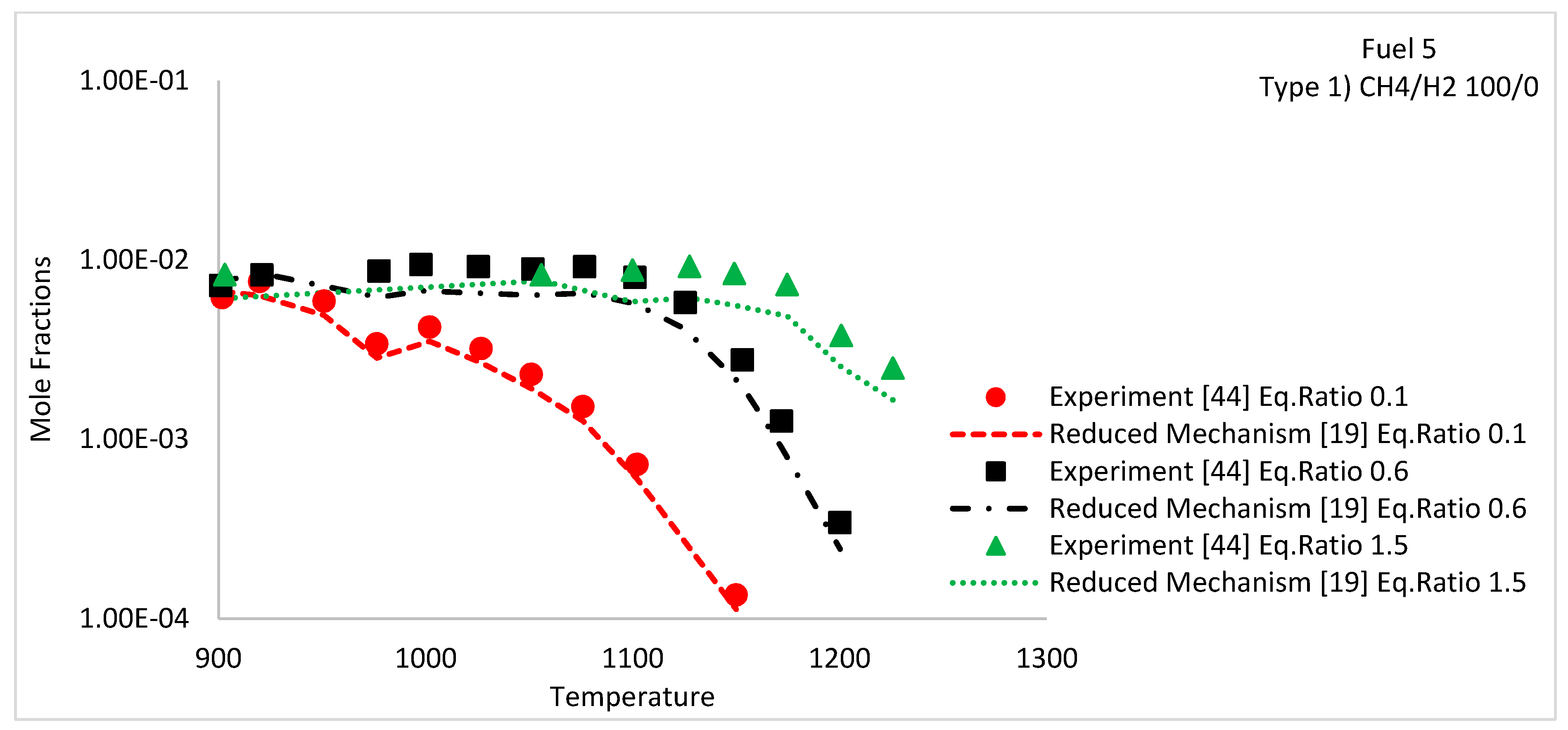
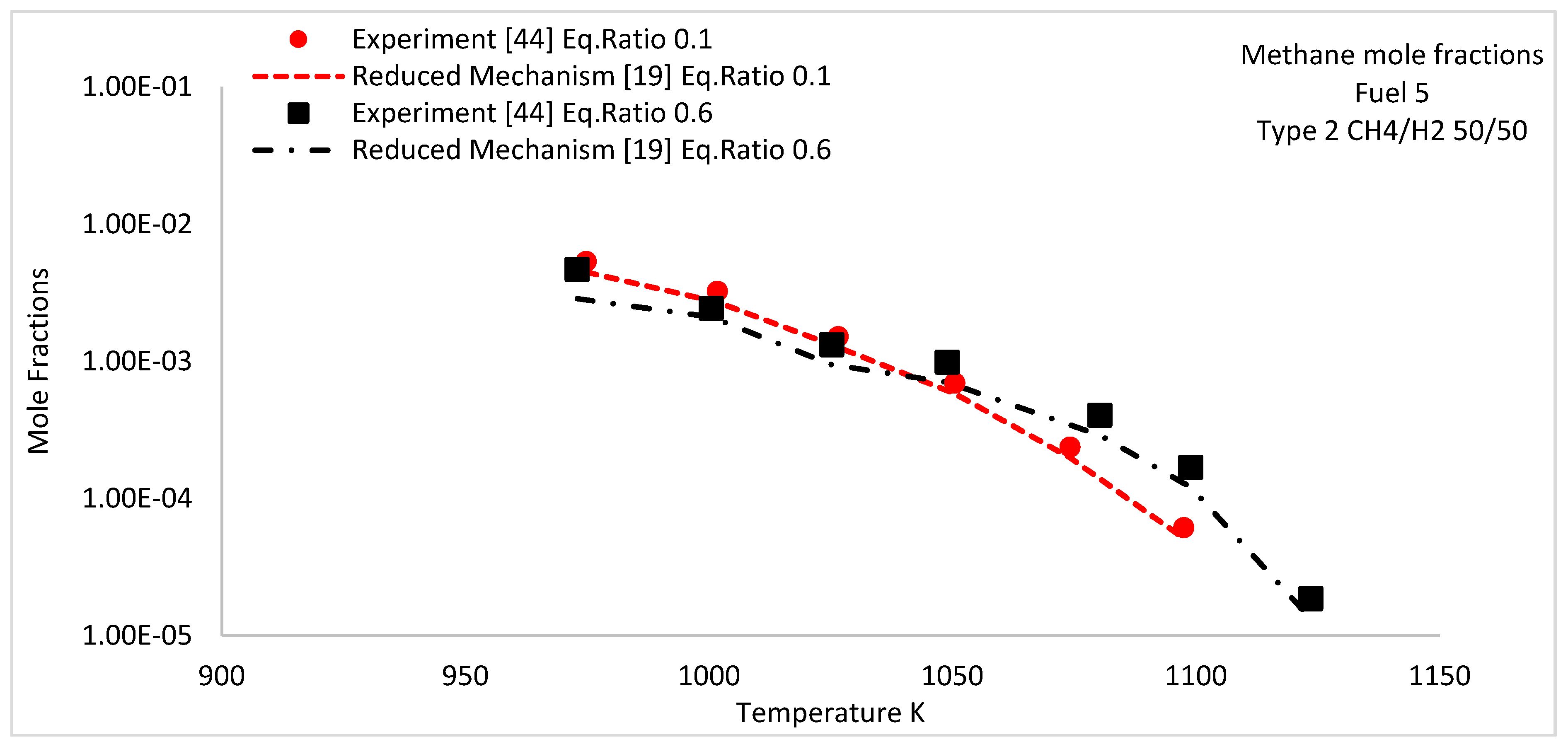
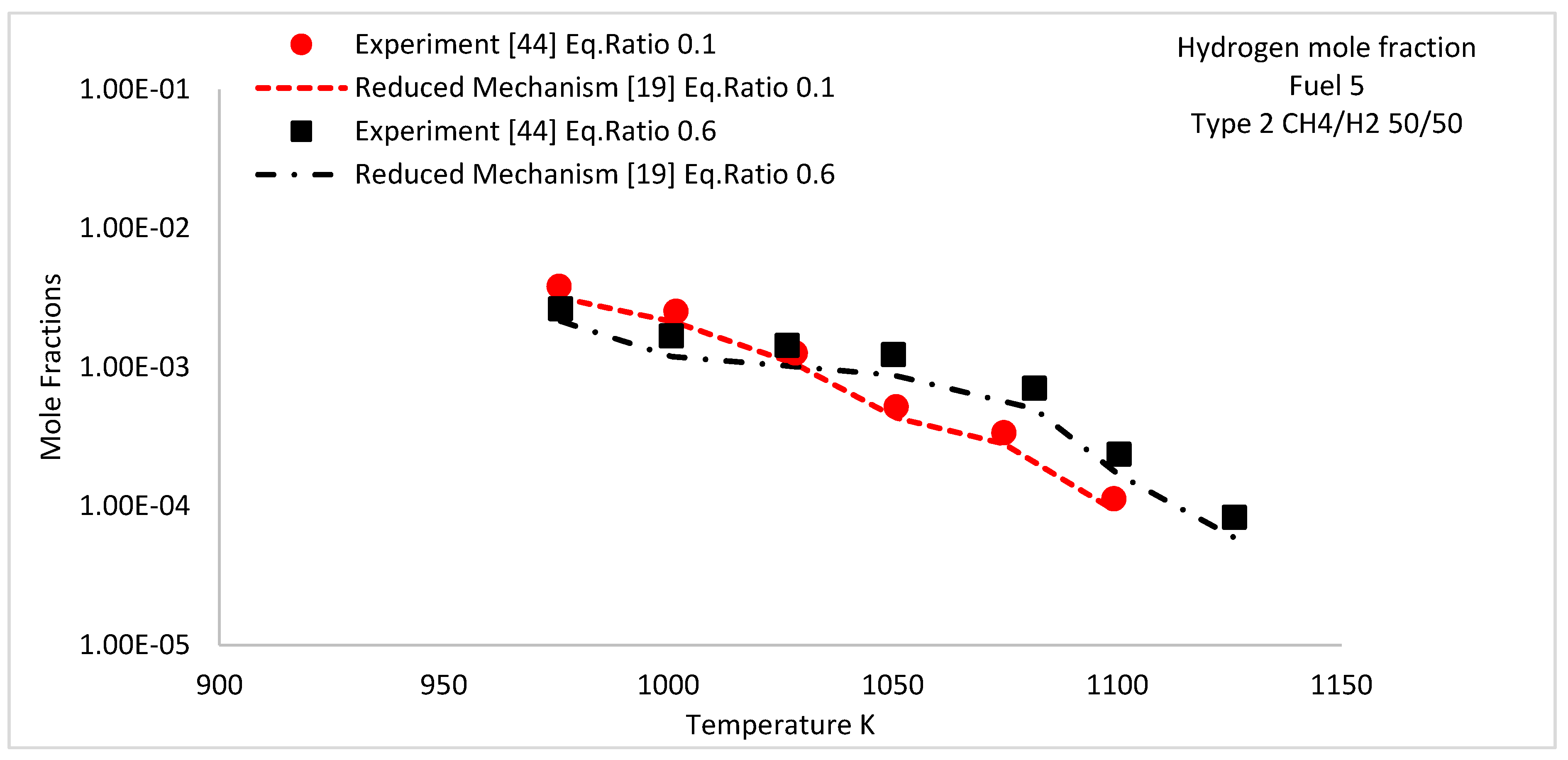
5.3. Species Concentration Profiles
5.4. Chemical Detailed Analysis
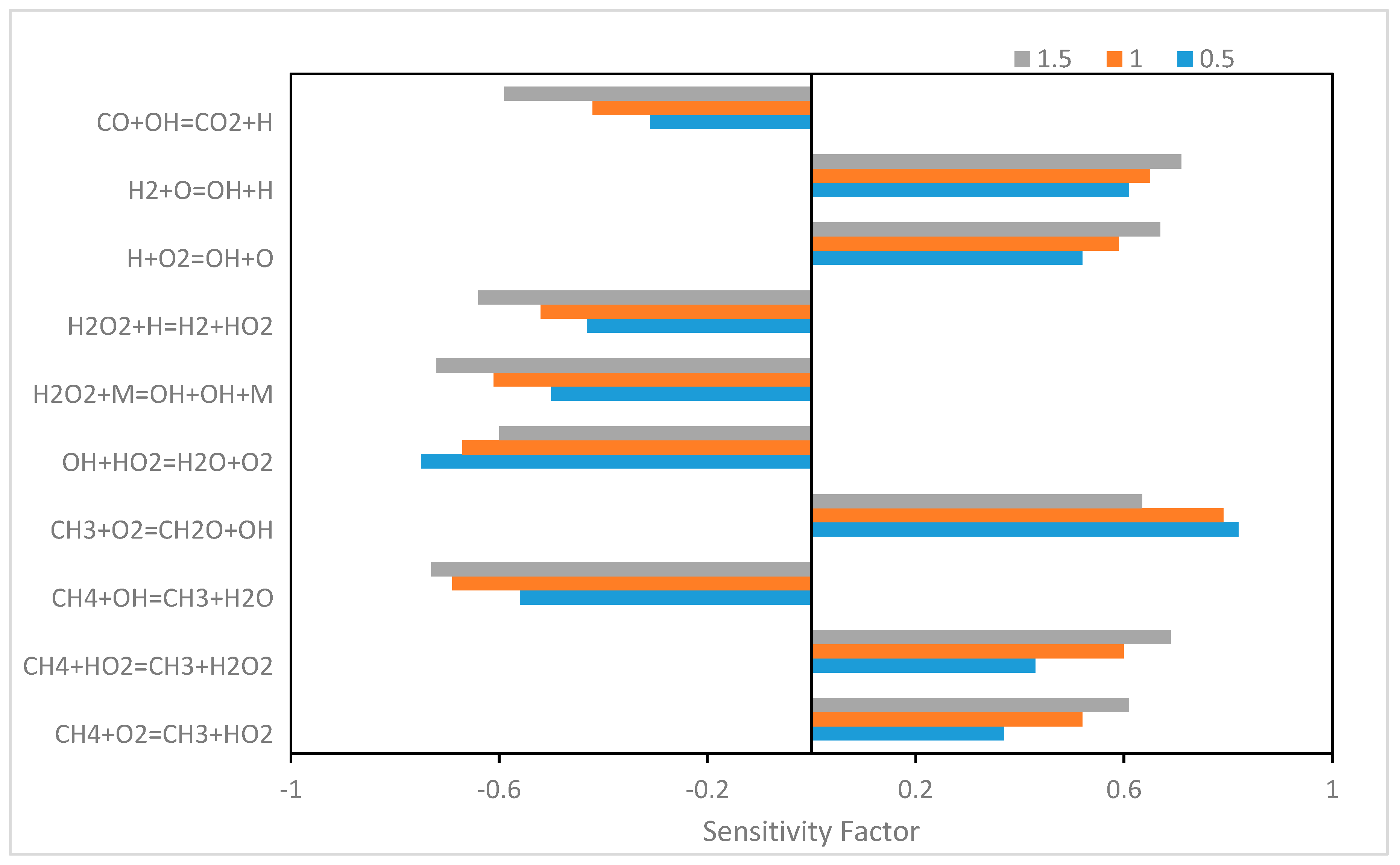
5.4.1. H2/CO/CH4 Reaction Sensitivity
Hydrogen-Based Reactions
Methane-Based Reactions
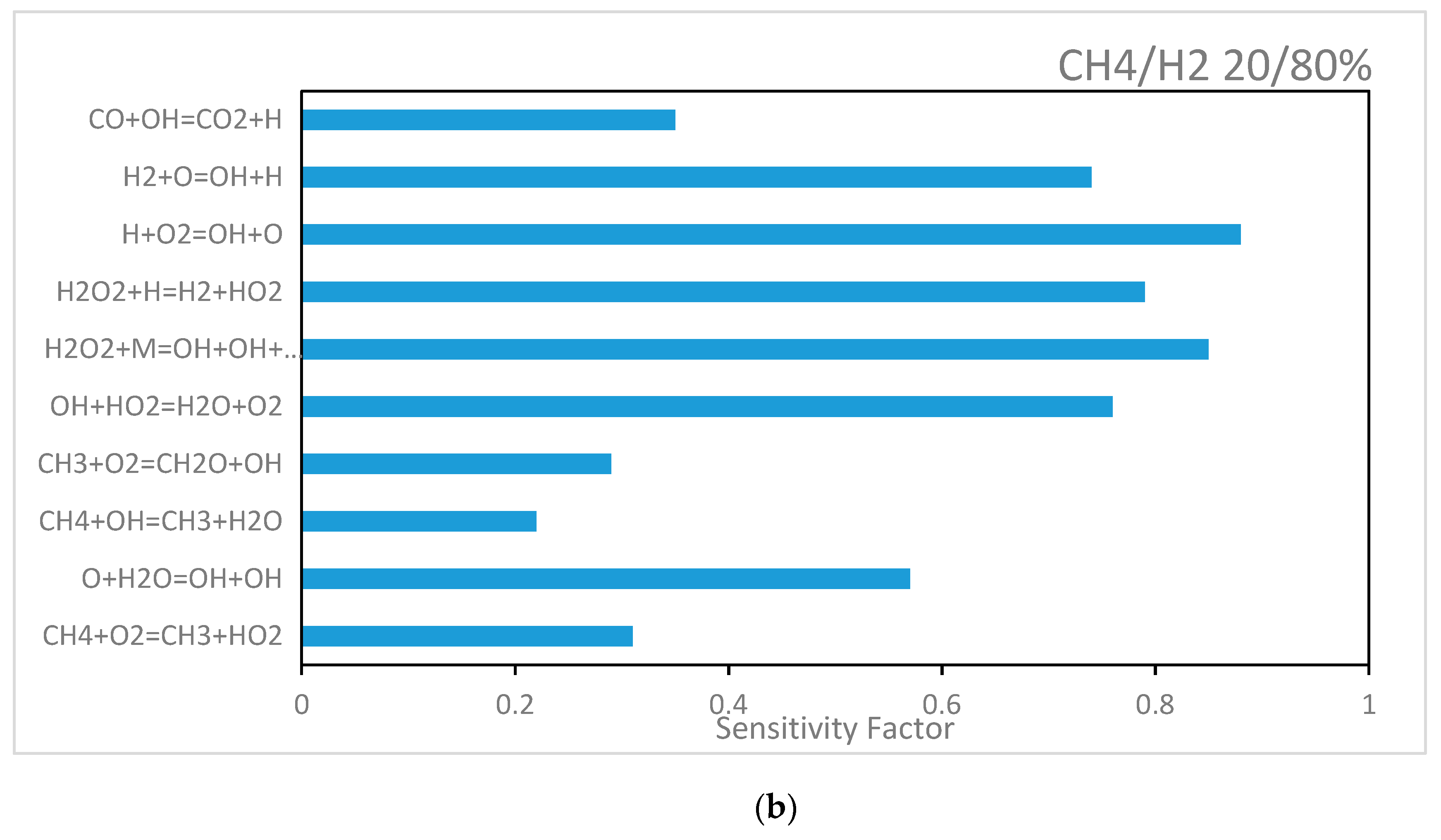
5.4.2. H2/CH4 Reaction Sensitivity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frassoldati, A.; Faravelli, T.; Ranzi, E.L. A wide range modeling study of NOx formation and nitrogen chemistry in hydrogen combustion. Int. J. Hydrog. Energy 2006, 31, 2310–2328. [Google Scholar] [CrossRef]
- Sridhar, G.; Paul, P.J.; Mukunda, H.S. Biomass derived producer gas as a reciprocating engine fuel—An experimental analysis. Biomass Bioenergy 2001, 21, 61–72. [Google Scholar] [CrossRef]
- Shilling, N.Z.; Lee, D.T. IGCC-clean power generation alternative for solid fuels. In Proceedings of the PowerGen Asia, Ho Chi Minh City, Vietnam, 23–25 September 2003. [Google Scholar]
- Liu, C.C.; Shy, S.S.; Chiu, C.W.; Peng, M.W.; Chung, H.J. Hydrogen/carbon monoxide syngas burning rates measurements in high-pressure quiescent and turbulent environment. Int. J. Hydrog. Energy 2011, 36, 8595–8603. [Google Scholar] [CrossRef]
- Brower, M.L.; Mathieu, O.; Petersen, E.L.; Donohoe, N.; Heufer, A.; Metcalfe, W.K.; Curran, H.J.; Bourque, G.; Güthe, F. Ignition Delay Time Experiments for Natural Gas/Hydrogen Blends at Elevated Pressures. In Proceedings of the ASME Turbo Expo 2013: Turbine Technical Conference and Exposition, San Antonio, TX, USA, 3–7 June 2013. GT2013-95151. [Google Scholar]
- Tahtouh, T.; Halter, F.; Samson, E.; Mounaïm-Rousselle, C. Effects of hydrogen addition and nitrogen dilution on the laminar flame characteristics of premixed methane–air flames. Int. J. Hydrog. Energy 2009, 34, 8329–8338. [Google Scholar] [CrossRef]
- Akansu, S.O.; Dulger, Z.; Kahraman, N.; Veziroǧlu, T.N. Internal combustion engines fueled by natural gas—Hydrogen mixtures. Int. J. Hydrog. Energy 2004, 29, 1527–1539. [Google Scholar] [CrossRef]
- Ilbas, M.; Crayford, A.P.; Yılmaz, I.; Bowen, P.J.; Syred, N. Laminar-burning velocities of hydrogen–air and hydrogen–methane–air mixtures: An experimental study. Int. J. Hydrog. Energy 2006, 31, 1768–1779. [Google Scholar] [CrossRef]
- Mathieu, O.; Kopp, M.; Petersen, E. Shock-tube study of the ignition of multi-component syngas mixtures with and without ammonia impurities. Proceed. Combust. Inst. 2013, 34, 3211–3218. [Google Scholar] [CrossRef]
- Watson, G.M.; Munzar, J.D.; Bergthorson, J.M. NO formation in model syngas and biogas blends. Fuel 2014, 124, 113–124. [Google Scholar] [CrossRef]
- Tomita, E. Combustion characteristics and performance of supercharged pyrolysis gas engine with micro-pilot ignition. In Proceedings of the 25th CIMAC World Congress on Combustion Engine Technology (CIMAC 2007), Vienna, Austria, 21–24 May 2007; Volume 178, pp. 1–10. [Google Scholar]
- Li, H.; Karim, G.A. Exhaust emissions from an SI engine operating on gaseous fuel mixtures containing hydrogen. Int. J. Hydrog. Energy 2005, 30, 1491–1499. [Google Scholar] [CrossRef]
- Gersen, S.; Darmeveil, H.; Levinsky, H. The effects of CO addition on the autoignition of H2, CH4 and CH4/H2 fuels at high pressure in an RCM. Combust. Flame 2012, 159, 3472–3475. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Laminar burning velocity of methane, hydrogen and their mixtures at extremely low-temperature conditions. Energy Fuels 2018, 32, 8830–8836. [Google Scholar] [CrossRef]
- Salzano, E.; Pio, G.; Ricca, A.; Palma, V. The effect of a hydrogen addition to the premixed flame structure of light alkanes. Fuel 2018, 234, 1064–1070. [Google Scholar] [CrossRef]
- Miao, H.; Jiao, Q.; Huang, Z.; Jiang, D. Effect of initial pressure on laminar combustion characteristics of hydrogen enriched natural gas. Int. J. Hydrog. Energy 2008, 33, 3876–3885. [Google Scholar] [CrossRef]
- Miao, H.; Jiao, Q.; Huang, Z.; Jiang, D. Measurement of laminar burning velocities and Markstein lengths of diluted hydrogen-enriched natural gas. Int. J. Hydrog. Energy 2009, 34, 507–518. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Giles, A.; Pugh, D.; Morris, S.; Pohl, M.; Ortwein, A. Investigation of combustion of emulated biogas in a gas turbine test rig. J. Therm. Sci. 2018, 27, 331–340. [Google Scholar] [CrossRef]
- Stylianidis, N.; Azimov, U.; Maheri, A.; Tomita, E.; Kawahara, N. Chemical kinetics and CFD analysis of supercharged micro-pilot ignited dual-fuel engine combustion of syngas. Fuel 2017, 203, 591–606. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.P.; Gardiner, W.C.; Lissianski, V.V.; Qin, Z.; Smith, G.P.; Golden, D.M.; Frenklach, M.; Eiteneer, B.; Goldenberg, M.; Moriarty, N.W.; et al. The gri-mechtm model for natural gas combustion and no formation and removal chemistry. In Proceedings of the 5th International Conference on Combustion Technologies for a Clean Environment, Lisbon, Portugal, 12–15 July 1999; Available online: http://www.me.berkeley.edu/gri_mech/ (accessed on 20 November 2018).
- Azimov, U.; Okuno, M.; Tsuboi, K.; Kawahara, N.; Tomita, E. Multidimensional CFD simulation of syngas combustion in a micro-pilot-ignited dual-fuel engine using a constructed chemical kinetics mechanism. Int. J. Hydrog. Energy 2011, 36, 13793–13807. [Google Scholar] [CrossRef]
- Li, S.-C.; Williams, F.A.; Gebert, K. A Reduced Reaction Mechanism for Predicting Knock in Dual-Fuel Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Li, S.; Williams, F. Reaction mechanisms for methane ignition. In Proceedings of the ASME Turbo Expo 2000: Power for Land, Sea and Air, Munich, Germany, 8–11 May 2000; American Society of Mechanical Engineers: New York, NY, USA, 2000. [Google Scholar]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. The ignition, combustion and flame structure of carbon monoxide/hydrogen mixtures. Note 1: Detailed kinetic modeling of syngas combustion also in presence of nitrogen compounds. Int. J. Hydrog. Energy 2007, 32, 3471–3485. [Google Scholar] [CrossRef]
- Fernández-Galisteo, D.; del Alamo, G.; Sánchez, A.L.; Linán, A. Zeldovich analysis of hydrogen-air premixed flames. In Proceedings of the Third European combustion Meeting, Crete, Greece, 11–13 April 2007. [Google Scholar]
- Sutherland, J.; Michael, J.; Pirraglia, A.; Nesbitt, F.; Klemm, R. Rate constant for the reaction of O (3P) with H2 by the flash photolysis—shock tube and flash photolysis—Resonance fluorescence techniques; 504K≤ T≤ 2495K. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.-J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef] [Green Version]
- Conaire, M.Ó.; Curran, H.J.; Simmie, J.M.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of hydrogen oxidation. Int. J. Chem. Kinet. 2004, 36, 603–622. [Google Scholar] [CrossRef]
- Konnov, A. Remaining uncertainties in the kinetic mechanism of hydrogen combustion. Combust. Flame 2008, 152, 507–528. [Google Scholar] [CrossRef]
- CD-Adapco Inc. DARS Basic 2.14; CD-Adapco Inc.: Melville, NY USA, 2019. [Google Scholar]
- Das, A.K.; Sung, C.J.; Zhang, Y.; Mittal, G. Ignition delay study of moist hydrogen/oxidizer mixtures using a rapid compression machine. Int. J. Hydrog. Energy 2012, 37, 6901–6911. [Google Scholar] [CrossRef]
- Lee, H.C.; Mohamad, A.A.; Jiang, L.-Y. Comprehensive comparison of chemical kinetics mechanisms for syngas/biogas mixtures. Energy Fuels 2015, 29, 6126–6145. [Google Scholar] [CrossRef]
- Goodwin, D. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics and Transport Processes; Caltech: Pasadena, CA, USA, 2009. [Google Scholar]
- Kathrotia, T.; Fikri, M.; Bozkurt, M.; Hartmann, M.; Riedel, U.; Schulz, C. Study of the H + O + M reaction forming OH∗: Kinetics of OH∗ chemiluminescence in hydrogen combustion systems. Combust. Flame 2010, 157, 1261–1273. [Google Scholar] [CrossRef]
- CD-Adapco Inc. DARS Manual, Book4: Flames; CD-Adapco Inc.: Melville, NY USA, 2018. [Google Scholar]
- CD-Adapco Inc. DARS Manual, Book5: Mechanism Reduction; CD-Adapco Inc.: Melville, NY USA, 2018. [Google Scholar]
- Løvås, T. Model reduction techniques for chemical mechanisms. IntechOpen 2012. [Google Scholar] [CrossRef]
- Soyhan, H.S.; Amnéus, P.; Løvås, T.; Nilsson, D.; Maigaard, P.; Mauss, F.; Sorusbay, C. Automatic Reduction of Detailed Chemical Reaction Mechanisms for Autoignition Under SI Engine Conditions; SAE Transactions; SAE International: Warrendale, PA, USA, 2000; pp. 1435–1444. [Google Scholar]
- Zhang, Y.; Huang, Z.; Wei, L.; Zhang, J.; Law, C.K. Experimental and modeling study on ignition delays of lean mixtures of methane, hydrogen, oxygen and argon at elevated pressures. Combust. Flame 2012, 159, 918–931. [Google Scholar] [CrossRef]
- Donohoe, N.; Heufer, A.; Metcalfe, W.K.; Curran, H.J.; Davis, M.L.; Mathieu, O.; Plichta, D.; Morones, A.; Petersen, E.L.; Güthe, F. Ignition delay times, laminar flame speeds and mechanism validation for natural gas/hydrogen blends at elevated pressures. Combust. Flame 2014, 161, 1432–1443. [Google Scholar] [CrossRef]
- Hermanns, R.T.E. Laminar Burning Velocities of Methanehydrogen-Air Mixtures. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2007. [Google Scholar]
- Coppens, F.H.V.; De Ruyck, J.; Konnov, A.A. The effects of composition on burning velocity and nitric oxide formation in laminar premixed flames of CH4 + H2 + O2 + N2. Combust. Flame 2007, 149, 409–417. [Google Scholar] [CrossRef]
- Lapalme, D.; Seers, P. Influence of CO2, CH4 and initial temperature on H2/CO laminar flame speed. Int. J. Hydrog. Energy 2014, 39, 3477–3486. [Google Scholar] [CrossRef]
- Cong, T.L.; Dagaut, P. Experimental and detailed kinetic modeling of the oxidation of methane and methane/syngas mixtures and effect of carbon dioxide addition. Combust. Sci. Technol. 2008, 180, 2046–2091. [Google Scholar] [CrossRef]
- Dufour, A.; Valin, S.; Castelli, P.; Thiery, S.; Boissonnet, G.; Zoulalian, A.; Glaude, P.A. Mechanisms and kinetics of methane thermal conversion in a syngas. Ind. Eng. Chem. Res. 2009, 48, 6564–6572. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Wang, T.; Hou, X.; Zhang, J.; Zheng, S. Numerical study of the chemical, thermal and diffusion effects of H2 and CO addition on the laminar flame speeds of methane–air mixture. Int. J. Hydrog. Energy 2015, 40, 8475–8483. [Google Scholar] [CrossRef]
- Roy, M.M.; Tomita, E.; Kawahara, N.; Harada, Y.; Sakane, A. Performance and emission comparison of a supercharged dual-fuel engine fueled by producer gases with varying hydrogen content. Int. J. Hydrog. Energy 2009, 34, 7811–7822. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Li, G.; Li, P. Effects of hydrogen addition on the performance of a pilot-ignition direct-injection natural gas engine: A numerical study. Energy Fuels 2017, 31, 4407–4423. [Google Scholar] [CrossRef]
- Hong, Z.; Davidson, D.F.; Hanson, R.K. An improved H2/O2 mechanism based on recent shock tube/laser absorption measurements. Combust. Flame 2011, 158, 633–644. [Google Scholar] [CrossRef]
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577. [Google Scholar] [CrossRef] [Green Version]
- Kappel, C.; Luther, K.; Troe, J. Shock wave study of the unimolecular dissociation of H2O2 in its falloff range and of its secondary reactions. Phys. Chem. Chem. Phys. 2002, 4, 4392–4398. [Google Scholar] [CrossRef]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Frank, P.; Hayman, G.; Just, T.; Kerr, J.A.; Murrells, T.; Pilling, M.J.; Troe, J.; et al. Summary table of evaluated kinetic data for combustion modeling: Supplement 1. Combust. Flame 1994, 98, 59–79. [Google Scholar] [CrossRef]
- Natarajan, K.; Roth, P. High temperature rate coefficient for the reaction of O (3P) with H2 obtained by the resonance absorption of O and H atoms. Combust. Flame 1987, 70, 267–279. [Google Scholar] [CrossRef]
- Javoy, S.; Naudet, V.; Abid, S.; Paillard, C.E. Rate constant for the reaction of O with H2 at high temperature by resonance absorption measurements of O atoms. Int. J. Chem. Kinet. 2000, 32, 686–695. [Google Scholar] [CrossRef]
- Nikolaou, Z.M.; Chen, J.Y.; Swaminathan, N. A 5-step reduced mechanism for combustion of CO/H2/H2O/CH4/CO2 mixtures with low hydrogen/methane and high H2O content. Combust. Flame 2013, 160, 56–75. [Google Scholar] [CrossRef]
- Hashemi, H.; Christensen, J.M.; Gersen, S.; Levinsky, H.; Klippenstein, S.J.; Glarborg, P. High-pressure oxidation of methane. Combust. Flame 2016, 172, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Giménez-López, J.; Millera, A.; Bilbao, R.; Alzueta, M.U. Experimental and kinetic modeling study of the oxy-fuel oxidation of natural gas, CH4 and C2H6. Fuel 2015, 160, 404–412. [Google Scholar] [CrossRef]
- Aul, C.J.; Metcalfe, W.K.; Burke, S.M.; Curran, H.J.; Petersen, E.L. Ignition and kinetic modeling of methane and ethane fuel blends with oxygen: A design of experiments approach. Combust. Flame 2013, 160, 1153–1167. [Google Scholar] [CrossRef]
- Srinivasan, N.K.; Su, M.C.; Sutherland, J.W.; Michael, J.V. Reflected shock tube studies of high-temperature rate constants for CH3 + O2, H2CO + O2 and OH + O2. J. Phys. Chem. A 2005, 109, 7902–7914. [Google Scholar] [CrossRef]
- Burke, M.P.; Chaos, M.; Ju, Y.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 kinetic model for high-pressure combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Chemical-Kinetic Mechanisms for Combustion Applications. Mechanical and Aerospace Engineering (Combustion Research), University of California at San Diego, 2011. Available online: http://combustion.ucsd.edu (accessed on 20 November 2018).





| Reactions | A (cal-cm-sec-K) | n | E (cal/mol) | Ref. | |
|---|---|---|---|---|---|
| R1 | CH4 + O2 = CH3 + HO2 | 3.98 × 1013 | 0.0 | 56,855.5 | [22] |
| R2 | CH4 + HO2 = CH3 + H2O2 | 0.964 × 1011 | 0.0 | 24,629.4 | [22] |
| R3 | CH4 + OH = CH3 + H2O | 1.60 × 107 | 1.83 | 2771.1 | [23] |
| R4 | CH3 + O2 = CH2O + OH | 3.30 × 1011 | 0.0 | 8934.4 | [22] |
| R5 | CH2O + OH = HCO + H2O | 3.90 × 1010 | 0.0 | 406.1 | [22] |
| R6 | CO + O (+M) = CO2 (+M) | 9.04 × 1012 | 0.89 | 3800.0 | [23] |
| /LOW / 0.2070 × 1027 −3.340 7610.0 /M/ H2O/12.00/ H2/2.00/ CO/1.50/ CO2/2.00/ AR/0.50/ | |||||
| R7 | CO + OH = CO2 + H | 0.9600 × 1012 | 0.14 | 7352.0 | [23] |
| R8 | CO + OH = CO2 + H | 0.7320 × 1011 | −1.00 | −16.0 | [23] |
| R9 | CO + HO2 = CO2 + OH | 0.1200 × 1018 | 0.00 | 17,000.0 | [23] |
| R10 | CO + H2O = CO2 + H2 | 0.2000 × 109 | 0.00 | 38,000.0 | [23] |
| R11 | HCO (+M) = CO + H (+M) | 0.3000 × 1014 | 0.03 | 23,000.0 | [23] |
| /M/ H2O/5.00/ CO2/3.00/ H2/1.90/ CO/1.90/ | |||||
| R12 | HCO + O = CO2 + H | 0.3000 × 1014 | 0.00 | 0.0 | [23] |
| R13 | HCO + H = H2 + CO | 0.1000 × 1013 | 0.00 | 0.0 | [23] |
| R14 | HCO + OH = H2O + CO | 0.5000 × 1014 | 0.00 | 0.0 | [23] |
| R15 | HCO + HO2 = H2O2 + CO | 0.4000 × 1012 | 0.00 | 0.0 | [23] |
| R16 | HCO + HO2 => H + OH + CO2 | 0.3000 × 1014 | 0.00 | 0.0 | [23] |
| R17 | O2 + CO = CO2 + O | 0.2530 × 1010 | 0.00 | 0.0 | [23] |
| R18 | O2 + HCO = HO2 + CO | 0.1000 × 1015 | 0.00 | 47,700.0 | [23] |
| R19 | OH + OH (+M) = H2O2 (+M) | 0.7400 × 1014 | −0.370 | 0.0 | [23] |
| /LOW / 0.2300 × 1019 −0.900 −1700.0 /TROE/ 0.7346 94.00 1756.0 5182.0 /M/ H2/2.00 /H2O/6.00/ CO/1.50/ CO2/2.00/ AR/0.70/ | |||||
| R20 | H + O2 = OH + O | 3.52 × 1016 | −0.7 | 17,061.4 | [24] |
| R21 | H2 + O = OH + H | 5.06 × 104 | 2.67 | 6287.6 | [25] |
| R22 | H2 + OH = H2O + H | 1.17 × 109 | 1.3 | 0.0 | [26] |
| R23 | H + O2 (+M) => HO2 + (M) | 4.6 × 1012 | 0.4 | 0.0 | [27] |
| /LOW / 1.737 × 1019 −1.23 0.0 /M/ AR/0.0/ H2/1.3/ H2O/10.0/ CO/1.9/ CO2/3.8/ | |||||
| R24 | H + H + (M) => H2 + (M) | 1.30 × 1018 | −1 | 0.0 | [25] |
| /M/ H2/2.5/ H2O/12.0/ CO/1.9 /CO2/3.8/ AR/0.5/ | |||||
| R25 | H + OH (+M) => H2O (+M) | 4.00 × 1022 | −2 | 0.0 | [25] |
| /M/ H2/2.5/ H2O/12.0/ CO/1.9/ CO2/3.8/ AR/0.38/ | |||||
| R26 | HO2 + H => OH + OH | 7.08 × 1013 | 0.0 | 298.8 | [28] |
| R27 | HO2 + H = H2 + O2 | 1.66 × 1013 | 0.0 | 821.8 | [27] |
| R28a | HO2 + OH = H2O + O2 | 2.89 × 1013 | 0.0 | −500 | [29] |
| R28b | HO2 + OH = H2O + O2 | 2.456 × 1013 | 0.0 | −497 | [27] |
| R29 | HO2 + HO2 = H2O2 + O2 | 1.300 × 1011 | 0.00 | −1.630 × 103 | [27] |
| R30 | H2O2 + H = H2 + HO2 | 7.7 × 1012 | 0.0 | 3755 | [29] |
| R31 | O + H2O = OH + OH | 2.97 × 106 | 2.02 | 1.340 × 104 | [28] |
| No. | Fuel Mixture | Model | Initial Pressure (bar) | Initial Temperature (K) | Composition in Volume Fractions (%) | Eq. Ratio | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Fuel 1 | CH4/H2 | Ignition delay time | 5, 10, 20 | 1050–1820 | Type1 | 80/20 | 0.5 | [39] | |
| Type 2 | 60/40 | ||||||||
| Type3 | 40/60 | ||||||||
| Type4 | 20/80 | ||||||||
| Fuel 2 | CH4/H2 | Laminar Flame speed | 1 | 298 | Type 1 | 80/20 | 0.5–1.3 | [40] | |
| Type 2 | 50/50 | ||||||||
| Type 3 | 10/90 | ||||||||
| Fuel 3 | CH4/H2 | Laminar Flame Speed | 1 | 300 | Type 1 | 100/0 | 0.65–1.1 | [41,42] | |
| Type 2 | 85/15 | ||||||||
| Type 3 | 70/30 | ||||||||
| Fuel 4 | CH4/H2/CO | Laminar Flame speed | 1 | 295 | Type1 | 20/40/40 | 0.75–1.3 | [43] | |
| Type 2 | 40/30/30 | ||||||||
| Fuel 5 | CH4/H2 | Species concentration profiles | 1, 10 | 900–1450 | Type 1 | 100/0 | 0.1, 0.6 and 1.5 | [44] | |
| 1 | 950–1125 | Type 2 | 50/50 | 0.1 and 0.6 | |||||
| Fuel 6 | CH4/H2/H2O/CO/CO2/N2 | Species concentration profiles | 1.3 | 990 | Type 1 | 14/16/25/19/14/12 | 0.5 | [45] | |
| Type 2 | 14/32/18/12/12/12 | ||||||||
| Fuel 7 | H2/CO/CH4 | Reaction sensitivity analysis | 10 | 1125 | 30/30/40 | 0.5, 1.0 and 1.5 | [43] | ||
| Fuel 8 | CH4/H2 | Reaction sensitivity analysis | 10 | 1100 | Type 1 | 20/80 | 0.5 | [40] | |
| Type 2 | 80/20 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianidis, N.; Azimov, U.; Birkett, M. Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism. Energies 2019, 12, 2442. https://doi.org/10.3390/en12122442
Stylianidis N, Azimov U, Birkett M. Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism. Energies. 2019; 12(12):2442. https://doi.org/10.3390/en12122442
Chicago/Turabian StyleStylianidis, Nearchos, Ulugbek Azimov, and Martin Birkett. 2019. "Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism" Energies 12, no. 12: 2442. https://doi.org/10.3390/en12122442
APA StyleStylianidis, N., Azimov, U., & Birkett, M. (2019). Investigation of the Effect of Hydrogen and Methane on Combustion of Multicomponent Syngas Mixtures using a Constructed Reduced Chemical Kinetics Mechanism. Energies, 12(12), 2442. https://doi.org/10.3390/en12122442






