Abstract
In reading, text difficulties increase the duration of eye fixation and the frequency of refixation and regression. The present article reviews previous attempts to quantify these effects based on the frequency of effect theory (FET), and links these effects to results from microstimulation of primate supplementary eye fields. Observed stimulation effects on the latency and frequency of visually-guided saccades depend on the onset time of electric current relative to target onset, and the strength of applied current. Resultant saccade delay was only observed for those made towards a highly predictive location ipsilateral to stimulated SEF sites. These findings are interpreted in the context of reading, where the detection of processing difficulty allows a suppression signal to supersede a forward saccade signal in a time race. This in turn permits a cognitively-based refixation/regression to be initiated in place of the suppressed forward saccade.
Introduction
Effects of Reading Difficulty on Eye Movements
Reading involves complex perceptual, linguistic, and ocular processes (Rayner and Pollatsek, 1989; Rayner, 1998). A skilled reader makes three to four jerky eye movements (saccades) along the line of text, with the duration of most eye fixations mostly between 150 and 300ms (Harris, Hainline, Abramov, Lemerise, Camenzuli, 1984, McConkie, Underwood, Zola, Wolverton, 1985; McConkie and Dyre, 2000; Suppes, 1989). About 80 to 90% of saccades in reading are made in the forward direction (Frazier and Rayner, 1982; Mitchell, Shen, Green, & Hodgson, 2008; Vitu and McConkie, 2000). In addition, 5 to 15% of saccades refixate on the same word depending on the initial fixation position on that word (Rayner and Pollatsek, 1987; McConkie, Kerr, Reddix, & Zola, 1989; Vitu, McConkie, & Zola, 1998).
The variability in fixation duration appears to reflect difficulties in processing text content (Just and Carpenter, 1980; Morrison, 1984; Raney and Rayner, 1996; Rayner and Duffy, 1987; Rayner and Pollatsek, 1981). Such an effect in fixation duration reflects a change to part of the frequency distribution of fixation duration (McConkie, Underwood, Zola, Wolverton, 1985; McConkie, Underwood, Wolverton, & Zola, 1988). When a low-frequency or contextually-ambiguous word is encountered, the duration of the initial fixation on that word increases and the following saccade is more likely to be a regressive one, and/or a refixation on the same word (Frazier and Rayner, 1982; Inhoff and Rayner, 1986; Pynte and Kennedy, 2006; Rayner and Frazier, 1987; Weger and Inhoff, 2007; van Gompel, Pickering, & Traxler, 2001).
Prominent models of eye movements in reading suggest that a saccade is delayed or cancelled, and a refixation to the difficult word is made, in order to increase the processing time for that word. Models of direct cognitive control assume that a planned forward saccade is triggered after achieving a certain benchmark of processing an attended word. Difficulty in processing that word cancels the planned saccade and replaces it with a refixation (e.g., Reichle, Pollatsek, Fisher, & Rayner, 1998; Reichle, Payner, & Pollatsek, 2003). Models of adaptive ocular strategy allow saccades to be triggered at predetermined times and frequencies; text difficulty interrupts such an ocular strategy and triggers refixation instead (Levy-schoen, 1981; O’Regan and Levy-Schoen, 1987; Vitu, O’Regan, Inhoff, & Topolski, 1995). Other models assume flexible modification of saccade plan that sends the gaze to the word with the highest lexical/linguistic attractiveness, which leads to refixation and regression to a difficult word (Engbert, Longtin, Kliegl, 2002; Engbert, Nuthmann, Richter, & Kliegl, 2005; Reilly and Radach, 2003).
George W. McConkie and colleagues have established a line of work addressing the issue of probabilistic cancellation or delay of planned saccades in response to reading difficulty. Based on their findings, McConkie and colleagues proposed a frequency of effect theory (FET) to qualitatively and quantitatively account for such a change in saccade latency (McConkie, Zola, Wolverton, 1985; McConkie, Reddix, & Zola, 1992; McConkie, Kerr, & Dyre, 1994). These earlier studies however did not address the effect of reading difficulty on regression/refixation frequency.
This article is a tribute to McConkie’s work. It reviews behavioral observations of saccade latency change underlying the FET, and evaluates new behavioral and neural evidence related to the occurrence of regression/refixation (Yang, 2005; Yang, Missal, & Heinen, 2008). These reviewed findings are then explained within the context of a behavioral/neural mechanism of eye movement control in reading. Predictions on eye movements in reading are also derived from these findings. Because of the limit of a review article, readers interested in detailed research methods involved in these evaluated studies should consult the original articles.
Frequency of Effect Theory
Original observations. The frequency of effect theory (FET) was initially proposed based on observed changes in the distribution of fixation duration in response to difficulties in passage reading (McConkie, Zola, & Wolverton, 1985). In the earlier study, selected words in text passage were replaced with pesudowords beforehand, and the duration of initial fixation on these alternate words were analyzed. A rightward (longer duration) shift in the frequency distribution of fixation duration was observed starting at 160ms. McConkie and colleagues concluded that the effect of the alternate pseudowords would be best accounted for with a frequency of effect analysis, which quantifies the effect of encountered difficulties as the change in saccade frequency (or fixation termination) at each time interval and the duration of delay for the affected saccade.
In a later article, McConkie, Reddix, and Zola (1992) laid out three assumptions of the FET: (a) Processing difficulty in reading can only delay saccades scheduled to occur at a time after the ocular system is informed of the difficulty and has adequate time to exert a delay; (b) only a portion of saccades occurring after the initial onset time of saccade delay are affected; (c) all effected saccades are delayed for a fixed amount of time. By manipulating these three parameters (initial onset time, affected proportion, and effect size of delay), they obtained an estimation of 160ms for the onset of saccade delay, 32% of affected saccades, and a delay of 28ms for data from the above study (McConkie et al., 1985). Note that these earlier studies and the original FET did not consider the direction of delayed saccades, such as whether the same portion of forward and regressive saccades is delayed and whether the delay has the same duration.
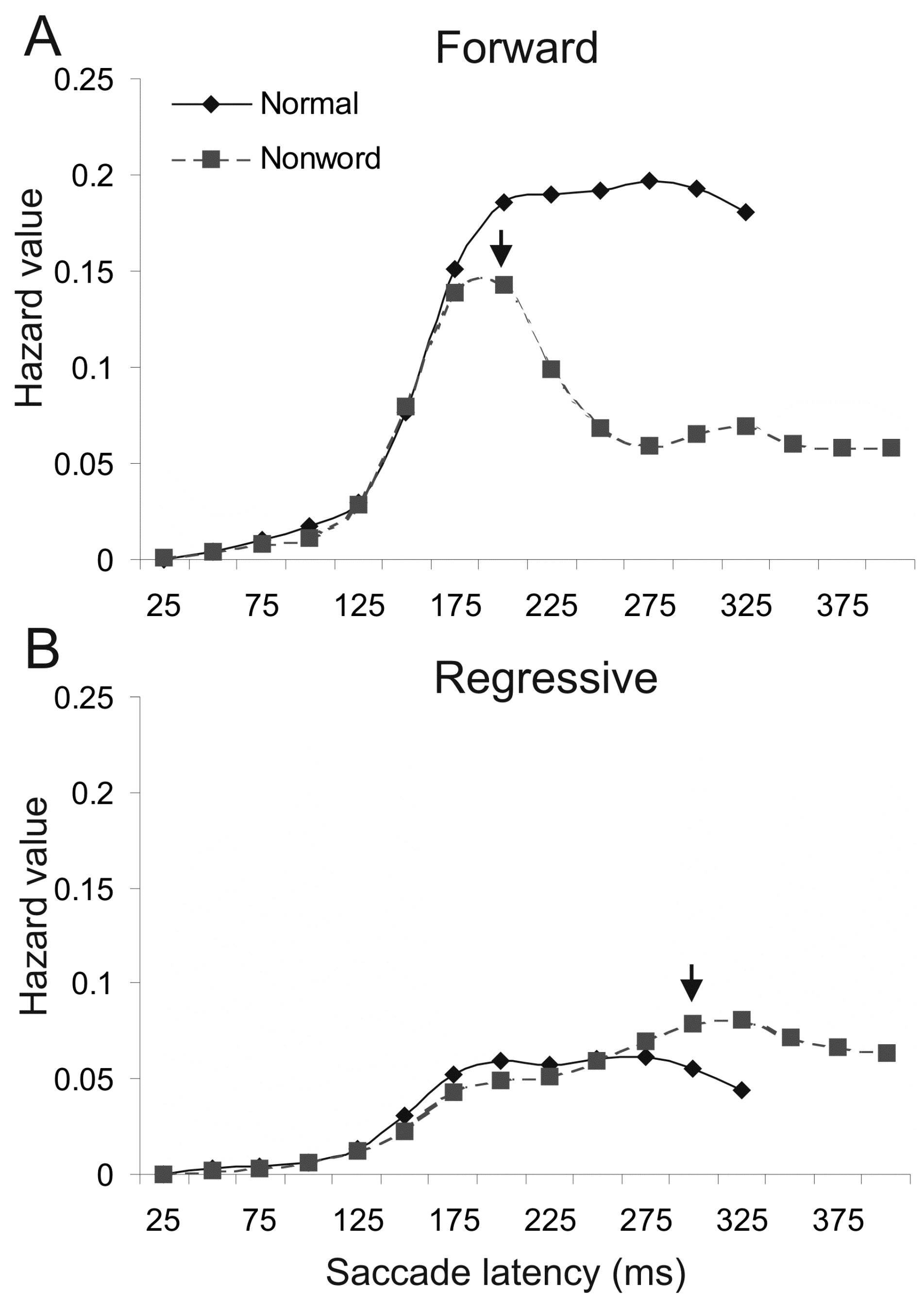
Differential effect on forward and regressive saccades. To investigate the saliency of text difficulties on saccade delay in reading, Yang and McConkie (2001, 2004) used a single-fixation displacement method to occasionally replace a page of normal text with nonwords for a single fixation. Different from the earlier studies, they analyzed the change in hazard value for forward and regressive saccades separately. Hazard value is a good indicator of saccade probability, as it is calculated based on the frequency of occurring saccades relative to that of saccades still having not occurred (Elandt-Johnson and Johnson, 1999; Janssen and Shadlen, 2005). Figure 1A shows the resultant hazard curves for forward saccades, computed from the frequency distribution for normal text and nonwords conditions (see Yang and McConkie 2001 for the method of calculation). The curves were cut off when 95% of saccades had occurred. Here the arrow indicates the earliest time when the curve for the nonword condition significantly departed from that for normal text. The initial onset time of reduced probability for forward saccade was 200ms for nonwords. Figure 1B shows the same conditions for regressive saccades, revealing no delay at 200ms but significantly increased probability of regressive saccades for nonwords at 300ms, a 100ms lag after the initial decrease in forward saccades. FET analysis based on the frequency distribution of fixation duration for the same data resulted in a 200ms onset time, 41% of affected saccades, and a delay of 52ms for affected saccades. Compared to the earlier results (McConkie et al., 1985), the nonwords condition resulted in a larger effect size (52 vs. 28ms) and a greater proportion of affected saccades (41% vs. 32%). The larger proportion and effect size of affected saccades likely reflects the greater stimulus saliency (single nonword vs. whole page of nonwords).
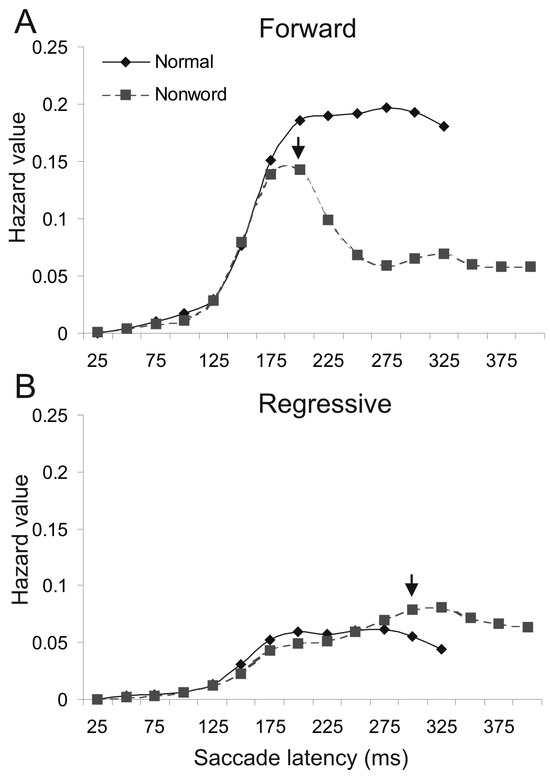
Figure 1.
Likelihood (hazard value) of forward and regressive saccades at different times during the critical fixation for normal text and nonwords conditions. Hazard value was calculated by dividing the frequency of forward or regressive saccade within each 25-ms time bin with that of not-yet-occurring all saccades. Z test was conducted to determine the difference between the two conditions at each time bins (α = .05). The earliest significant difference was marked with an arrow. A. Forward saccade hazard curves. B. Regressive saccade hazard curves.
The increase of regressive saccades with nonwords suggests saccades were not merely delayed; rather, regressions were planned and executed in response to the detection of nonwords. The readers likely attempted to make a refixation to re-inspect the foveated words and some of them were regressive. This is confirmed by the accompanying finding that the additional regressions were mostly 1to 4-letter length (Yang and McConkie, 2001).
The 100ms lag between the onset of forward saccade reduction and the initial increase of regressive saccade is much longer than the estimated 52ms with the FET. This is not surprising, as the original estimate of saccade delay was calculated based on both forward and regressive saccades. Note half of saccades were regressive after the 250ms interval for the nonwords condition (see hazard levels in Figure 1A and 1B), and regressive saccades were not reduced but increased with nonwords. The actual proportion of delayed saccade likely would be at least doubled when only forward saccades were taken into account, thus the 52ms is an underestimation.
To investigate how the detection latency of reading difficulties affects saccade latency, McConkie and Yang analyzed a set of data obtained from a study in which various types of text difficulty were created by replacing single original words with alternate text stimuli (McConkie and Yang, 2003; Yang, 2002). Subjects read alternate versions of passages containing either the original words or the alternate stimuli without utilizing any gaze-contingent manipulation. Hazard curves for initial fixations in normal (original), nonwords, syntactic, and discourse difficulties were calculated for forward and regressive saccades. Results show that frequency reduction to forward saccades first occurred at 225ms for nonwords, 250ms for syntactic difficulties, and at 275ms for discourse difficulties. The initial increase of regression probability was found at 300, 350, and 350ms for these three conditions, although the increase was quite small, with no significant departure from the normal condition. These additional regressive saccades were either short with 1- to 3-letter length, or longer between 6-to 8-letter lengths. Some of these regressive saccades were likely refixations and the rest were aimed at a previously fixated word.
Together, the above findings suggest that eye movement changes in response to processing difficulty in reading can be best described as a reduction in the frequency of forward saccades and a later increase of refixations or regressions. The latency of detecting the difficulty (nonwords, syntactic or discourse related) and the saliency of difficulty (e.g., single word in fovea vs. whole page) likely determine the initial onset time of reduced saccade frequency as well as the proportion of affected saccades.
Neurophysiological Bases for Cognitive Control
SEF involvement in cognitive control. Cognitive control of motor responses is chiefly mediated by the frontal cortex (Passingham, 1985, 1993). Among the frontal cortical structures involved in ocular control, the supplementary eye field (SEF) is a good candidate for exerting the effects of processing difficulty described above (Schlag and Schlag-Rey, 1987; Tehovnik, 1995; Tehovnik and Lee, 1993; Tehovnik and Sommer, 1997). Anatomically, the SEF is a frontal premotor structure involved in object-centric ocular responses (Russo and Bruce, 1993, 1996, 2000; Park, Schlag-Rey, & Schlag, 2006; Schall, Morel, & Kass, 1993), helping specify an ocular response relative to a visual landmark or decision boundary (Olson, 2003; Olson and Gettner, 1995, 1996; Trembley, Gettner, Olson, 2002). It receives inputs from visual structures such as the occipito-temporal and inferior-temporal (IT) cortex where visual/orthographic information is encoded (Nobre, Allison, & McCarthy, 1994; Pandya and Yeteria, 2002), and from the lateral intra-parietal (LIP) cortex where spatial location of visual objects is processed and updated for saccade guidance (Luppino, Matelli, Camarda, & Rizzolatti, 1993). The SEF also projects to the frontal eye field (FEF), superior colliculus (SC), and brainstem where saccade preparation is carried out and saccade metrics are specified (Fries, 1985; Hartmann-von Monakow, Akert, & Kunzle, 1979; Huerta and Kaas 1990; Schall, Morel, Kaas, 1993; Shook, Schlag-Ray, & Schlag, 1990). These observations point to a likely SEF role in transforming the signal for a nonspatial processing difficulty into a specific ocular command relative to a visual landmark.
Functionally, the SEF has been shown to participate in ocular go/nogo decision and in initiating antisaccades. Human SEF is more active when making the decision to withhold a saccade/pursuit than it is for either the same visual stimulation or eye movements but no decision is required (Heinen, Rowland, Lee, & Wade, 2006). Separate populations of neurons in monkey’s SEF signal go or nogo decision respectively (Heinen, Hwang, & Yang, 2011; Kim, Badler, Heinen, 2005; Yang, Ford, Hwang, & Heinen, 2010). In performing the antisaccade task, neurons in the SEF signal the withheld of ipsilateral prosaccade saccade, as well as the planning and execution of contralateral antisaccades (Park, Schlag-Rey, & Schlag, 2006; Schlag-Rey, Amador, Sanchez, & Schlag, 1997; Stuphorn and Schall, 2006; Stuphorn, Taylor, & Schall, 2000). Abolition of the SEF leads to little deficit in visually-guided saccades, but heightens difficulties in executing go/nogo ocular decision and antisaccade tasks (Schiller and Chou, 1998; Tehovnik, Sommer, Chou, Slocum, & Schiller, 2000).
Potential SEF involvement in human reading is supported by a recent optical imaging study (Yang, Tai, & McConkie, 2003). Using the same single-fixation replacement method documented by Yang and McConkie (2001), the study showed that human SEF displayed heightened activity when human readers encountered reading difficulties. The heightened neural activity was temporally linked to the critical fixations when nonwords were present, compared to activity with normal text. The functional location of the SEF region in this study was confirmed with the antisaccade paradigm.
While the above findings are not direct evidence of human SEF involvement in exerting the cognitive signal in response to text difficulty, they suggest likely SEF involvement in the needed cognitive control of eye movements in reading.
SEF microstimulation studies. To assess whether the SEF can exert changes on saccades in a manner consistent with the frequency of effect findings reported above, a series of microstimulation studies were conducted (Yang et al., 2008; Yang et al., 2010). These experiments specifically addressed the following questions: (a) Does stimulating the SEF at different times affect the latency and proportion of affected saccades in a manner consistent with the observed effects with various types of processing difficulty in reading? (b) Does the strength of SEF stimulation mimick the saliency effect of processing difficulty on the proportion and effect size of affected saccades? (c) Would the effect of SEF stimulation on saccades be directionally selective and dependent on the anticipated direction of visual target as in reading?
To these ends, monkeys were trained to make a saccade to a target (white circle extended 0.5° of visual angle), which randomly appeared at horizontal locations 10° to the left or right of a fixation point ((see Yang et al., 2008 for details). In each experimental session, an electrode was first lowered into an implanted chamber above the SEF, and an effective site was found by delivering a train of stimulating current and observing its effect with online monitoring of saccade initiation time. Most sites were identified with current amplitudes of 50 to75 microA and stimulation duration of 100ms. After an effective site was identified, trials with various stimulation parameters (current onset time, current amplitude, and target locations) were conducted. Note all sessions did not result in an equal number of trials; about 300 to 600 trials were conducted for each site.
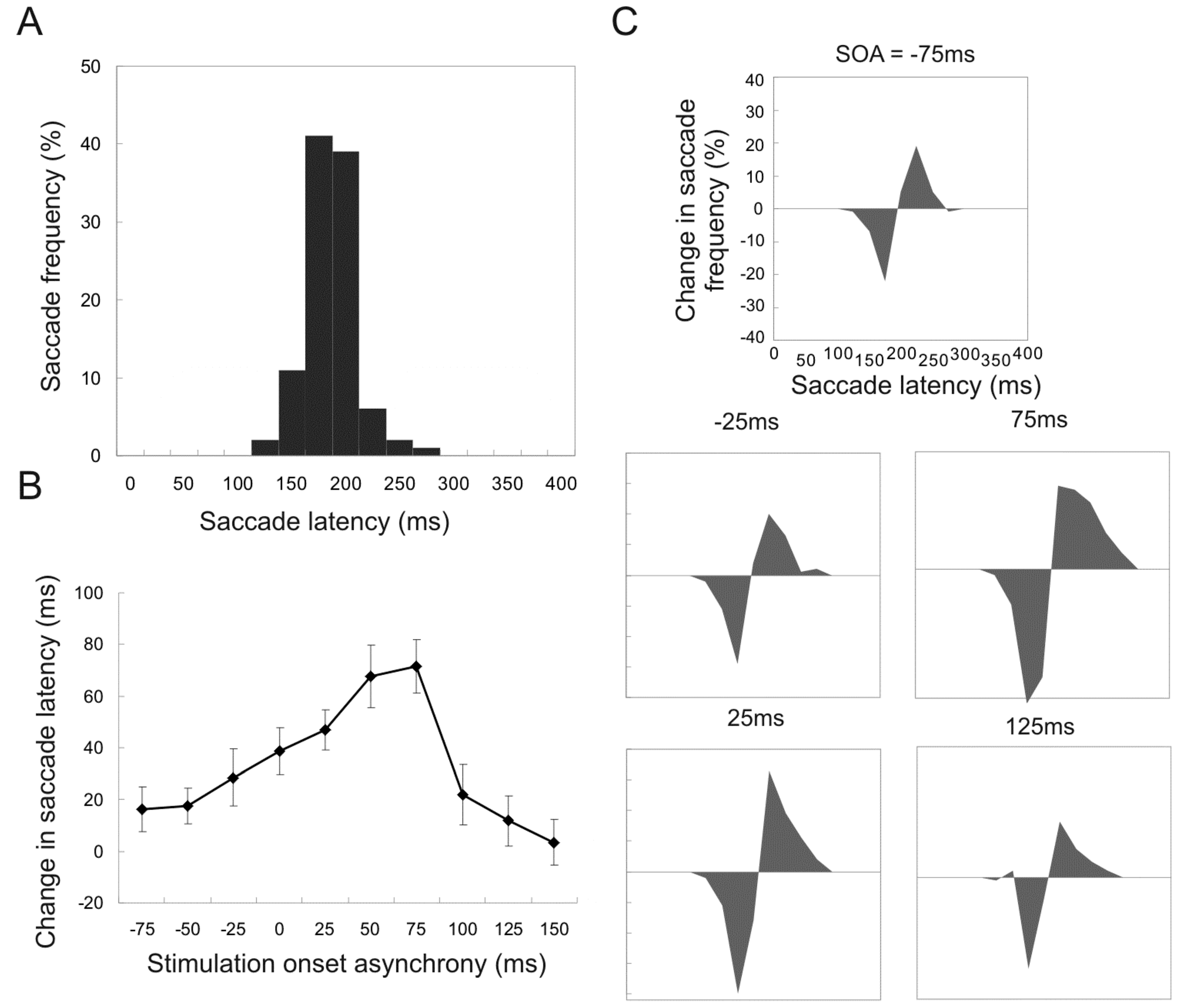
Stimulation onset time. In the first experiment, 7 experimental sessions were conducted in which the stimulation onset times (SOA) was varied but the current amplitude (75 to 100microA) and duration (100ms) were kept constant. Figure 2A shows the frequency of saccade latency for control trials (no current stimulation) pooled from 7 sessions. Note the peak frequency was about 175ms.
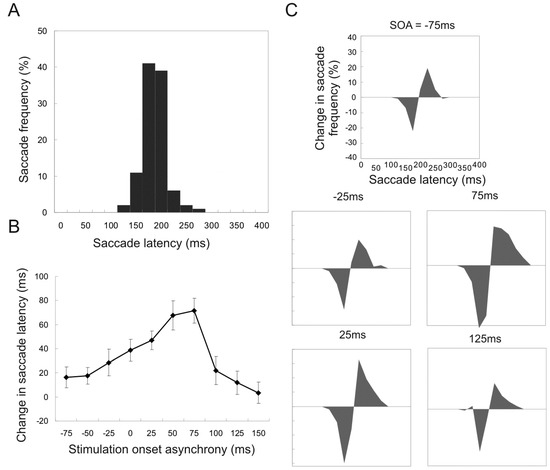
Figure 2.
Changes in the frequency of affected saccades and mean saccade latency for trials with different stimulation onset times relative to target onset (stimulation onset asynchrony, or SOA). A. Frequency of saccade latency for control trials (no stimulation). B. Differences in mean saccade latency between control trials and stimulation trials with various SOAs. Error bars indicate the 95% confidence intervals. C. Changes in saccade frequency for simulated trials relative with different SOAs, compared to the frequency for control trials. The area below the zero indicates reduced frequency and that above increased frequency.
Figure 2B shows the difference in mean saccade latency between control and stimulated conditions. The difference in saccade latency increased and then decreased as SOA was shifted to the right, and the maximal difference was found with +75ms SOA, when the duration of stimulation coincides with the period of highest saccade frequencies without stimulation. This is based on the assumption that it requires a latency of 50 to 75ms to generate the delay effect on saccade initiation (see Yang et al., 2008). Figure 2C shows the frequency of affected saccades for different SOAs; the area below zero indicates reduced frequency and that above zero increased frequency. Data were pooled from all 7 sessions, with the frequency for control trials subtracted from that of stimulated trials recorded in the same session. Resultant distributions show maximal reduction in frequency at 175ms, and with +75ms SOA.
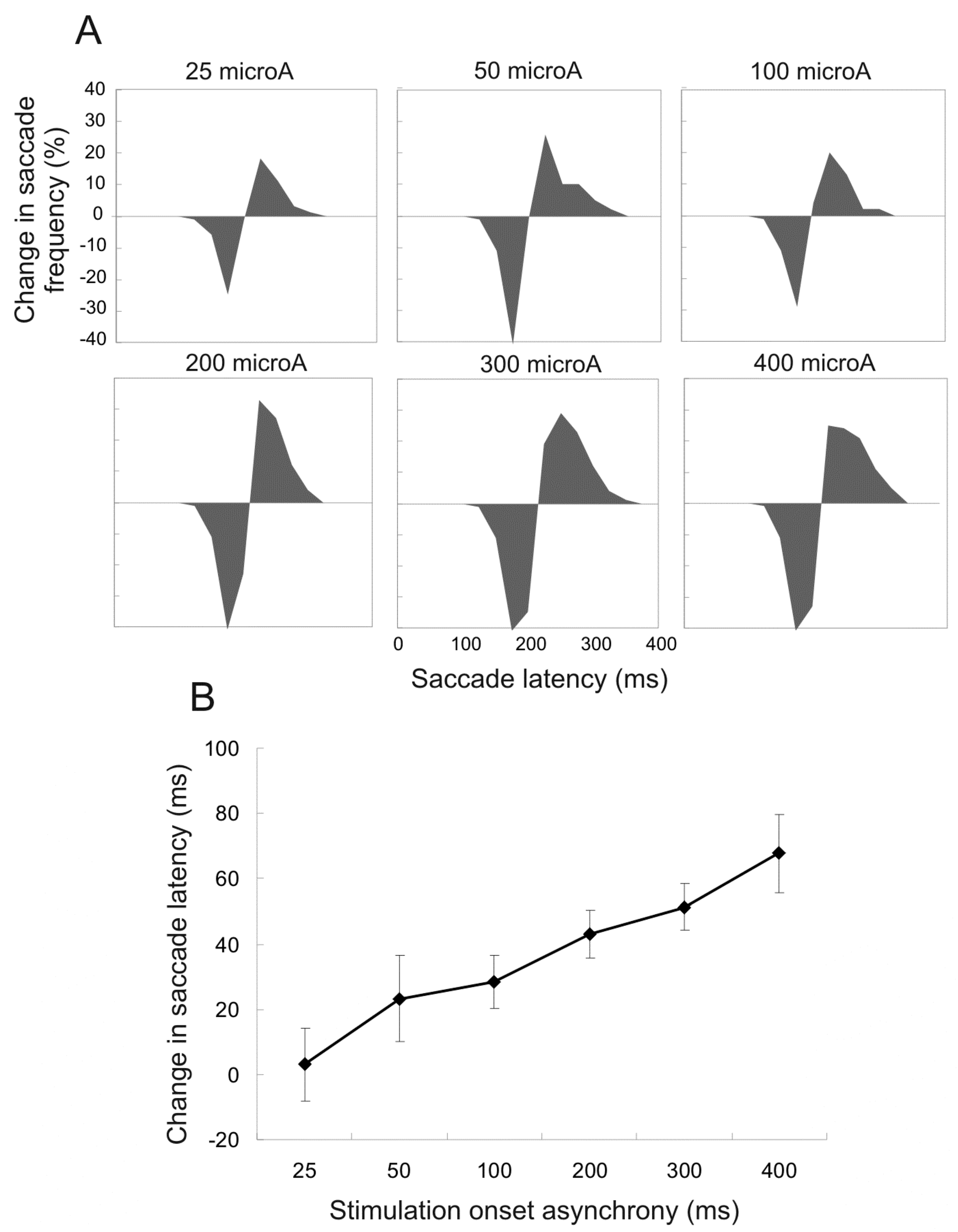
Current amplitude. Current amplitude was also systematically manipulated in 6 sessions with the SOA kept constant at +75ms and duration at 100ms. Figure 3A shows the proportion of affected saccades with different current amplitudes (25 to 400microA). As the current was heightened, the proportion of affected saccades also increased; the difference in distribution grew narrower but was not shifted further to the right (longer latencies). Figure 3B shows the mean difference in saccade latency. The increase of current amplitude (20 to 400microA) was accompanied by greater increase of saccade latency; this was due to a greater proportion of delayed saccades rather than an increase of effect size.
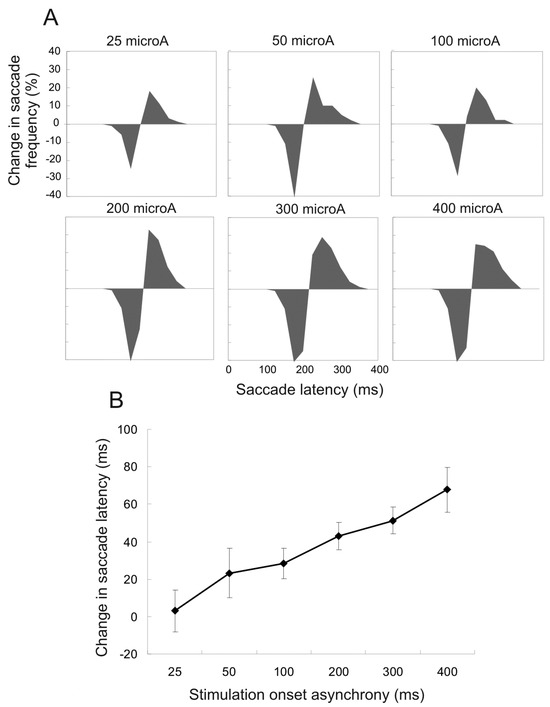
Figure 3.
Changes in saccade frequency and mean saccade latency for trials with various amplitudes of stimulation current, compared to control trials. All stimulations had +75ms SOA and 100ms duration. Illustrations are the same as in Figure 2. A. Changes in saccade frequency for different current amplitudes. B. Changes in mean saccade latency for corresponding trials. Error bars indicate 95% confidence intervals.
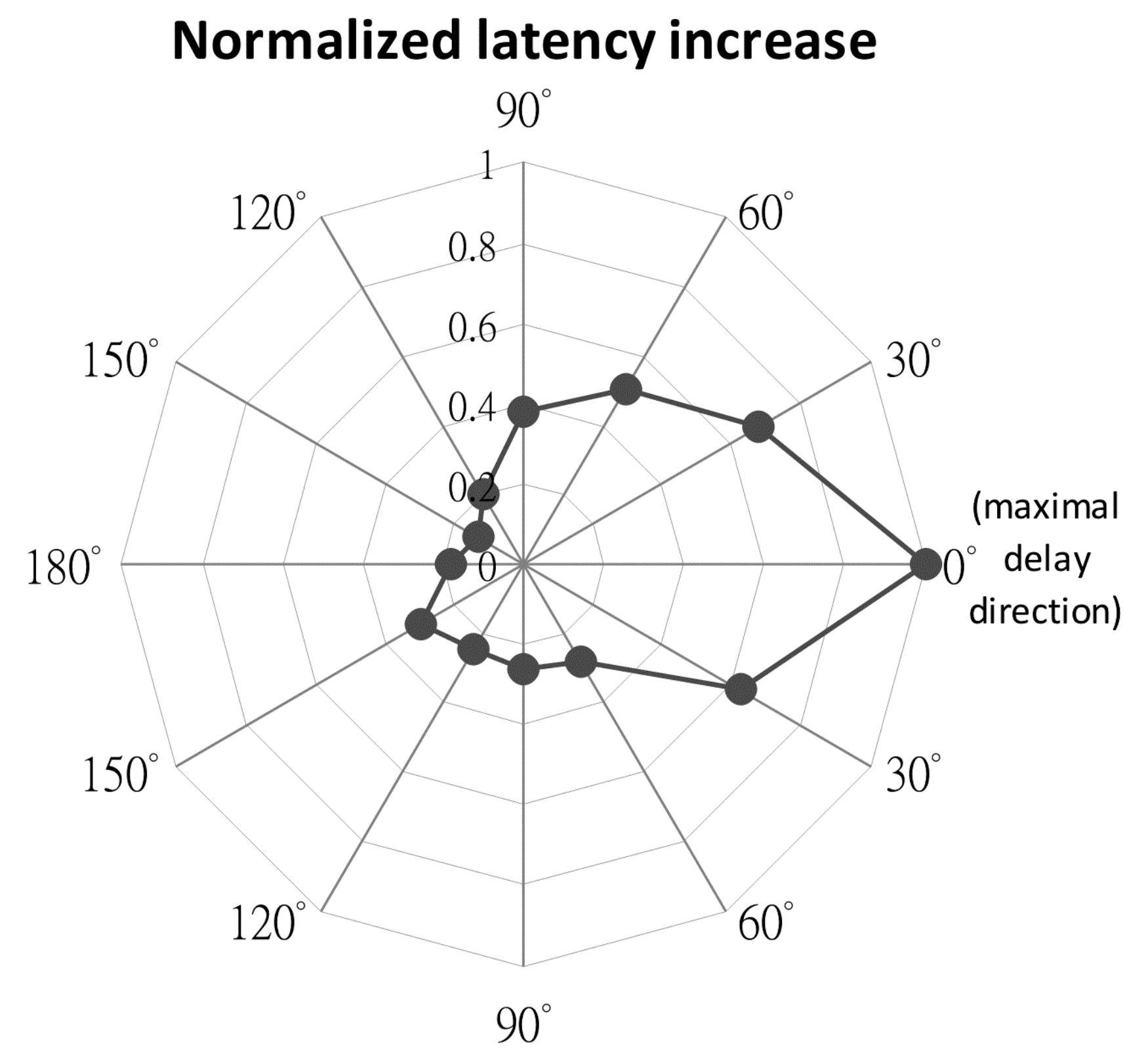
Effected saccade direction. To investigate the spatial extent of the stimulation effect, stimulation (50 or 75microA, +75ms SOA, 100ms duration) was delivered while the monkey was making a saccade toward a target randomly presented at 10° eccentricity and in one of 12 directions (equally spaced by 30° radial angles). Results were recorded from 11 SEF sites. To quantify the spatial tuning of the stimulation effect, the latency change (stimulated - control) at different target locations was normalized relative to the location with the maximum difference. To combine results from all 11 sites, the normalized magnitude of difference for the 12 directions was first rotated and aligned to the direction with the maximal delay. The normalized scores were then averaged for the 12 aligned angles for all sites. Figure 4 shows the normalized mean latency differences. Each point represents the average of the normalized scores of the realigned angles for all sites, with 0° indicating the direction with maximum latency change. Gaussian estimation reveals that the spatial extent of significant saccade delay (SD = 21.4°) is limited to 172° of radial angle (α = .05). Note that the maximal delay direction for all 21 sites was ipsilateral to the stimulation SEF site.
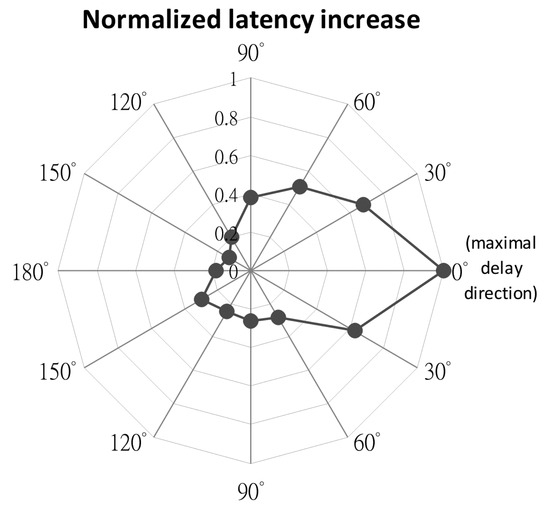
Figure 4.
Normalized change in saccade latency between stimulated and control trials. The mean increase in saccade latency recorded from 21 sessions was normalized in relation to the maximal delay recorded from each site. Rotation of target direction was done to align the results to the maximal delay direction (at 0°) before averaged across all sites.
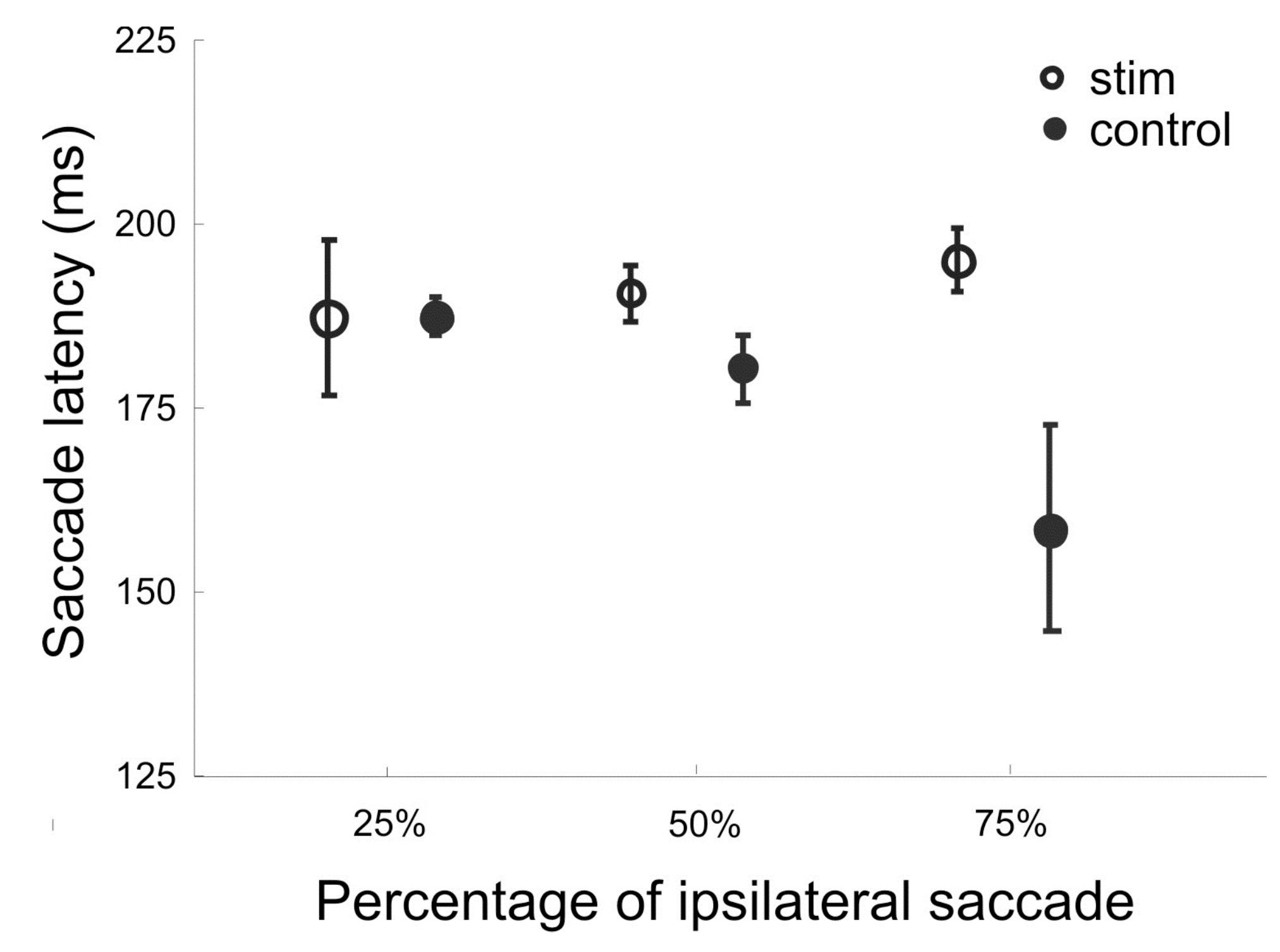
In reading, there is clear expectation about saccade direction. To determine whether effect of SEF stimulation depends on such directional anticipation, in 10 stimulation sessions the target was displayed at one of the two horizontal locations at 10° eccentricity. In three separate blocks with 200 trials in each block, the target appeared at the ipsilateral location in 50%, 75%, or a 25% frequency. Target direction was randomly interleaved in each trial block.
Figure 5 shows the mean saccade latency for stimulated and control trials pooled from 9 ipsilateral delay sites. Results from trial blocks with different frequencies of target location were plotted separately. It reveals that without stimulation, the mean latency of ipsilateral saccades was shorter with higher target frequency. With stimulation, saccade latency was increased more with 75% of target frequency, and less so with 50% frequency. As a result, the mean latency was the same for all three frequencies with stimulation applied to the ipsilateral SEF. Therefore, the effect of current stimulation is best characterized as the removal of any facilitating effect associated with anticipated target location.
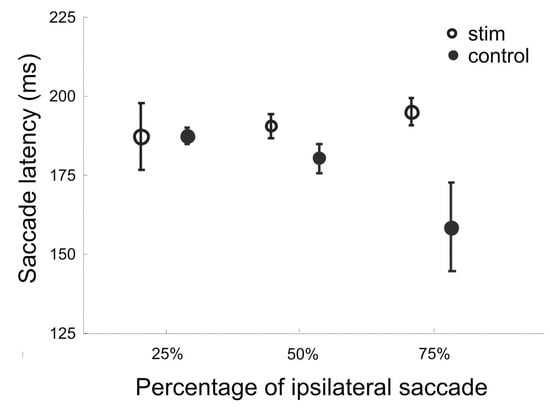
Figure 5.
Mean saccade latency for blocks of trials with different target frequencies. The target appeared at an ipsilateral location of stimulated SEF sites with 25%, 50%, or 75% in separate blocks. Stimulated and unstimulated control trials were interleaved within a block. Trials from 10 sessions were pooled together to compute the means and 95% confidence intervals. Only data from trials with ipsilateral target locations were included in the analysis.
Together, these microstimulation experiments reveal several important findings regarding the SEF role in saccade control. First, the latency change induced by SEF stimulation depends on when the current is delivered. The stimulation current affects more saccades when its duration optimally overlaps the time intervals with the highest saccade frequency without stimulation. This is consistent with the observed different onset times and proportions of affected saccades for various types of processing difficulty (McConkie and Yang, 2003). Second, the effect size of saccade delay and the proportion of affected saccades increase in relation to the amplitude of stimulation current. These can account for the longer delay and more affected saccades for the condition with whole-page nonwords than a single nonword. Third, the stimulation effect is spatially tuned. Visually-guided saccades toward a direction ipsilateral to the stimulated SEF site are maximally delayed, but there is little delay effect for the opposite, contralateral direction. Finally, SEF stimulation delays visually-guided saccades by removing the facilitation of location anticipation. There was little effect on saccade latency when the target appeared at the less anticipated location. The last two findings are consistent with the selective cancellation of forward saccades and the addition of regressive saccades in response to reading difficulty.
A Neural/Behavioral Mechanism for Cognitive Control in Reading
The above observations show several parallel similarities between effects of reading difficulty and SEF stimulation. They suggest that the SEF can help resolve reading difficulty by preventing forward saccades, hence slowing down the progression of gaze shift alone the line of text in reading. To illustrate how a neural mechanism involving the SEF can exact the theorized cognitive control within the context of reading, the following sections provide an account on how neural processes are coordinated to exact the observed effects on eye movements. The illustrated mechanism is not intended to be a welldefined model of neural control, but an illustration of likely SEF involvement based on presently known neural processes.
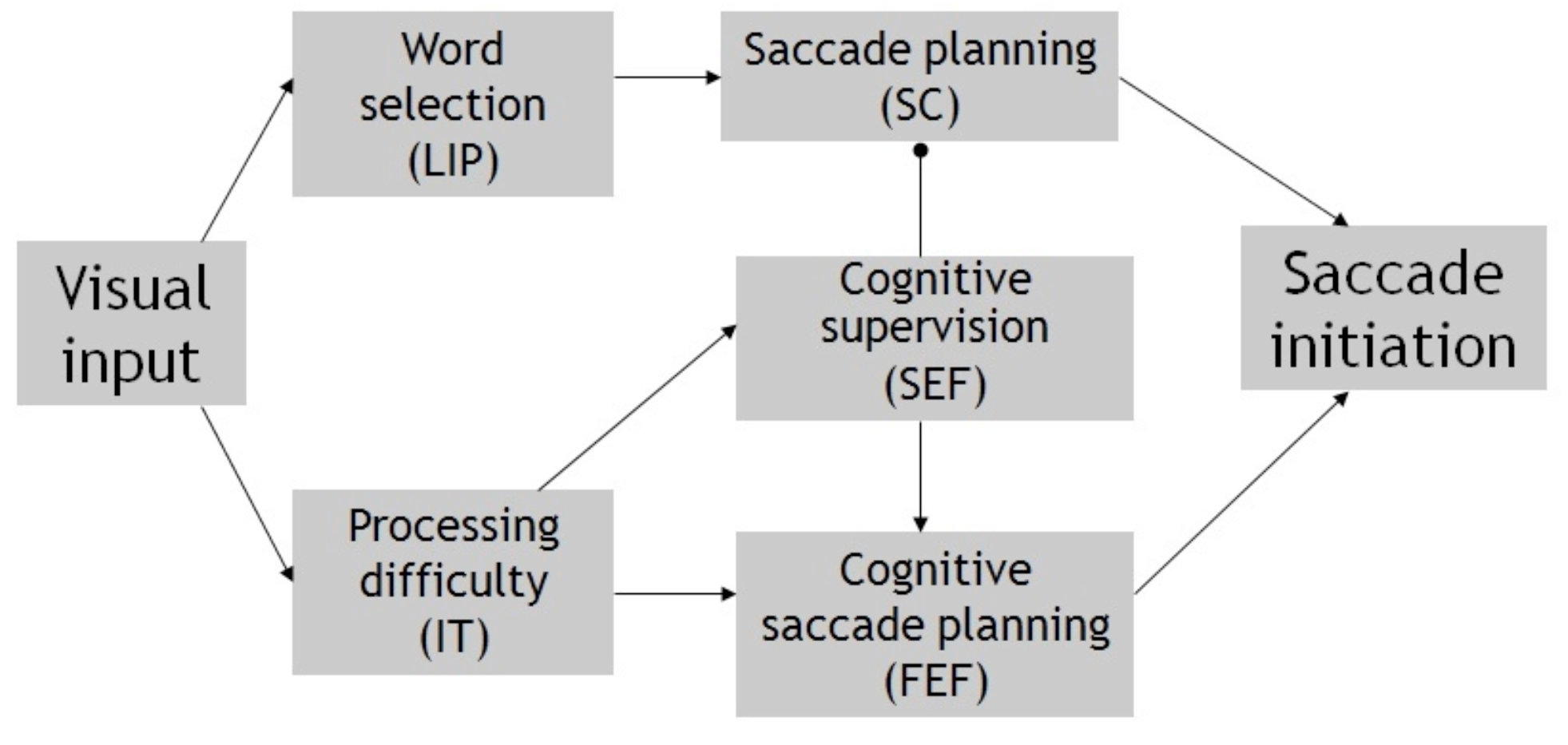
Neural processes for cognitive control. For the purpose of describing the neural control of saccade delay in reading, one can simplify the underlying neural mechanism of saccade initiation as consisting of two components: cortico-collicular and cortical (Trappenberg, Doris, Munoz, & Klein, 2001; Findley and Walker, 1999). Figure 6 illustrates the involved neural substrates and processes. The cortico-collicular component, including posterior cortical areas (e.g., lateral intra-parietal lobe, LIP) and the superior colliculus (SC), carries out the processes of selecting a visual target and converting the retinal error of the selected target into neural pulses that subsequently drive the ocular muscle (Colby, Duhamel, & Goldberg, 1995; Ferraina, Pare, & Wurtz, 2002; Konen, Kleiser, Bremmer, & Seitz, 2007; Schiller, 1998; Wurtz, Sommer, Pare, & Ferraina, 2001).
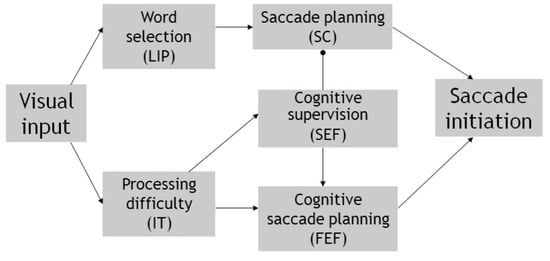
Figure 6.
A diagram summarizing the likely neural/behavioral mechanism of cognitive control in reading. Difficulty in orthographic difficulty is used as an example. Behavioral functions and underlying neural substrates (in parenthesis) are illustrated. Arrows indicate signal flows for saccade planning; the filled end circle indicates saccade suppression.
The frontal cortical system carries out the function of transforming cognitive, top-down decisions into an ocular signal that modifies or overrides the subcortical one (Stuphorn and Schall, 2006; Schall, Stuphorn, & Brown, 2002). In it, the frontal eye field (FEF) specifies the metrics (direction and amplitude) of an endogenously prepared saccade, whereas the supplementary eye field (SEF) encodes the preferred ocular response (e.g., go vs. nogo, left vs. right of a landmark) based on object-centric coordinates (Schall, 1991a, 1991b; Schall, Morel, & Kaas, 1993). The SEF achieves cognitive control by suppressing or reducing the cortico-subcortical activity augmented by direction anticipation in a spatially selective manner, while allowing an alternate saccade to be planned and executed by other regions such as the frontal eye field (FEF), which is also spatially selective. Such fine-tuned coordination is illustrated by the coinvolvement of the SEF and FEF in no/nogo and antisaccade tasks, and their differential involvement in saccade execution, with the SEF signaling the cognitively-based choice and the FEF executing the actual saccade plan (Yang, Heinen, Hwang, & Badler, 2006).
Eye movement control in reading. Figure 6 summarizes the possible involvement of the above neural substrates in effecting a change in saccade latency and direction in reading. It entails the scenario of encountering an orthographic difficulty (e.g., a low-frequency word) during an eye fixation. Spatial information afforded by multiple word stimuli is processed in parallel at the LIP, and a saccade is planned toward the center of a selected word, the gravity center of letter strings, or that of a visual blob, dependent on the assumed visual selection processing in reading (McConkie et al., 1988; O’Regan & LevySchoen, 1987; Vitu et al., 1995; Yang & Vitu, 2006). Because of attentional or ocular biases caused by directional anticipation, word stimuli in the forward direction (i.e., to the right in English reading) are more likely to be selected. The SC encodes the retinal error for the selected visual stimuli, and the retinal error of the selected word is transformed into temporal neural pulses in the brainstem (Keller and Edelman, 1994; Waitzman, Ma, Optican, Wurtz, 1991; Wurtz and Optican, 1995; Optican, 1996).
Taking place in parallel to the above process is the orthographic processing of word(s) present in the effective foveal region. This involves neural areas such as the inferior-temporal (IT) lobe, where neural activities reflect the processing of word stimulus and is heightened when more words and greater difficulty in word recognition is encountered (Hagoort, Indefrey, Brown, Herzog, Steinmetz, & Seitz, 1999; Nobre, Allison, & McCarthy, 1994; Rumsey, Horwitz, Donohue, Nace, Maisog, & Andreason, 1997).
Cognitive control in reading can be achieved by first generating a distressing (heightened) signal at IT that indicates significant orthographic difficulty. The SEF directly or indirectly receives the signal and in turn suppresses the visually-based forward saccade; it also signals the decision to refixate the current or previously fixated word. The FEF plans and executes a saccade that reflects the choice preferred by the SEF, and therefore its activity reflects the eventual saccade decision (see Heinzle, Hepp, & Martin, 2010 for the modeling of such saccade decisions).
Note that the above description serves to illustrate the likely neural mechanism of cognitive control in response to processing difficulty. It is not intended to account for the all aspects of eye movement control in reading. For detailed models of neural involvements in eye movement control, please consult other more comprehensive models (e.g., Heinzle et al., 2010; Reichle et al., 2003).
Implications and novel predictions. The above mechanism accounts for some critical behavioral observations in reading. First, the frequency of effect on fixation duration for different types of processing difficulty can be explained by the race between the visually-based saccadic signal and the cognitive control (suppressing) signal generated by the SEF. A more elementary processing center can detect the difficulty earlier and allow the SEF to exert a suppression signal faster. This explains why more saccades are cancelled at earlier times when encountering a nonword than a syntactic ambiguity. Second, the observed tendency of making refixations and regressive saccades in response to processing difficulty can be accounted for by the spatial tuning and objectcentric coordinates of cortically-based neural control (McPeek and Keller, 2002; Walker, Deubel, Schneider, & Findlay, 1997). Such spatial tuning allows a forward saccade to be suppressed and a regressive saccade or refixation be executed despite the ongoing inhibition of forward saccades. Third, the larger effect size and proportion of affected saccade with more salient orthographic difficulty can be explained by the stronger SEF signal relative to a constant threshold for saccade suppression. This allows the probability of affected saccade to increase, and/or the latency of reaching the threshold to be shortened (Carpenter and Williams, 1995).
The proposed neural/behavioral mechanism also generates several novel predictions about cognitive control of eye movements in reading. First, the original FET predicts a constant effect size of saccade delay, without regard to the direction of the executed saccades. This assumption is based on the idea that a saccade is delayed to allow more processing time (McConkie et al., 1994).
The present observations suggest that the delay reflects the cancellation of a planned forward saccade, and the planning and execution of refixation or regression independently of the cancelled saccade. Consequently, it predicts a longer delay when a regression has to be generated to a specific word that requires the update of word location. In comparison, a regression to the same word, requiring no updated word location, should result in a shorter latency. The amount of delay does not depend on the nature of processing difficulty (nonwords or syntactic ambiguity), but the resultant response to the difficulty (refixation vs. longer regression). This is in contrast with the assumption that fixation duration is predicted based on the difficulty of currently processed word. This hypothesis can be readily examined by looking into the amount of time lag between the onset of reduced saccade frequency and the increase of refixation and regression for different processing difficulties.
The second novel prediction is that the same type of difficulty with various severity or saliency should result in different onset times and proportions of delayed saccades. A nonword composed of random letters should delay more saccades than a pseudoword, compared to a high-frequency real word. This is due to the higher probability of detecting nonwords than pseudowords, which can be quantitatively predicted based on the saliency of processing difficulty. This is in contrast with the theory of saccades being delayed to allow more processing time, as it predicts the same amount of delay due to the fact that they are both not real words.
Finally, the hypothesized race between forward saccade signal and suppression signal predicts that the effect of processing difficulty could spread over consecutive eye fixations. This is because the latency of initial saccade delay for some types of text difficulty could be very long, and the cancellation of forward saccades and a regression can be executed only during the following fixation. In some cases, the processing difficulty can also have a delay effect when the eye fixates at the word to the left of the one causing difficulty, but this would only occur when the difficulty can be detected early enough and when the fixation on that word is long enough. These would account for the peripheral to foveal effect observed in previous studies (e.g., Kennedy and Pynte, 2005). Conducting a frequency of effect analysis on data from these studies should confirm whether this is the case.
Conclusions
In reading, cognitive control of eye movements is necessary to cope with text difficulty. Here a neural mechanism is outlined to cancel visually-based, anticipation-facilitated forward saccades while allowing refixations or regressions to be executed based on processing difficulty. Frontal cortical areas such as the SEF likely carry out these processes, and a certain amount of time is needed to accomplish such control, dependent on the type of cognitively-based eye movements. Future research or reanalysis of existing findings is needed to validate novel predictions based on the proposed mechanism.
References
- Amador, N., M. Schlag-Rey, and J. Schlag. 2004. Primate antisaccade. II. Supplementary eye field neuronal activity predicts correct performance. Journal of Neurophysiology 91: 1672–1689. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R. H. S., and MLL. Williams. 1995. Neural computation of log likelihood in control of saccadic eye movements. Nature 377: 59–62. [Google Scholar] [PubMed]
- Colby, C. L., J. R. Duhamel, and M. E. Goldberg. 1995. Oculocentric spatial representation in parietal cortex. Cerebral Cortex 5, 5: 470–481. [Google Scholar]
- Elandt-Johnson, R., and N. Johnson. 1999. Survival Models and Data Analysis. New York: John Wiley & Sons. [Google Scholar]
- Engbert, R., A. Longtin, and R. Kliegl. 2002. A dynamical model of saccade generation in reading based on spatially distributed lexical processing. Vision Research 42: 621–636. [Google Scholar]
- Engbert, R., A. Nuthmann, E. M. Richter, and R. Kliegl. 2005. SWIFT: A dynamical model of saccade generation during reading. Psychological Review 112: 777–813. [Google Scholar] [CrossRef]
- Ferraina, S., M. Pare, and R. H. Wurtz. 2002. Comparison of cortico-cortical and cortico-collicular signals for the generation of saccadic eye movements. Journal of Neurophysiology 87, 2: 845–858. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J. M., and R. Walker. 1999. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral & Brain Sciences 22, 4: 661–721. [Google Scholar]
- Frazier, L., and K. Rayner. 1982. Making and correcting errors during sentence comprehension: Eye movements in the analysis of structurally ambiguous sentences. Cognitive Psychology 14: 178–210. [Google Scholar]
- Fries, W. 1985. Inputs from motor and premotor cortex to the superior colliculus of the macaque monkey. Behavioral and Brain Research 18: 95–105. [Google Scholar]
- Hagoort, P., P. Indefrey, C. Brown, H. Herzog, H. Steinmetz, and R. J. Seitz. 1999. The neural circuitry involved in the reading of German words and pseudowords: A PET study. Journal of Cognitive Neuroscience 11, 4: 383–398. [Google Scholar]
- Hanes, D.P., *!!! REPLACE !!!* W.F., and Schall Patterson J.D. 1998. The role of frontal eye field in countermanding saccades: Visual, movement and fixation activity. Journal of Neurophysiology 79: 817–834. [Google Scholar] [CrossRef]
- Harris, C. M., L. Hainline, I. Abramov, E. Lemerise, and C. Camenzuli. 1988. The distribution of fixation durations in infants and naïve adults. Vision Research 28: 419–432. [Google Scholar]
- Harris, C.M., L. Hainline, I. Abramov, E. Lemerise, and C. Camenzuli. 1988. The distribution of fixation durations in infants and naïve adults. Vision Research 28: 419–432. [Google Scholar] [PubMed]
- Hartmann-von Monakow, K Akert. 1979. Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Experimental Brain Research 34: 91–105. [Google Scholar]
- Heinen, S. J., H. Hwang, and S.-N. Yang. 2011. Flexible interpretation of a decision rule by supplementary eye field neurons. Journal of Neurophysiology 106, 6: 2992–3000. [Google Scholar] [PubMed]
- Heinen, S. J., J. Rowland, B.-T. Lee, and A. R. Wade. 2006. An oculomotor decision process revealed by functional magnetic resonance imaging. Journal of Neuroscience 26: 13515–13522. [Google Scholar] [PubMed]
- Heinzle, J., K. Hepp, and K. A. C. Martin. 2010. A biologically realistic cortical model of eye movement control in reading. Psychological Review 117, 3: 808–830. [Google Scholar]
- Huerta, M. F., and J. H. Kaas. 1990. Supplementary eye field as defined by intracortical microstimulation: connections in macaques. Journal of Comparative Neurology 293: 299–330. [Google Scholar]
- Inhoff, A. W., and K. Rayner. 1986. Parafoveal word processing during eye fixations in reading: Effects of word frequency. Perception and Psychophysics 40, 6: 431–439. [Google Scholar]
- Inhoff, A. W., and U. W. Weger. 2005. Memory for word location during reading: Eye movements to previously read words are spatially selective but not precise. Memory & Cognition 33, 3: 447–461. [Google Scholar]
- Inhoff, A. W., B. M. Eiter, and R. Radach. 2005. Time course of linguistic information extraction from consecutive words during eye fixations in reading. Journal of Experimental Psychology: Human Perception and Performance 31, 5: 979–995. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P., and M. N. Shadlen. 2005. A representation of the hazard rate of elapsed time in macaque area LIP. Nature Neuroscience 8, 2: 234–241. [Google Scholar] [CrossRef]
- Just, M. A., and P. A. Carpenter. 1980. A theory of reading: From eye fixations to comprehension. Psychological Review 87: 329–54. [Google Scholar] [CrossRef] [PubMed]
- Keller, E. L., and J. A. Edelman. 1994. Use of interrupted saccade paradigm to study spatial and temporal dynamics of saccadic burst cells in superior colliculus in monkey. Journal of Neurophysiology 72, 6: 2754–2770. [Google Scholar] [CrossRef]
- Kennedy, A., and J. Pynte. 2005. Parafoveal-on-foveal effects in normal reading. Vision Research 45, 2: 153–168. [Google Scholar] [CrossRef]
- Kim, Y.-G., J. B. Badler, and S. J. Heinen. 2005. Trajectory interpretation by supplementary eye field neurons during ocular baseball. Journal of Neurophysiology 94, 2: 1385–1391. [Google Scholar] [CrossRef]
- Konen, C. S., R. Kleiser, F. Bremmer, and R. J. Seitz. 2007. Different cortical activations during visuospatial attention and the intention to perform a saccade. Experimental Brain Research 182, 3: 333–341. [Google Scholar] [CrossRef] [PubMed]
- Levy-Schoen, A. 1981. Flexible and/or rigid control of oculomotor scanning behavior. In Eye movements: Cognition and visual perception. Edited by D. F. Fisher, R. A. Monty and J. W. Senders. Hillsdale, NJ: Erlbaum, pp. 299–316. [Google Scholar]
- Luppino, G., M. Matelli, R. Camarda, and G. Rizzolatti. 1993. Corticocortical connections of area F3 (SMAproper) and area F6 (pre-SMA) in the macaque monkey. Journal of Comparative Neurology 338, 114–140. [Google Scholar] [CrossRef]
- McConkie, G. W., N. R. Underwood, D. Zola, and G. S. Wolverton. 1985. Some temporal characteristics of processing during reading. Journal of Experimental Psychology: Human Perception & Performance 11, 2: 168–186. [Google Scholar]
- McConkie, G. W., D. Zola, and G. S. Wolverton. 1985. Estimating frequency and size of effects due to experimental manipulations in eye movement research. In Eye mvoements and human information processing. Edited by R. Groner, G. W. McConkie and C. Menz. North Holland: Amsterdam: pp. 137–148. [Google Scholar]
- McConkie, G. W., and B. P. Dyre. 2000. Eye fixation durations in reading: Models of frequency distributions. In Reading as a perceptual process. Edited by A. Kennedy, R. Radach, D. Heller and J. Pynte. Oxford, UK: Elseriver Science, pp. 683–700. [Google Scholar]
- McConkie, G. W., and K. Rayner. 1975. The span of the effective stimulus during a fixation in reading. Perception & Psychophysics 17, 6: 578–586. [Google Scholar]
- McConkie, G. W., and S.-N. Yang. 2003. How cognition affects eye movements during reading. In The mind's eye: Cognitive and applied aspects of eye movement research. Edited by J. Hyönä, R. Radach and H. Deubel. Oxford, UK: Elsevier, pp. 413–427. [Google Scholar]
- McConkie, G. W., P. W. Kerr, and B. P. Dyre. 1994. What are 'normal 'eye movements during reading: Toward a mathematical description. In Eye Movements in Reading. Edited by J. Ygge and Lennerstrand. Oxford, England: Elsevier, pp. 331–343. [Google Scholar]
- McConkie, G. W., P. W. Kerr, M. D. Reddix, and D. Zola. 1988. Eye movement control during reading: I. The locations of initial eye fixations in words. Vision Research 28, 10: 1107–1118. [Google Scholar] [PubMed]
- McConkie, G. W., P. W. Kerr, M. D. Reddix, and D. Zola. 1989. Eye movement control during reading: II. Frequency of refixating a word. Perception & Psychophysics 46, 3: 245–253. [Google Scholar]
- McConkie, G. W., M. R. Reddix, and D. Zola. 1992. Perception and cognition in reading: Where is the meeting point? In Eye movement and visual cognition. Edited by K. Rayner. New York: Springer-Verlag: pp. 293–303. [Google Scholar]
- McConkie, G. W., N. R. Underwood, G. S. Wolverton, and D. Zola. 1988. Some properties of eye movement control during reading. In Eye movement research: Physiological and psychological aspects. Edited by G. Luer, U. Lass and J. Shallo-Hoffmann. Toronto: C.J. Hogrefe, pp. 226–245. [Google Scholar]
- McPeek, R. M., and E. L. Keller. 2002. Superior colliculus activity related to concurrent processing of saccade goals in a visual search task. Journal of Neurophysiology 87: 1805–1815. [Google Scholar] [PubMed]
- Mitchell, D. C., X. Shen, M. J. Green, and T. L. Hodgson. 2008. Accounting for regressive eye-movements in models of sentence processing: A reappraisal of the selective reanalysis hypothesis. Journal of Memory and Language 59: 266–293. [Google Scholar]
- Morrison, R. E. 1984. Manipulation of stimulus onset delay in reading: Evidence for parallel programming of saccades. Journal of Experimental Psychology: Human Perception and Performance 10: 667–82. [Google Scholar]
- Nachev, P., G. Rees, A. Parton, C. Kennard, and M. Husain. 2005. Volition and conflict in human medial frontal cortex. Current Biology 15: 122–128. [Google Scholar]
- Nobre, A. C., G. Allison, and T. McCarthy. 1994. Word recognition in the human inferior temporal lobe. Nature 372, 17: 260–262. [Google Scholar]
- Olson, C. R. 2003. Brain representation of objectcentered space in monkeys and humans. Annual Review of Neuroscience 26: 331–354. [Google Scholar]
- Olson, C. R., and S. N. Gettner. 1995. Object-centered direction selectivity in the macaque supplementary eye field. Science 269, 5226: 985–988. [Google Scholar]
- Olson, C. R., and S. N. Gettner. 1996. Representation of object-centered space in the primate frontal lobe. Cognitive Brain Research 5, 1–2: 147–156. [Google Scholar]
- Optican, L. M. 1995. A field theory of saccade generation: Temporal-to-spatial transform in the superior colliculus. Vision Research 35, 23–24: 3313–3320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- O'Regan, J. K., and A. Levy-Schoen. 1987. Eyemovement strategy and tactics in word recognition and reading. In Attention and performance 12: The psychology of reading. Edited by M. Coltheart. Hove, UK: Lawrence Erlbaum Associates, pp. 363–383. [Google Scholar]
- Pandya, D. N., and E. H. Yeterian. 1990. Prefrontal cortex in relation to other cortical areas in rhesus monkey: Architecture and connections. Progression in Brain Research 85: 63–94. [Google Scholar]
- Park, J., M. Schalg-Rey, and J. Schlag. 2005. Frames of reference for saccadic command tested by saccade collision in the supplementary eye field. Journal of Neurophysiology 95: 159–170. [Google Scholar]
- Passingham, R. E. 1985. Cortical mechanisms and cues for action. Philosophical Transcription of Royal Society London: B Biological Science 308: 101–111. [Google Scholar]
- Passingham, R. E. 1993. The Frontal Lobes and Voluntary Action. Oxford Psychology Series, no. 21. Oxford, England UK: Oxford University Press, vol. no. 21. [Google Scholar]
- Pynte, J., and A. Kennedy. 2006. An influence over eye movements in reading exerted from beyond the level of the word: Evidence from reading English and French. Vision Research 46, 22: 3786–3801. [Google Scholar] [CrossRef]
- Raney, G. E., and K. Rayner. 1995. Word frequency effects and eye movements during two readings of a text. Canadian Journal of Experimental Psychology 49: 151–172. [Google Scholar]
- Rayner, K., and A. Pollatsek. 1981. Eye movement control during reading: Evidence for direct control. Quarterly Journal of Experimental Psychology 33A: 351–373. [Google Scholar]
- Rayner, K., and G. E. Raney. 1996. Eye movement control in reading and visual search: Effects of word frequency. Psychonomic Bulletin and Review 3: 238–244. [Google Scholar] [CrossRef]
- Rayner, K. 1998. Eye movements in reading and information processing: 20 years of research. Psychological Bulletin 124, 3: 372–422. [Google Scholar]
- Rayner, K., and A. Pollatsek. 1989. The Psychology of Reading. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- Rayner, K., and S. A. Duffy. 1987. Eye movements and lexical ambiguity. In Eye movements: From physiology to cognition. Edited by J. K. O'Regan and A. Levy-Schoen. Amsterdam, Netherlands: Elsevier Science, pp. 521–529. [Google Scholar]
- Rayner, K., and L. Frazier. 1987. Parsing temporarily ambiguous complements. Quarterly Journal of Experimental Psychology, A (Human Experimental Psychology)39, 657–673. [Google Scholar] [CrossRef]
- Rayner, K., and G. W. McConkie. 1976. What guides a reader's eye movements? Vision Research 16(8), 1976: 829–837. [Google Scholar] [CrossRef]
- Reichle, E. D., A. Pollatsek, D. L. Fisher, and K. Rayner. 1998. Toward a model of eye movement control in reading. Psychological Review 105, 1: 125–157. [Google Scholar] [CrossRef]
- Reichle, E. D., K. Rayner, and A. Pollatsek. 2003. The E-Z Reader model of eye movement control in reading: comparisons to other models. Behavioral and Brain Sciences 26: 445–526. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R. G., and R. Radach. 2003. Foundations of an interactive activation model of eye movement control in reading. In The mind's eye: Cognitive and applied aspects of eye movement research. Edited by J. Hyönä, R. Radach and H. Deubel. Oxford: Elsevier Science, pp. 429–455. [Google Scholar]
- Rumsey, J. M., B. Horwitz, B. C. Donohue, K. Nace, J. M. Maisog, and P. Andreason. 1997. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain 120, 5: 739–759. [Google Scholar] [CrossRef]
- Russo, G. S., and C. J. Bruce. 1993. Effect of eye position within the orbit on electrically elicited saccadic eye movements: A comparison of the macaque monkey's frontal and supplementary eye fields. Journal of Neurophysiology 69: 800–818. [Google Scholar] [CrossRef] [PubMed]
- Russo, G. S., and C. J. Bruce. 1996. Neurons in the supplementary eye field of rhesus monkeys code visual targets and saccadic eye movements in an oculocentric coordinate system. Journal of Neurophysiology 76: 825–848. [Google Scholar] [CrossRef] [PubMed]
- Russo, G. S., and C. J. Bruce. 2000. Supplementary eye field: Representation of saccades and relationship between neural response fields and elicited eye movements. Journal of Neurophysiology 84: 2605–2621. [Google Scholar] [CrossRef]
- Schall, J. D. 1991a. Neuronal activity related to visually guided saccades in the frontal eye fields of rhesus monkeys: Comparison with supplementary eye fields. Journal of Neurophysiology 66, 2: 559–579. [Google Scholar] [CrossRef]
- Schall, J. D. 1991b. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. Journal of Neurophysiology 66: 530–558. [Google Scholar] [CrossRef]
- Schall, J. D., A. Morel, and J. H. Kaas. 1993. Topography of supplementary eye field afferents to frontal eye field in macaque: Implications for mapping between saccade coordinate systems. Vision Neuroscience 10: 385–393. [Google Scholar] [CrossRef]
- Schall, J. D., V. Stuphorn, and J. W. Brown. 2002. Monitoring and control of action by the frontal lobes. Neuron 36, 309–322. [Google Scholar]
- Schiller, P. H. 1998. The neural control of visually guided eye movements. In Cognitive neuroscience of attention: A developmental perspective. Edited by J. E. Richards. Lawrence erlbaum associates.: Mahwah, NJ, USA: pp. 3–50. [Google Scholar]
- Schiller, P. H., and I. Chou. 1998. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nature Neuroscience 1: 248–253. [Google Scholar] [PubMed]
- Schlag, J., and M. Schlag-Rey. 1987. Evidence for a supplementary eye field. Journal of Neurophysiology 57: 179–200. [Google Scholar]
- Schlag-Rey, M., N. Amador, H. Sanchez, and J. Schlag. 1997. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature 390: 398–401. [Google Scholar]
- Shook, B. L., M. Schlag-Rey, and J. Schlag. 1988. Direct projection from the supplementary eye field to the nucleus raphé interpositus. Experimental Brain Research 73: 215–218. [Google Scholar] [PubMed]
- Shook, B. L., M. Schlag-Rey, and J. Schlag. 1990. Primate supplementary eye field: I. Comparative aspects of mesencephalic and pontine connections. Journal of Comparative Neurology 301, 4: 618–642. [Google Scholar]
- Stuphorn, V, and J. D. Schall. 2006. Executive control of countermanding saccades by the supplementary eye field. Nature Neuroscience 9, 7: 925–931. [Google Scholar]
- Stuphorn, V., T. L. Taylor, and J. D. Schall. 2000. Performance monitoring by the supplementary eye field. Nature 408, 6814: 857–860. [Google Scholar]
- Suppes, P. 1989. Eye-movement models for arithmetic and reading performance. In Eye movements and their role in visual and cognitive processes. Edited by E. Kowler. Amsterdam: Elsevier: pp. 455–477. [Google Scholar]
- Tehovnik, E. J. 1995. The dorsomedial frontal cortex: eye and forelimb fields. Behavior and Brain Research 67: 147–163. [Google Scholar]
- Tehovnik, E. J., and K. Lee. 1993. The dorsomedial frontal cortex of the rhesus monkey: topographic representation of saccades evoked by electrical stimulation. Experimental Brain Research 96, 430–442. [Google Scholar]
- Tehovnik, E. J., and M. A. Sommer. 1997. Electrically evoked saccades from the dorsomedial frontal cortex and frontal eye fields: a parametric evaluation reveals differences between areas. Experimental Brain Research 117, 369–378. [Google Scholar] [CrossRef]
- Trappenberg, T. P., M. C. Dorris, D. P. Munoz, and R. M. Klein. 2001. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. Journal of Cognitive Neuroscience 13, 256–271. [Google Scholar]
- Tremblay, L., S. N. Gettner, and C. R. Olson. 2002. Neurons with object-centered spatial selectivity in macaque SEF: Do they represent locations or rules? Journal of Neurophysiology 87, 1: 333–350. [Google Scholar]
- Van Gompel, R. P. G., M. J. Pickering, and M. J. Traxler. 2001. Reanalysis in sentence processing: Evidence against current constraint-based and two-stage models. Journal of Memory and Language 45: 225–258. [Google Scholar] [CrossRef]
- Vitu, F., and G.W. McConkie. 2000. Regressive saccades and word perception in adult reading. In Reading as a perceptual process. Edited by A. Kennedy, R. Radach, D. Heller and J. Pynte. Oxford: Elsevier, pp. 301–326. [Google Scholar]
- Vitu, F., G. W. McConkie, and D. Zola. 1998. About regressive saccades in reading and their relation to word identification. In Eye guidance in reading and scene perception. Edited by & Geoffrey Underwood. Oxford, England: Elsevier Science, pp. 101–124. [Google Scholar]
- Vitu, F., J.K. O’Regan, A.W. Inhoff, and R. Topolski. 1995. Mindless reading: Eye movement characteristics are similar in scanning strings and reading texts. Perception and Psychophysics 57, 352–364. [Google Scholar]
- Vitu, F., J. K. O'Regan, and M. Mittau. 1990. Optimal landing position in reading isolated words and continuous text. Perception & Psychophysics 47, 6: 583–600. [Google Scholar]
- Waitzman, D. M., T. P. Ma, L. M. Optican, and R. H. Wurtz. 1991. Superior colliculus neurons mediate the dynamic characteristics of saccades. Journal of Neurophysiology 66, 5: 1716–1737. [Google Scholar]
- Walker, R., H. Deubel, W. X Schneider, and J. M. Findlay. 1997. Effect of Remote Distractors on Saccade Programming: Evidence for an Extended Fixation Zone. Journal of Neurophysiology 78, 2: 1108–1119. [Google Scholar]
- Weger, U. W., and A. W. Inhoff. 2007. Long-range regressions to previously read words are guided by spatial and verbal memory. Memory & Cognition 35, 6: 1293–1306. [Google Scholar]
- Wurtz, R. H., and L. M. Optican. 1994. Superior colliculus cell types and models of saccade generation. Current Opinion in Neurobiology 4, 6: 857–861. [Google Scholar] [PubMed]
- Wurtz, R. H., M. A. Sommer, M. Pare, and S. Ferraina. 2001. Signal transformations from cerebral cortex to superior colliculus for the generation of saccades. Vision Research 41, 25–26: 3399–3412. [Google Scholar] [PubMed]
- Yang, S.-N. 2002. Inhibitory control of saccadic eye movements in reading: A neurophysiologically based interaction-competition theory of saccade programming. Unpublished doctoral dissertation, 2002, University of Illinois at Urbana-Champaign, Urbana, Illinois, Urbana, Illinois. [Google Scholar]
- Yang, S.-N. 2005. Stimulation at the supplementary eye field modulates the initiation of directionally selective anticipatory saccades. Presented in the 13th European Conference for Eye Movements, Bern, Switzerland, September. [Google Scholar]
- Yang, S.-N. 2006. An oculomotor-based model of eye movements in reading: The competition/interaction model. Cognitive Systems Research 7: 56–69. [Google Scholar]
- Yang, S.-N. 2007. The dynamic coding of saccade metrics: Integrated visual and intrinsic signals. In Eye movements: A window on mind and brain. Edited by Gompel Roger van. Oxford, England: Oxford University Press. [Google Scholar]
- Yang, S.-N., and G. W. McConkie. 2004. Saccade generation during reading: Are words necessary? European Journal of Cognitive Psychology 16, 1/2: 226–261. [Google Scholar]
- Yang, S.-N., and F. Vitu. 2006. Dynamic coding of saccade length in reading. In Eye movements: A window on mind and brain. Edited by R.P.G. Gompel, M.H. Fischer, W.S. Murray and R.L. Hill. Elsevier Science: Oxford: pp. 293–318. [Google Scholar]
- Yang, S.-N., and G. W. McConkie. 2001. Eye movements during reading: a theory of saccade initiation times. Vision Research 41: 3567–3585. [Google Scholar]
- Yang, S.-N., and G. W. McConkie. 2005. New directions in theories of eye movement control during reading. In Cognitive processes in eye guidance. Edited by G. Underwood. Oxford University Press: Oxford, UK: pp. 105–130. [Google Scholar]
- Yang, S.-N., S. J. Heinen, H. Hwang, and J. Badler. 2006. The different involvement of the supplementary and frontal eye fields in rule-based visuomotor control. Presented at the annual conference for the Society for Neuroscience, Oct, Atlanta, GA. [Google Scholar]
- Yang, S.-N., S. Heinen, and M. Missal. 2008. Effects of Microstimulation of the Dorsomedial Frontal Cortex in Macaque Monkeys. Journal of Neurophysiology 99: 1857–1870. [Google Scholar]
- Yang, S.-N., S. Heinen, and H. Hwang. 2010. Supplementary eye field activity reflects a decision rule governing smooth pursuit but not the movement decision itself. Journal of Neurophysiology 103, 5: 2458–2469. [Google Scholar]
- Yang, S.-N., Y.-C. Tai, and G. W. McConkie. 2003. The application of gaze-contingent display changes and event-related optical signals (EROS) for measuring inhibitory neural signal. Presented at the annual conference of American Educational Research Association, April, Chicago. [Google Scholar]
© 2012 by the authors. This article is licensed under a Creative Commons Attribution 4.0 International License.