OKN and Pupillary Response Modulation by Gaze and Attention Shifts
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
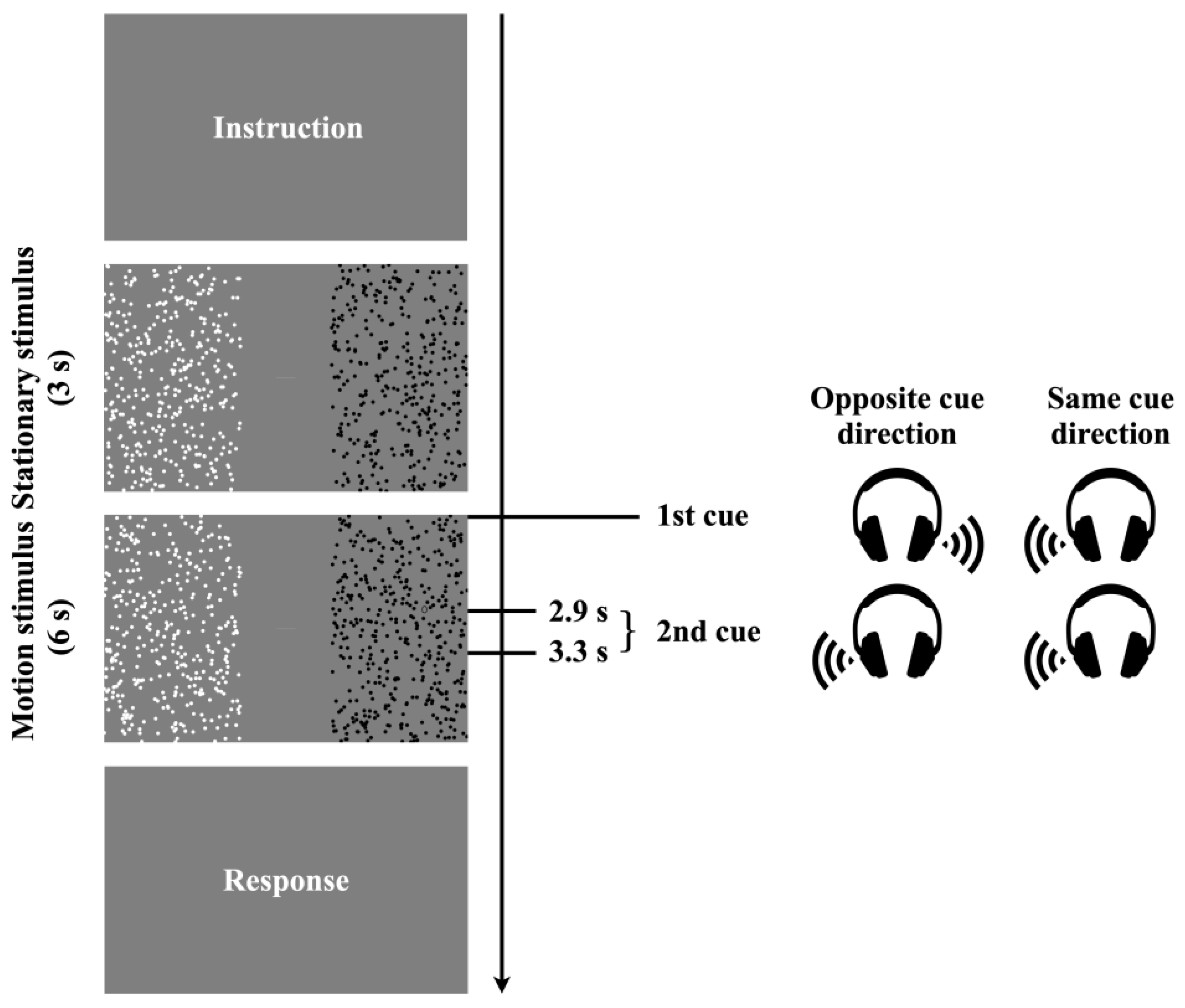
2.3. Procedure
2.4. Apparatus
2.5. Analysis
3. Results
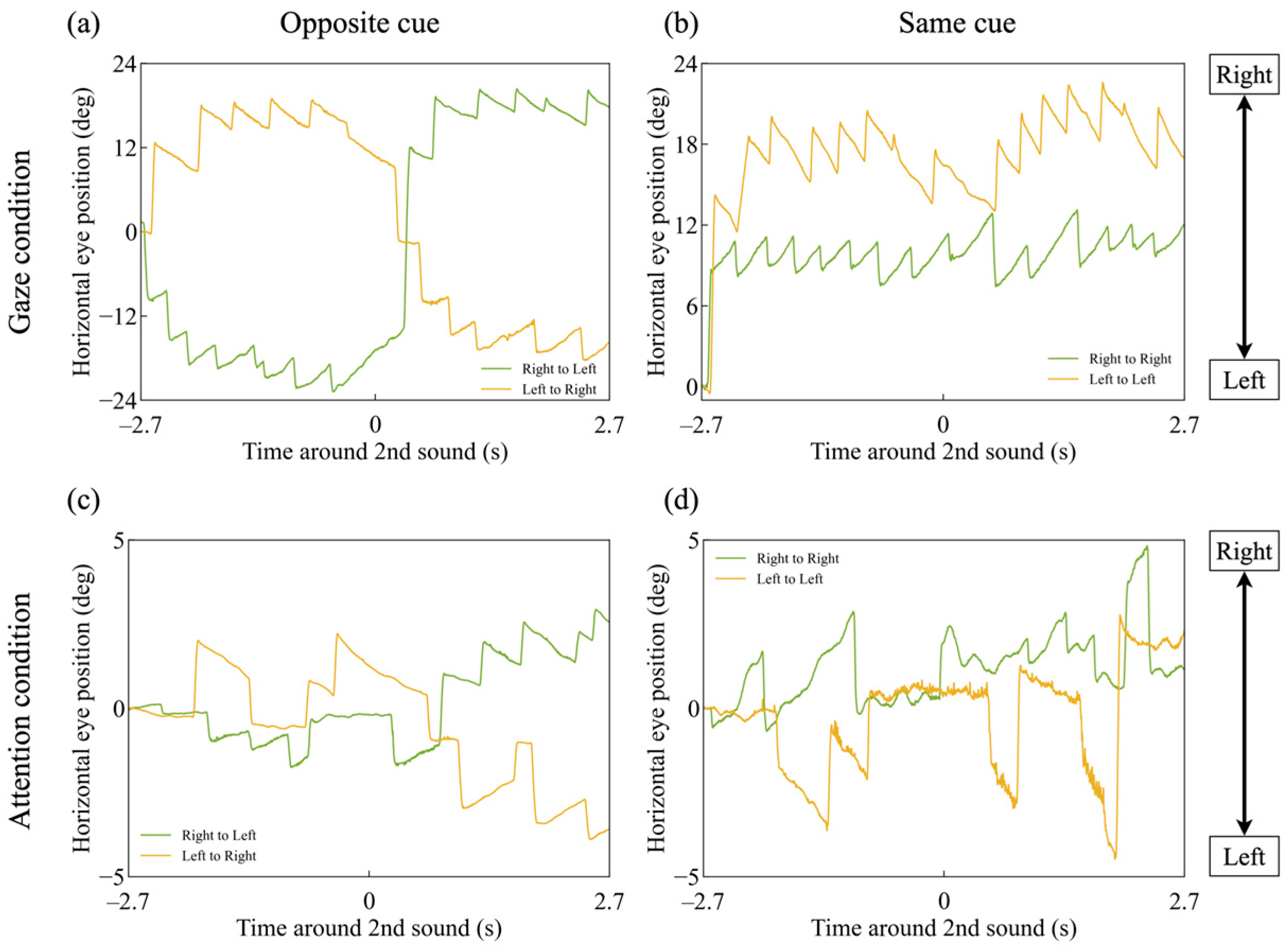
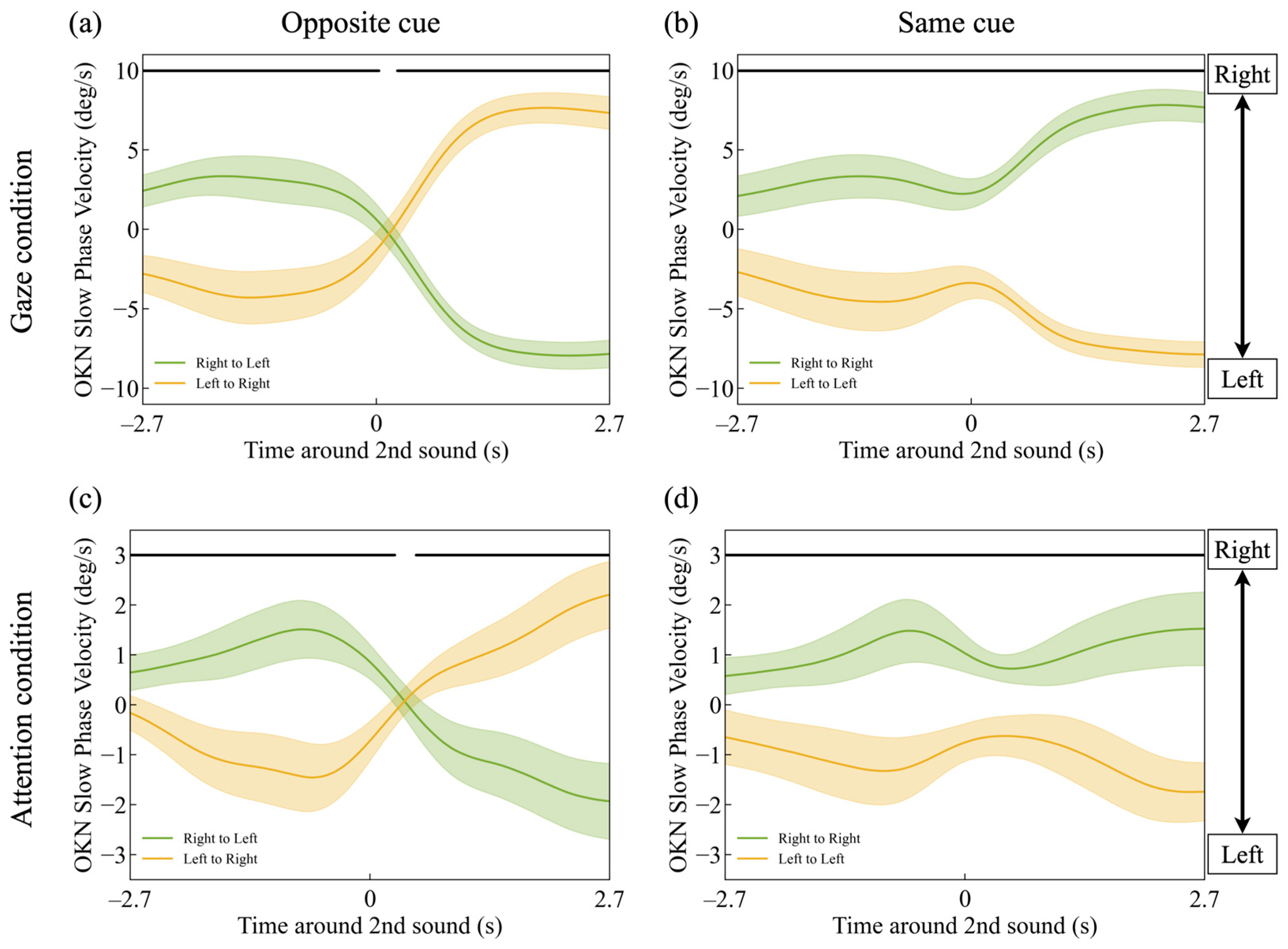
3.1. Slow-Phase Velocity of OKN
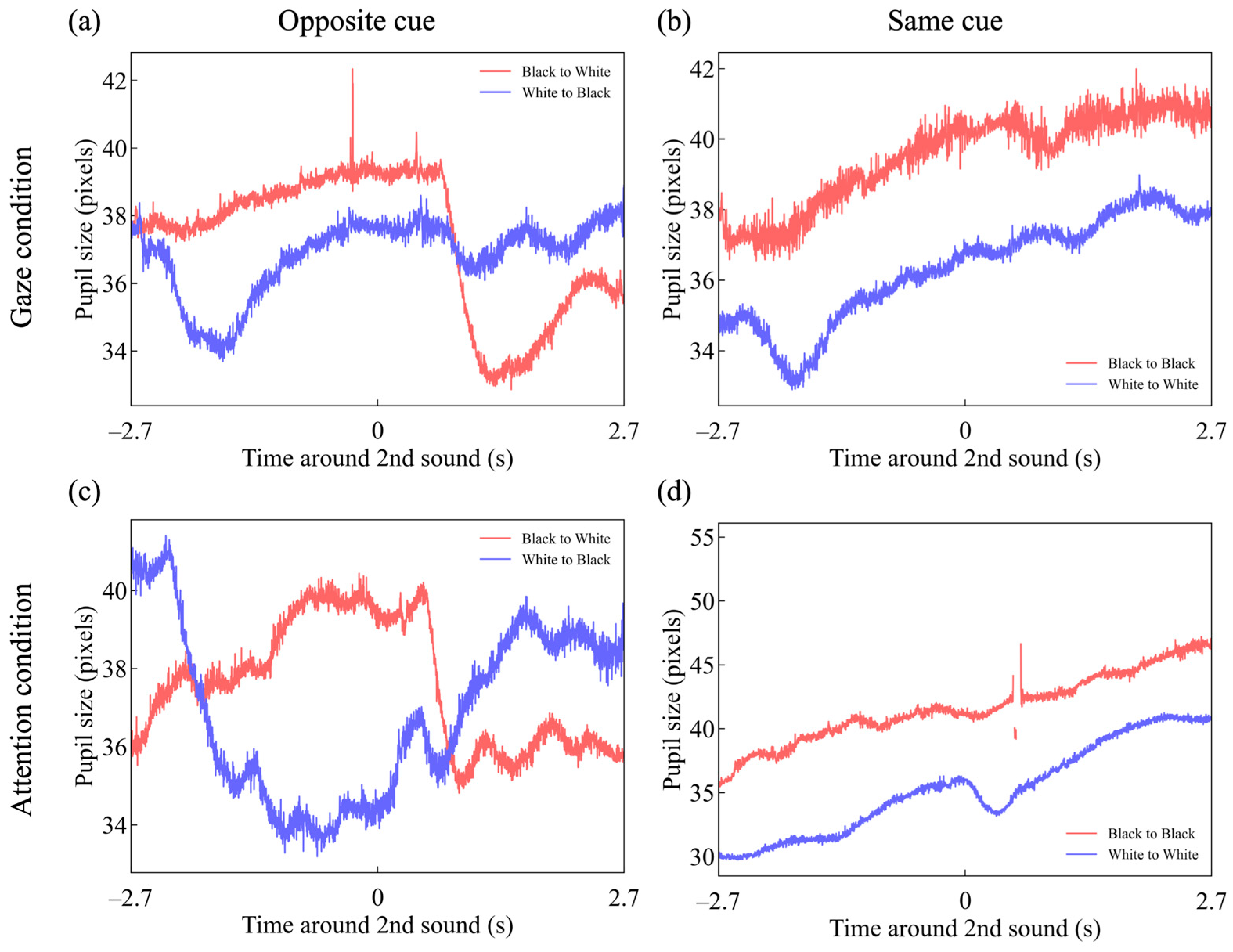
3.2. Pupil Response
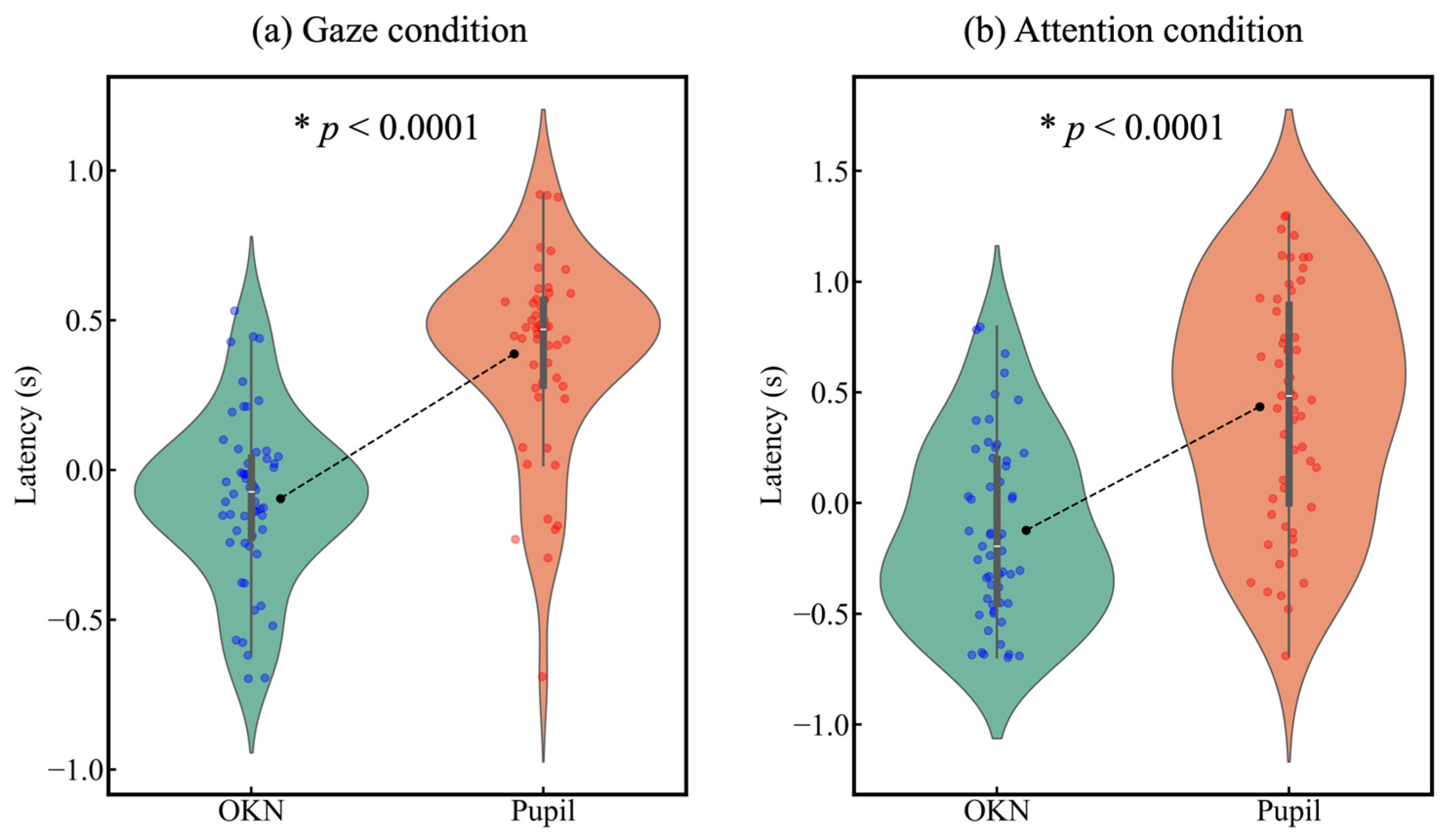
3.3. Latency in Gaze and Attentional Shift
4. Discussion
4.1. OKN Modulation by Gaze and Attention
4.2. Pupillary Modulation by Gaze and Attention
4.3. Distinct Control Mechanisms for OKN and Pupillary Responses
4.4. Relationship Between OKN Slow-Phase and Smooth Pursuit
4.5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, F.W.; Gregory, A.H. Effect of size of pupil on visual acuity. Nature 1960, 187, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, J.M. The effect of pupil size on grating detection at various contrast levels. Vis. Res. 1975, 15, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S. Pupillometry: Psychology, physiology, and function. J. Cogn. 2018, 1, 1–23. [Google Scholar] [CrossRef]
- Walls, G.L. The evolutionary history of eye movements. Vis. Res. 1962, 2, 69–80. [Google Scholar] [CrossRef]
- Land, M.F. Motion and vision: Why animals move their eyes. J. Comp. Physiol. A 1999, 185, 341–352. [Google Scholar] [CrossRef]
- Purkinje, J. Beobachtungen und Versuche zur Physiologie der Sinne Neue Beitrage zur Kenntnis des Sehens in Subjektiver Hinsicht; Tiskem a Nakladenn G. Reimera: Berlin, Germany, 1825. [Google Scholar]
- Posner, M.I. Orienting of attention. Q. J. Exp. Psychol. 1980, 32, 3–25. [Google Scholar] [CrossRef]
- Binda, P.; Pereverzeva, M.; Murray, S.O. Attention to bright surfaces enhances the pupillary light reflex. J. Neurosci. 2013, 33, 2199–2204. [Google Scholar] [CrossRef]
- Mathôt, S.; Van Der Linden, L.; Grainger, J.; Vitu, F. The pupillary light response reveals the focus of covert visual attention. PLoS ONE 2013, 8, e78168. [Google Scholar] [CrossRef]
- Binda, P.; Murray, S.O. Spatial attention increases the pupillary response to light changes. J. Vis. 2015, 15, 1. [Google Scholar] [CrossRef]
- Binda, P.; Pereverzeva, M.; Murray, S.O. Pupil size reflects the focus of feature-based attention. J. Neurophysiol. 2014, 112, 3046–3052. [Google Scholar] [CrossRef]
- Cheng, M.; Outerbridge, J.S. Optokinetic nystagmus during selective retinal stimulation. Exp. Brain Res. 1975, 23, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.F.W.; Collewijn, H. Optokinetic reactions in man elicited by localized retinal motion stimuli. Vis. Res. 1979, 19, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.P.; Gonzalez, E.G. Human optokinetic nystagmus in response to moving binocularly disparate stimuli. Vis. Res. 1987, 27, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Kanari, K.; Sakamoto, K.; Kaneko, H. Effect of visual attention on the properties of optokinetic nystagmus. PLoS ONE 2017, 12, e0175453. [Google Scholar] [CrossRef]
- Kanari, K.; Kaneko, H. Effect of visual attention and horizontal vergence in three-dimensional space on occurrence of optokinetic nystagmus. J. Eye Mov. Res. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Kanari, K. Pupil response is modulated by attention shift in optokinetic nystagmus. J. Opt. Soc. Am. A 2020, 37, 361–367. [Google Scholar] [CrossRef]
- Kanari, K.; Kaneko, H. Pupil response is modulated with optokinetic nystagmus in transparent motion. J. Opt. Soc. Am. A 2021, 38, 149–156. [Google Scholar] [CrossRef]
- Naber, M.; Frässle, S.; Einhäuser, W. Perceptual rivalry: Reflexes reveal the gradual nature of visual awareness. PLoS ONE 2011, 6, e20910. [Google Scholar] [CrossRef]
- Konen, C.S.; Kleiser, R.; Seitz, R.J.; Bremmer, F. An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans. Exp. Brain Res. 2005, 165, 203–216. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, Y.; Zhang, H. Ocular autonomic nervous system: An update from anatomy to physiological functions. Vision 2022, 6, 6. [Google Scholar] [CrossRef]
- Kato, I.; Harada, K.; Hasegawa, T.; Igarashi, T.; Koike, Y.; Kawasaki, T. Role of the nucleus of the optic tract in monkeys in relation to optokinetic nystagmus. Brain Res. 1986, 364, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Cohen, B.; Büttner-Ennever, J.; Matsuo, V. Effects of lesions of the nucleus of the optic tract on optokinetic nystagmus and after-nystagmus in the monkey. Exp. Brain Res. 1990, 79, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.; Kerzel, D. Visual selective attention and the control of tracking eye movements: A critical review. J. Neurophysiol. 2021, 125, 1552–1576. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P.D.; Zhang, H.; Clarke, R.J. Luminance neurons in the pretectal olivary nucleus mediate the pupillary light reflex in the rhesus monkey. Exp. Brain Res. 1995, 106, 177–180. [Google Scholar]
- Hoffman, J.E.; Subramaniam, B. The role of visual attention in saccadic eye movements. Percept. Psychophys. 1995, 57, 787–795. [Google Scholar] [CrossRef]
- Kowler, E.; Anderson, E.; Dosher, B.; Blaser, E. The role of attention in the programming of saccades. Vis. Res. 1995, 35, 1897–1916. [Google Scholar] [CrossRef]
- Deubel, H.; Schneider, W.X. Saccade target selection and object recognition: Evidence for a common attentional mechanism. Vis. Res. 1996, 36, 1827–1837. [Google Scholar] [CrossRef]
- Mathôt, S.; van Der Linden, L.; Grainger, J.; Vitu, F. The pupillary light response reflects eye-movement preparation. J. Exp. Psychol. Hum. Percept. Perform. 2015, 411, 28–35. [Google Scholar]
- Scala, N.P.; Spiegel, E.A. Subcortical (passive) optokinetic nystagmus in lesions of the midbrain and of the vestibular nuclei. Confin. Neurol. 1940, 3, 53–73. [Google Scholar] [CrossRef]
- Smith, K.U.; Bridgman, M. The neural mechanisms of movement vision and optic nystagmus. J. Exp. Psychol. 1943, 33, 165–187. [Google Scholar] [CrossRef]
- Arimoto, K. Superior colliculus, as center of nystagmus. Wakayama Med. Rep. 1958, 4, 1–35. [Google Scholar]
- Brainard, D.H. The psychophysics toolbox. Spat. Vis. 1997, 10, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat. Vis. 1997, 10, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Nagami, T.; Sugase, Y.; Takemura, A.; Kawano, K. A widely applicable real-time mono/binocular eye tracking system using a high frame-rate digital camera. In International Conference on Human-Computer Interaction; Springer: Cham, Switzerland, 2017; Volume 10271, pp. 593–608. [Google Scholar] [CrossRef]
- Einhäuser, W.; Stout, J.; Koch, C.; Carter, O. Pupil dilation reflects perceptual selection and predicts subsequent stability in perceptual rivalry. Proc. Natl. Acad. Sci. USA 2008, 105, 1704–1709. [Google Scholar] [CrossRef]
- Frässle, S.; Sommer, J.; Jansen, A.; Naber, M.; Einhäuser, W. Binocular rivalry: Frontal activity relates to introspection and action but not to perception. J. Neurosci. 2014, 34, 1738–1747. [Google Scholar] [CrossRef]
- Tychsen, L.; Lisberger, S.G. Visual motion processing for the initiation of smooth-pursuit eye movements in humans. J. Neurophysiol. 1986, 56, 953–968. [Google Scholar] [CrossRef]
- Ferrera, V.P. Task-dependent modulation of the sensorimotor transformation for smooth pursuit eye movements. J. Neurophysiol. 2000, 84, 2725–2738. [Google Scholar] [CrossRef][Green Version]
- Bergamin, O.; Kardon, R.H. Latency of the pupil light reflex: Sample rate, stimulus intensity, and variation in normal subjects. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1546–1554. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Frattini, D.; Wibble, T. Alertness and visual attention impact different aspects of the optokinetic reflex. Investig. Ophthalmol. Vis. Sci. 2021, 62, 16. [Google Scholar] [CrossRef]
- van Die, G.C.; Collewijn, H. Control of human optokinetic nystagmus by the central and peripheral retina: Effects of partial visual field masking, scotopic vision and central retinal scotomata. Brain Res. 1986, 383, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.H.; Polt, J.M. Pupil size in relation to mental activity during simple problem-solving. Science 1964, 143, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D.; Beatty, J. Pupil diameter and load on memory. Science 1966, 154, 1583–1585. [Google Scholar] [CrossRef]
- Steinhauer, S.R.; Siegle, G.J.; Condray, R.; Pless, M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int. J. Psychophysiol. 2004, 52, 77–86. [Google Scholar] [CrossRef]
- van der Wel, P.; van Steenbergen, H. Pupil dilation as an index of effort in cognitive control tasks: A review. Psychon. Bull. Rev. 2018, 25, 2005–2015. [Google Scholar] [CrossRef]
- Thompson, H.S.; Montague, P.; Cox, T.A.; Corbett, J.J. The relationship between visual acuity, pupillary defect and visual field loss. Am. J. Ophthalmol. 1982, 93, 681–688. [Google Scholar] [CrossRef]
- Johnson, L.N.; Hill, R.A.; Bartholomew, M.J. Correlation of afferent pupillary defects with visual field loss on automated perimetry. Ophthalmology 1988, 95, 1649–1655. [Google Scholar] [CrossRef]
- Lowenstein, O.; Kawabata, H.; Loewenfeld, I.E. The pupil as indicator of retinal activity. Am. J. Ophthalmol. 1964, 57, 569–596. [Google Scholar] [CrossRef]
- Wang, C.A.; Boehnke, S.E.; White, B.J.; Munoz, D.P. Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. J. Neurosci. 2012, 3211, 3629–3636. [Google Scholar] [CrossRef]
- Wang, C.A.; Munoz, D.P. Coordination of pupil and saccade responses by the superior colliculus. J. Cogn. Neurosci. 2021, 33, 919–932. [Google Scholar] [CrossRef]
- Krauzlis, R.J. Recasting the smooth pursuit eye movement system. J. Neurophysiol. 2004, 91, 591–603. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanari, K.; Kikuchi, M. OKN and Pupillary Response Modulation by Gaze and Attention Shifts. J. Eye Mov. Res. 2025, 18, 11. https://doi.org/10.3390/jemr18020011
Kanari K, Kikuchi M. OKN and Pupillary Response Modulation by Gaze and Attention Shifts. Journal of Eye Movement Research. 2025; 18(2):11. https://doi.org/10.3390/jemr18020011
Chicago/Turabian StyleKanari, Kei, and Moe Kikuchi. 2025. "OKN and Pupillary Response Modulation by Gaze and Attention Shifts" Journal of Eye Movement Research 18, no. 2: 11. https://doi.org/10.3390/jemr18020011
APA StyleKanari, K., & Kikuchi, M. (2025). OKN and Pupillary Response Modulation by Gaze and Attention Shifts. Journal of Eye Movement Research, 18(2), 11. https://doi.org/10.3390/jemr18020011





