Maintaining Fixation by Children in a Virtual Reality Version of Pupil Perimetry
Abstract
:Introduction
Aims
Methods
Participants
Apparatus
Fixation target conditions
Environment & stimuli
Procedure
Analysis
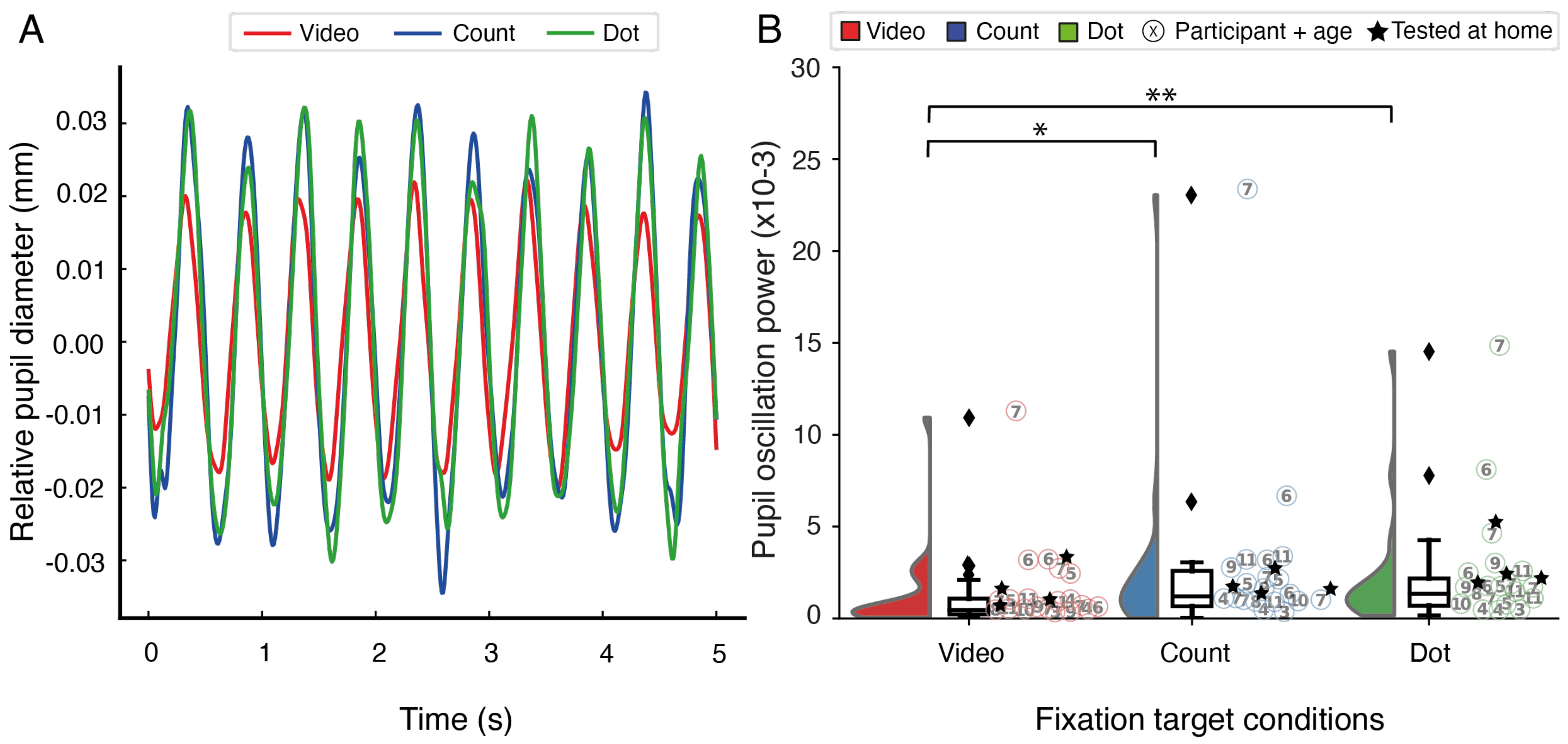
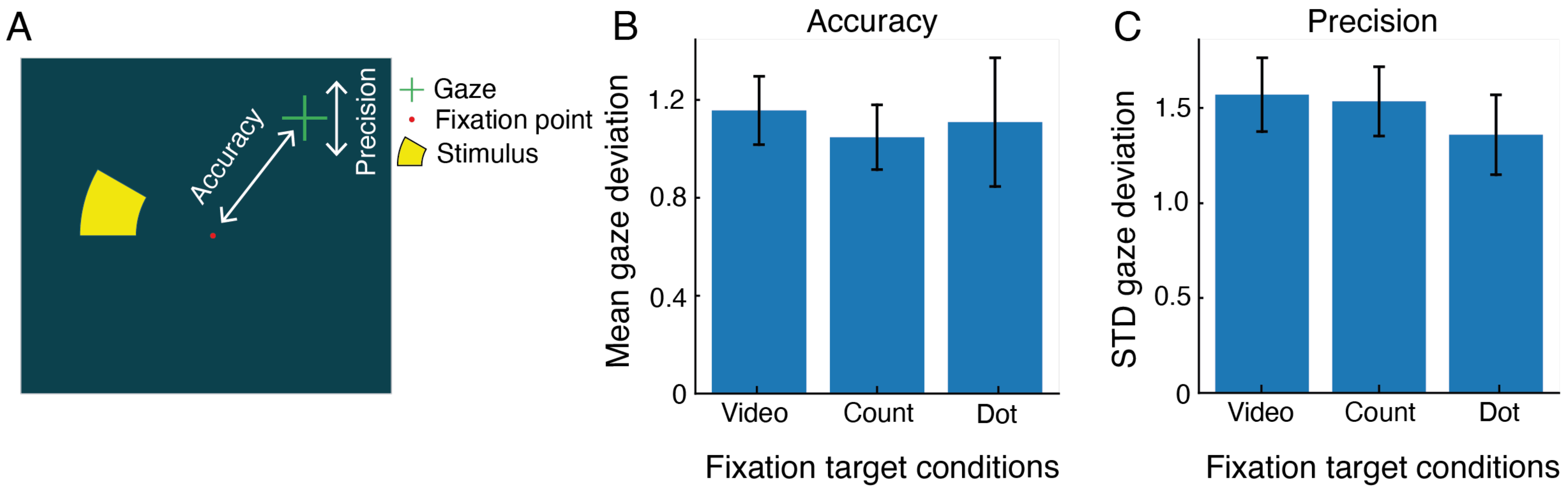
Results
Discussion
Ethics and Conflict of Interest
Acknowledgements
References
- Alawa, K. A., R. P. Nolan, E. Han, A. Arboleda, H. Durkee, M. S. Sayed, M. C. Aguilar, and R. K. Lee. 2021. Low-cost, smartphone-based frequency doubling technology visual field testing using a head-mounted display. British Journal of Ophthalmology 105, 3: 440–444. [Google Scholar] [CrossRef] [PubMed]
- Allen, M., D. Poggiali, K. Whitaker, T. R. Marshall, J. van Langen, and R. A. Kievit. 2021. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Research 4: 63. [Google Scholar] [CrossRef]
- Barbur, Harlow, and Sahraie. 1992. Pupillary responses to stimulus structure, colour and movement. Ophthalmic and Physiological Optics 12, 2: 137–141. [Google Scholar] [CrossRef]
- Binda, P., and S. O. Murray. 2015. Spatial attention increases the pupillary response to light changes. Journal of Vision 15, 2: 1–1. [Google Scholar] [CrossRef] [PubMed]
- Binda, P., M. Pereverzeva, and S. O. Murray. 2013. Attention to bright surfaces enhances the pupillary light reflex. Journal of Neuroscience 33, 5: 2199–2204. [Google Scholar] [CrossRef]
- Deiner, M. S., B. E. Damato, and Y. Ou. 2020. Implementing and Monitoring At-Home Virtual Reality Oculo-kinetic Perimetry During COVID-19. In Ophthalmology. Elsevier: Vol. 127, Issue 9, p. 1258. [Google Scholar] [CrossRef]
- Gamlin, P. D. R., H. Zhang, A. Harlow, and J. L. Barbur. 1998. Pupil responses to stimulus color, structure and light flux increments in the rhesus monkey. Vision Research 38, 21: 3353–3358. [Google Scholar] [CrossRef]
- He, J., S. Zhang, P. Wu, Y. Zhang, X. Zheng, and L. Zhou. 2019. A Novel Virtual Reality Design of Portable Automatic Perimetry. IEEE MTT-S 2019 International Microwave Biomedical Conference, IMBioC 2019 - Proceedings, May 1. [Google Scholar] [CrossRef]
- Kardon, R. H. 1992. Pupil perimetry. In Current Opinion in Ophthalmology. Vol. 3, Issue 5, pp. 565–570. [Google Scholar] [CrossRef]
- Kelbsch, C., J. Lange, H. Wilhelm, B. Wilhelm, T. Peters, M. Kempf, L. Kuehlewein, and K. Stingl. 2020. Chromatic pupil campimetry reveals functional defects in exudative age-related macular degeneration with differences related to disease activity. Translational Vision Science and Technology 9, 6: 5–5. [Google Scholar] [CrossRef]
- Kelbsch, C., K. Stingl, R. Jung, M. Kempf, P. Richter, T. Strasser, T. Peters, B. Wilhelm, H. Wilhelm, and F. Tonagel. 2021. How lesions at different locations along the visual pathway influence pupillary reactions to chromatic stimuli. Graefe’s Archive for Clinical and Experimental Ophthalmology 1: 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kelbsch, C., K. Stingl, M. Kempf, T. Strasser, R. Jung, L. Kuehlewein, H. Wilhelm, T. Peters, B. Wilhelm, and K. Stingl. 2019. Objective measurement of local rod and cone function using gaze-controlled chromatic pupil campimetry in healthy subjects. Translational Vision Science and Technology 8, 6. [Google Scholar] [CrossRef]
- Koenraads, Y., K. P. J. Braun, D. C. P. van der Linden, S. M. Imhof, and G. L. Porro. 2015. Perimetry in young and neurologically impaired children: the Behavioral Visual Field (BEFIE) Screening Test revisited. JAMA Ophthalmology 133, 3: 319–325. [Google Scholar] [CrossRef]
- Maeda, F., C. Kelbsch, T. Straßer, K. Skorkovská, T. Peters, B. Wilhelm, and H. Wilhelm. 2017. Chromatic pupillography in hemianopia patients with homonymous visual field defects. Graefe’s Archive for Clinical and Experimental Ophthalmology 255, 9: 1837–1842. [Google Scholar] [CrossRef]
- Mathôt, S., L. van der Linden, J. Grainger, and F. Vitu. 2013. The pupillary light response reveals the focus of covert visual attention. PloS One 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Mathôt, S., and S. Van der Stigchel. 2015. New Light on the Mind’s Eye: The Pupillary Light Response as Active Vision. Current Directions in Psychological Science 24, 5: 374–378. [Google Scholar] [CrossRef]
- Mees, L., S. Upadhyaya, P. Kumar, S. Kotawala, S. Haran, S. Rajasekar, D. S. Friedman, and R. Venkatesh. 2020. Validation of a Headmounted Virtual Reality Visual Field Screening Device. Journal of Glaucoma 29, 2: 86–91. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M. A., D. B. Henson, C. Fenerty, S. Biswas, and T. Aslam. 2016. Development of a pediatric visual field test. Translational Vision Science and Technology 5, 6: 13–13. [Google Scholar] [CrossRef]
- Morales, J., and S. M. Brown. 2001. The feasibility of short automated static perimetry in children. Ophthalmology 108, 1: 157–162. [Google Scholar] [CrossRef] [PubMed]
- Murray, I. C., C. Schmoll, A. Perperidis, H. M. Brash, A. D. McTrusty, L. A. Cameron, A. G. Wilkinson, A. O. Mulvihill, B. W. Fleck, and R. A. Minns. 2018. Detection and characterisation of visual field defects using Saccadic Vector Optokinetic Perimetry in children with brain tumours. Eye 32, 10: 1. [Google Scholar] [CrossRef]
- Naber, M., G. A. Alvarez, and K. Nakayama. 2013. Tracking the allocation of attention using human pupillary oscillations. Frontiers in Psychology 4: 919. [Google Scholar] [CrossRef]
- Naber, M., and K. Nakayama. 2013. Pupil responses to high-level image content. Journal of Vision 13, 6: 7–7. [Google Scholar] [CrossRef]
- Naber, M., C. Roelofzen, A. Fracasso, D. P. Bergsma, M. van Genderen, G. L. Porro, S. O. Dumoulin, and Y. T. van der Schouw. 2018. Gaze-Contingent Flicker Pupil Perimetry Detects Scotomas in Patients With Cerebral Visual Impairments or Glaucoma. Frontiers in Neurology 9, July: 558. [Google Scholar] [CrossRef]
- Neumayr, L., T. Pieper, M. Kudernatsch, S. TrauzettelKlosinski, and M. Staudt. 2020. Uncovering homonymous visual field defects in candidates for pediatric epilepsy surgery. European Journal of Paediatric Neurology 25: 165–171. [Google Scholar] [CrossRef] [PubMed]
- Patel, D. E., P. M. Cumberland, B. C. Walters, I. RussellEggitt, J. S. Rahi, and O. study. OPTIC study group. 2015. Study of Optimal Perimetric Testing in Children (OPTIC): Feasibility, Reliability and Repeatability of Perimetry in Children. PloS One 10, 6: e0130895. [Google Scholar] [CrossRef]
- Porro, G., E. M. Dekker, O. Van Nieuwenhuizen, D. Wittebol-Post, M. B. H. Schilder, A. J. F. SchenkRootlieb, and W. F. Treffers. 1998. Visual behaviours of neurologically impaired children with cerebral visual impairment: An ethological study. British Journal of Ophthalmology 82, 11: 1231–1235. [Google Scholar] [CrossRef]
- Porro, G., J. Hofmann, D. Wittebol-Post, O. Van Nieuwenhuizen, Y. T. Van Der Schouw, M. B. H. H. Schilder, M. E. M. M. Dekker, and W. F. Treffers. 1998. A new behavioral visual field test for clinical use in pediatric neuroophthalmology. Neuro-Ophthalmology 19, 4: 205–214. [Google Scholar] [CrossRef]
- Portengen, B. L., Y. Koenraads, S. M. Imhof, and G. L. Porro. 2020. Lessons Learned from 23 Years of Experience in Testing Visual Fields of Neurologically Impaired Children. NeuroOphthalmology 44, 6: 361–370. [Google Scholar] [CrossRef] [PubMed]
- Portengen, B. L., C. Roelofzen, G. L. Porro, S. M. Imhof, A. Fracasso, and M. Naber. 2021. Blind spot and visual field anisotropy detection with flicker pupil perimetry across brightness and task variations. Vision Research 178, October 2020: 79–85. [Google Scholar] [CrossRef]
- Razeghinejad, R., A. Gonzalez-Garcia, J. S. Myers, and L. J. Katz. 2021. Preliminary Report on a Novel Virtual Reality Perimeter Compared with Standard Automated Perimetry. Journal of Glaucoma 30, 1: 17–23. [Google Scholar] [CrossRef] [PubMed]
- Reason, J. T. 1978. Motion sickness adaptation: A neural mismatch model. Journal of the Royal Society of Medicine 71, 11: 819–829. [Google Scholar] [CrossRef] [PubMed]
- Rosli, Y., C. F. Carle, Y. Ho, A. C. James, M. Kolic, E. M. F. Rohan, and T. Maddess. 2018. Retinotopic effects of visual attention revealed by dichoptic multifocal pupillography. Scientific Reports 8, 1: 1–13. [Google Scholar] [CrossRef]
- Sipatchin, A., S. Wahl, and K. Rifai. 2021. Eye-tracking for clinical ophthalmology with virtual reality (Vr): A case study of the htc vive pro eye’s usability. Healthcare (Switzerland) 9, 2: 180. [Google Scholar] [CrossRef]
- Tan, L., M. Kondo, M. Sato, N. Kondo, and Y. Miyake. 2001. Multifocal pupillary light response fields in normal subjects and patients with visual field defects. Vision Research 41, 8: 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, S., D. Papaconstantinou, A. Diagourtas, K. Droutsas, K. Andreanos, M. M. Moschos, and D. Brouzas. 2017. Visual field examination method using virtual reality glasses compared with the humphrey perimeter. Clinical Ophthalmology 11: 1431–1443. [Google Scholar] [CrossRef]
- Tsapakis, S., D. Papaconstantinou, A. Diagourtas, S. Kandarakis, K. Droutsas, K. Andreanos, and D. Brouzas. 2018. Home-based visual field test for glaucoma screening comparison with Humphrey perimeter. Clinical Ophthalmology 12: 2597–2606. [Google Scholar] [CrossRef]
- Tschopp, C., A. B. Safran, P. Viviani, A. Bullinger, M. Reicherts, and C. Mermoud. 1998. Automated visual field examination in children aged 5–8 years: Part I: Experimental validation of a testing procedure. Vision Research 38, 14: 2203–2210. [Google Scholar] [CrossRef]
- Tsujimura, S. I., J. S. Wolffsohn, and B. Gilmartin. 2006. Pupil response to color signals in cone-contrast space. Current Eye Research 31, 5: 401–408. [Google Scholar] [CrossRef] [PubMed]
- Ukai, K. 1985. Spatial pattern as a stimulus to the pupillary system. Journal of the Optical Society of America A 2, 7: 1094–1100. [Google Scholar] [CrossRef]
- Walkey, H. C., A. Hurden, I. R. Moorhead, J. A. F. Taylor, J. L. Barbur, and J. A. Harlow. 2005. Effective contrast of colored stimuli in the mesopic range: a metric for perceived contrast based on achromatic luminance contrast. Journal of the Optical Society of America A 22, 1: 17–28. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, H., J. Neitzel, B. Wilhelm, S. Beuel, H. Lüdtke, U. Kretschmann, and E. Zrenner. 2000. Pupil Perimetry using M-Sequence Stimulation Technique. Investigative Ophthalmology & Visual Science 41, 5: 1229–1238. [Google Scholar]
- Wroblewski, D., B. A. Francis, A. Sadun, G. Vakili, and V. Chopra. 2014. Testing of visual field with virtual reality goggles in manual and visual grasp modes. BioMed Research International, 2014. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Portengen, B.L.; Naber, M.; Jansen, D.; van den Boomen, C.; Imhof, S.M.; Porro, G.L. Maintaining Fixation by Children in a Virtual Reality Version of Pupil Perimetry. J. Eye Mov. Res. 2022, 15, 1-10. https://doi.org/10.16910/jemr.15.3.2
Portengen BL, Naber M, Jansen D, van den Boomen C, Imhof SM, Porro GL. Maintaining Fixation by Children in a Virtual Reality Version of Pupil Perimetry. Journal of Eye Movement Research. 2022; 15(3):1-10. https://doi.org/10.16910/jemr.15.3.2
Chicago/Turabian StylePortengen, Brendan L., Marnix Naber, Demi Jansen, Carlijn van den Boomen, Saskia M. Imhof, and Giorgio L. Porro. 2022. "Maintaining Fixation by Children in a Virtual Reality Version of Pupil Perimetry" Journal of Eye Movement Research 15, no. 3: 1-10. https://doi.org/10.16910/jemr.15.3.2
APA StylePortengen, B. L., Naber, M., Jansen, D., van den Boomen, C., Imhof, S. M., & Porro, G. L. (2022). Maintaining Fixation by Children in a Virtual Reality Version of Pupil Perimetry. Journal of Eye Movement Research, 15(3), 1-10. https://doi.org/10.16910/jemr.15.3.2


