Abstract
Mild traumatic brain injury (mTBI), also known as concussion, is a common injury which affects patients of all demographics. There is a global effort to accurately diagnose and identify patients at highest risk of prolonged symptom burden to facilitate appropriate rehabilitation efforts. Underreporting is common with large numbers not engaging with services, in addition to differences in treatment outcomes according to ethnicity, age, and gender. As patients recover, symptomology evolves which challenges rehabilitative efforts with no clear definition of ‘recovered’. This review describes key areas in mTBI such as diagnostic challenges, epidemiology, prognosis, and pathophysiology which serves as an introduction to “Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers.”
Introduction
Mild traumatic brain injury (mTBI), commonly referred to as concussion, is a complex neurobehavioural phenomenon resulting from mechanical trauma (Carroll et al., 2004). Despite increased knowledge of the biomechanics and pathophysiology of concussion, no standardized biomarkers exist (either clinical or serological). Health professionals currently rely on symptom reporting which many patients are not willing to disclose (e.g. athletes, military personnel, or patients pressured into return to work). Likewise, prognosis is variable and it is not possible to predict which patients will require prolonged rehabilitation therapy. In recent years, researchers have shifted their focus to eye tracking due to the widespread neural pathways responsible for ocular motor control. These ocular motor abnormalities may serve as a useful tool in everyday clinical practice for not only diagnosis, but also serve as a biomarker for recovery (Ventura et al., 2015). This review will provide a comprehensive introduction to “Eye Movements in Mild Traumatic Brain Injury: Ocular Biomarkers”, to summarize the current state of mTBI epidemiology, diagnosis (including subtypes), prognosis, and pathophysiology.
mTBI Diagnosis and Challenges
A single unifying diagnostic nosology for classifying mTBI represents one of the greatest challenges within the field. The term concussion and mTBI are often used interchangeably, however, some authorities suggest that concussion should be considered a subset (milder form) of mTBI, although there is no consensus of such a classification (Mayer et al., 2017). Currently there are no distinct symptom diagnostic criteria that differentiate concussion from mTBI. Current guidelines from the Centers for Disease Control (CDC) promote the single term “mild traumatic brain injury”, instead of concussion (Lumba-Brown, 2018; Sharp & Jenkins, 2015).
Although there is extensive discussion regarding the operational definition of mTBI, the definition by the American Congress of Rehabilitation Medicine, revised by the World Health Organization (WHO), seems to be increasingly accepted by clinicians and researchers in the field (Lefevre-Dognin et al., 2021). The definition of mTBI requires a Glasgow Coma Scale score between 13 and 15 at 30 minutes post-injury, and one or more of the following symptoms: <30 min loss of consciousness; <24 hours post-traumatic amnesia; impaired mental state at time of accident (confusion, disorientation, etc.); and/or transient neurological deficits (McCrory, 2013). Neuroimaging is typically normal as standard techniques are not sensitive enough to detect damage in the majority of cases (10% sensitivity for CT and 30% for MRI) (Borg et al., 2004; Mittl et al., 1994; Rugg-Gunn et al., 2001).
Sport-related concussion (SRC) is considered by some investigators to have its own nosological framework. The Consensus Statement on Concussion in Sport at the 4th International Conference on Concussion qualifies SRC as a direct blow to the head, face, neck, or elsewhere on the body with an ‘impulsive’ force transmitted to the head (McCrory, 2013). Typically, this results in rapid onset of short-lived impairment of neurological function that resolves spontaneously. However, in some cases, signs and symptoms evolve over a number of minutes to hours. Standard neuroimaging is normal as the acute clinical signs and symptoms are considered to largely reflect a functional disturbance rather than a structural injury. It is important to note that clinical features should not be explained by drug, alcohol, medication, other injuries (e.g. cervical injuries or peripheral vestibular dysfunction), or other comorbidities (e.g. psychological factors or coexisting medical conditions). Loss of consciousness is not a requirement. While resolution of the clinical and cognitive features typically follows a sequential course, some cases experience a prolonged symptom burden.
Hence, one of the challenges of mTBI research is that the present definition encompasses a broad spectrum of injury. From the above definitions it is clear that the limits of the definitions may overlap with ‘moderate’ TBI at the upper end and trivial head trauma at the lower end. This has a significant impact in interpreting the research in particular with respect to prognosis and management.
Epidemiology
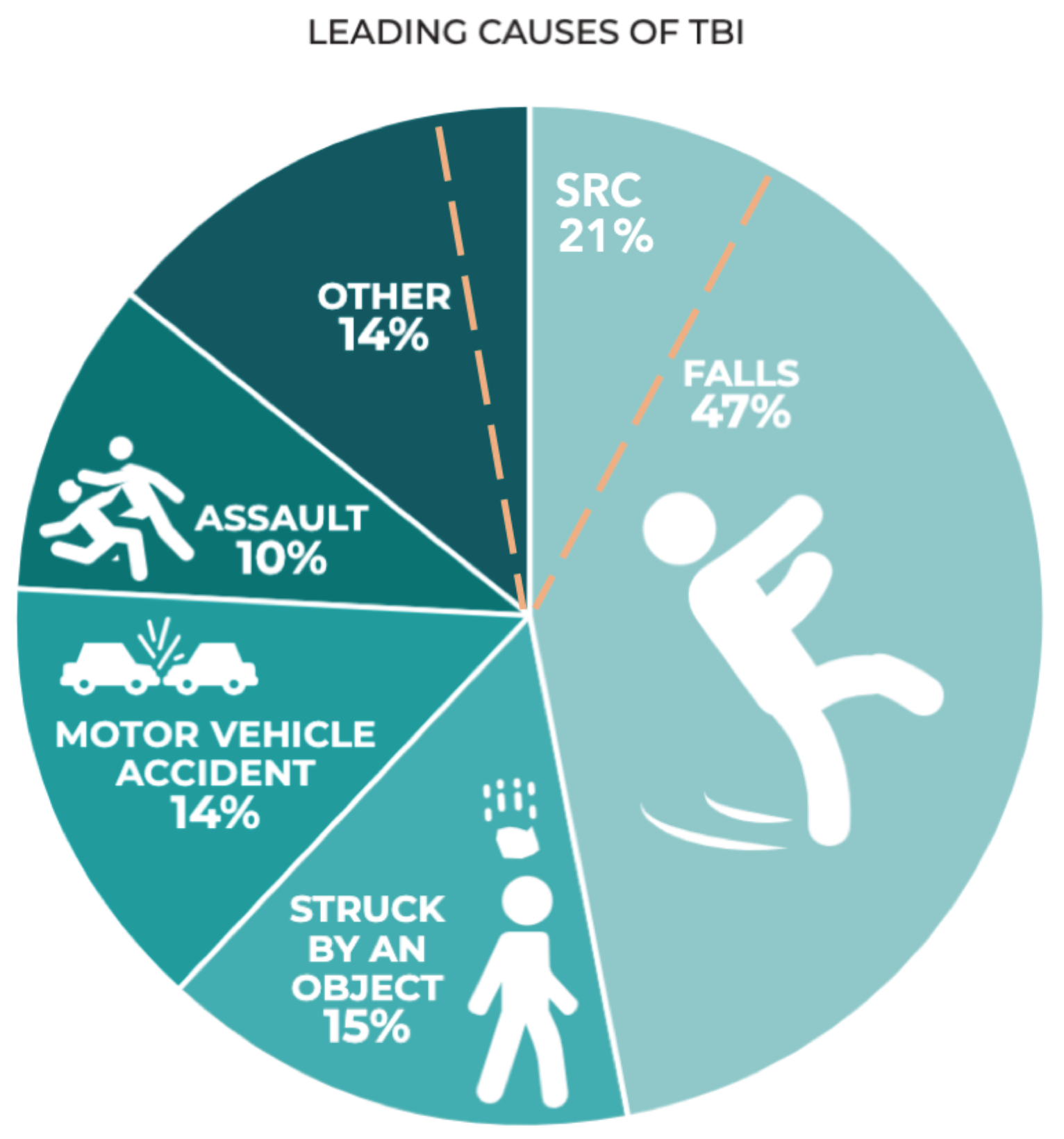
Over 50 million people suffer traumatic brain injury each year (at least 6 per 1,000 globally) (Hon et al., 2019; Maas, 2017) and it is estimated that half the global population will experience a form of TBI during their lifespan (Maas, 2017). Mild traumatic brain injury forms 60-95% of TBIs (Maas, 2017) when averaged across the US, Middle East, Eastern Europe, and Asia with a global incidence of 939/100,000 for all-cause TBI when averaged across African region, Latin America, US/ Canada, Eastern Mediterranean, Europe, Southeast Asia, and Western Pacific (Dewan et al., 2018). In higher income countries, elderly fall-related TBIs are increasing, whilst trauma from road traffic accidents is increasing in lower-income countries (Figure 1) (Maas, 2017). UK data suggests 30% of people aged over 65 will fall at least once per year and for those over 80, the risk is over 50% (England, 2020). Likewise, the Center for Disease Control in the USA cites a prevalence of 27.5% of people aged over 65 per year (35.6 million falls per year) (Moreland B, 2020). Although specific data is not provided with regard to injury subtype following these events, a range of TBI will inevitably arise due to direct or indirect transmission of force.
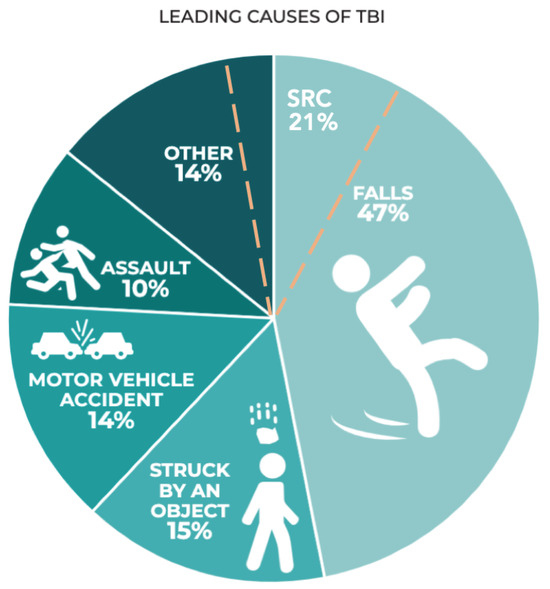
Figure 1.
Leading causes of TBI in the 2014 CDC TBI surveillance report (published in 2019) based on hospital admissions in the US(Prevention, 2019). Sport-related concussion (SRC) has been added by authors (dashed orange line) based on community data from Theadom et al. (Theadom et al., 2014).
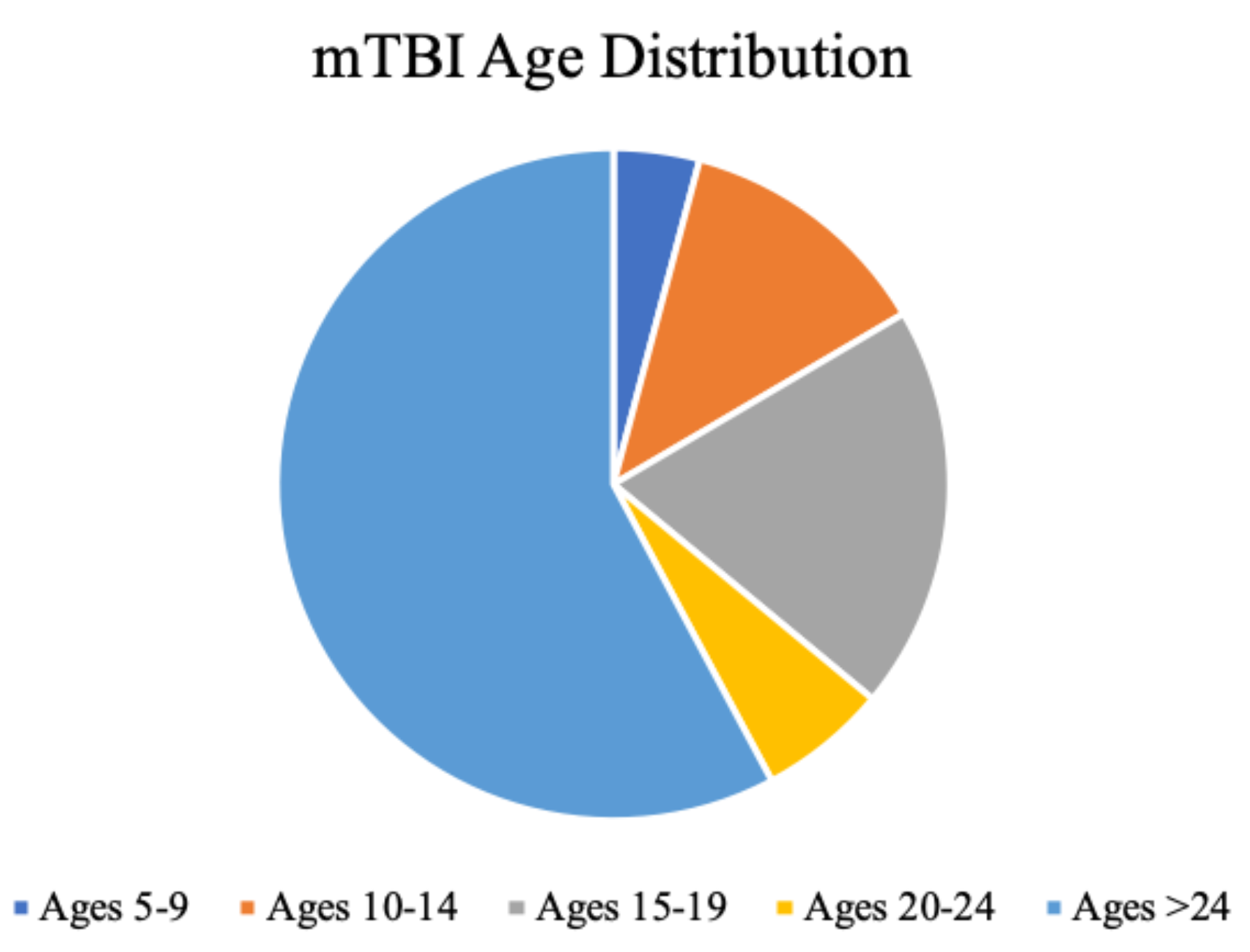
A meta-analysis of 15 prevalence studies (25,134 adults) found that 12% of the sample experienced TBI with loss of consciousness, with men being at more than double the risk of women (Frost et al., 2013). Another epidemiological review of US health insurance companies by Zhang and colleagues analyzed 8,828,248 patients and reported an overall incidence of TBI of 4.9%, with a third falling between ages of 10-19 (29% overall rate of loss of conciousness) (Zhang et al., 2016). These numbers increased by 60% from 2007 to 2014 when the study was conducted, reflecting both population growth and increased health-seeking behaviour (Zhang et al., 2016). Of these subjects, male gender comprised 55% of the sample with the highest incidence of mTBI between the ages of 15 to 19 (16.5/1000), followed by ages 10-14 (10.5/1000), ages 20-24 (5.2/1000), and 5-9 (3.5/1000) (Figure 2). This sample consisted of 56% being diagnosed in the emergency department and 29% at primary care (the remainder were in urgent care and inpatient settings) (Zhang et al., 2016). A major limitation to this review was its inability to detect milder brain injuries in other community settings, such as sports fields, where these are underreported and hence remain undetected.
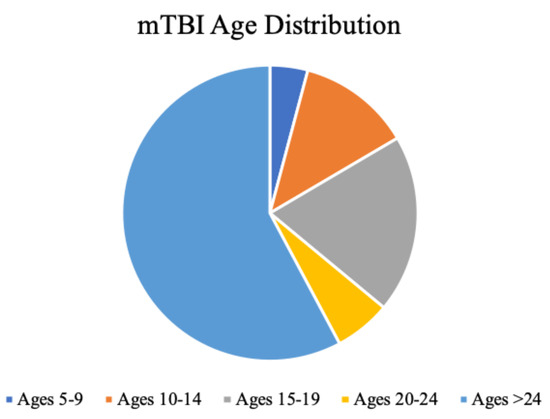
Figure 2.
Distribution of mTBI incidence. Ages 5-24, less than two decades, account for 42% of the entire mTBI population, with ages 15-19 most dominant. Data adapted from (Zhang et al., 2016). .
A significant burden of mTBI is related to sportsrelated concussion (McCrory, 2013). In the United states, this incidence is at least 15.4/100,000 people per year based on an epidemiological study of sport-related mTBI presenting to hospital departments (Selassie et al., 2013).
The majority of studies with this type of selection bias from hospital data cite sport-related concussion as anywhere from 5.4% (Austria) (Mauritz et al., 2014) to 26.7% (USA) (Leibson et al., 2011) of all-cause mTBI. These figures are conservative as they do not account for presentations to other health services and unreported mTBI.
A community-based study exploring multiple sources of referrals revealed a significantly higher incidence of 170/100,000 and sport-related causes comprised 21% of these (Theadom et al., 2014). In a cohort of athletes, a third experienced previous undiagnosed mTBI (Meehan et al., 2013) and it is estimated as many as 70% of head injuries go unreported (Sye et al., 2006). Similarly, in a survey of 133 rugby union players (age under 20), 48% reported experiencing at least one mTBI (mean 2.25) for which half did not seek medical attention (Baker et al., 2013). This is particularly relevant in adolescent populations; those who began contact sports before the age of 12 were deemed twice as likely to have long term dysregulation in behaviour and three times as likely to experience apathy or depression (Alosco et al., 2017).
Gender
mTBI predominantly impacts young males (66% to 76% of all cases) which is due to higher rates of contactsports and riskier maneuvers such as tackling (Theadom et al., 2020). While male athletes have a higher concussion incidence overall, concussion incidence for gendercomparable sports is higher among females (Covassin et al., 2016; Dick, 2009; Gessel et al., 2007; Lincoln et al., 2011; Marar et al., 2012). Further studies on gender differences in mTBI have shown females report a higher number of mild symptoms at baseline testing (Covassin et al., 2006). Colvin and colleages noted slower reaction times, increased symptoms, and lower neurocognitive scores in a cohort of 234 soccer players, aged 8 to 24 years (141 females, 93 males) (Colvin et al., 2009) while another study involving 260 youth (adult cohort: 47 males, 31 females, aged 18-59; paediatric cohort: 97 males, 40 females, aged 4-17) concluded prolonged symptoms were more frequent in adult females, but not minors (Preiss-Farzanegan et al., 2009). These limited gender studies highlight the need for more research in this area.
Ethnicity
Some studies have investigated the relationship between racial disparities and mTBI as well as sportrelated concussion and have identified that there appears to be differences in concussion incidence, awareness, outcome, and morbidity. African American children under the age of 4 years old who sustained TBI have been shown to have mortality rates twice those of Caucasians (Langlois et al., 2005). Other research has shown African American athletes are at a greater risk of neurocognitive impairment (over twice as likely to experience at least one cognitive decline measure on the ImPACT test along with lower processing speed compared to their baseline) 7 days following sports-related concussion (Kontos et al., 2010).
Inequities in healthcare based on ethnicity have been identified in the entire spectrum of mTBI. African American patients were less likely than Caucasian patients to have emergency department visits for head injuries and were less likely to be diagnosed with a concussion during an emergency department visit (Gessel et al., 2007). African American children also have higher rates of concussion/mTBI from assault compared to sports injuries. Bloodgood et al. have also highlighted that African Americans and Hispanics in the USA had less awareness of mTBI than non-Hispanic Caucasians which may lead to underreporting (Bloodgood et al., 2013) in addition to differences in cognitive-related scores between African Americans and Caucasian Americans (Wallace & Mannix, 2021). This may further bias population-based studies from higher under-reporting in these groups. The situation is echoed in New Zealand where underreporting is highest amongst the Māori population who were also found to have a 23% greater risk of mild TBI than New Zealand Europeans (Feigin et al., 2013). Of those that are reported, they are more severe in nature and have lasting effects, including increased health care needs (King, 2014).
Future epidemiological studies are required to investigate incidence longitudinally with consideration of factors such as age, gender, ethnicity, and particular riskfactors in sport-related concussion such as level of competition (elite vs non-elite) and player position which is known to affect risk (Gardner et al., 2019).
mTBI Subtypes
mTBI is a heterogenous injury resulting in diverse clinical presentations which cluster into broad domains (McCrory, 2013). These domains are also dynamic; patients who present with one dominate symptom cluster may evolve into another as recovery progresses. The five most commonly recognized subtypes are described below from a 2019 literature review and meta-analysis by experts from the 2015 “Targeted Evaluation and Active Management” meeting (Lumba-Brown et al., 2019):
Concussion-associated conditions are common symptoms which may be present irrespective of subtype. These include sleep disturbance and cervical strain. These patients may experience insomnia or hypersomnolence (Lumba-Brown et al., 2019). Cervical strain consists of neck pain, stiffness, weakness, or chronic occipital/ suboccipital pain, which is often caused by whiplash injury (Bogduk & Govind, 2009), particularly prevalent in wheelchair athletes (Hollander et al., 2020).
However, these domains (and assocated conditions) have recently been challenged following a systematic review and meta-cluster analysis by Langdon and colleagues of 5592 athletes across 22 carefully selected studies (Langdon et al., 2020). Their analysis revised these five subtypes (based on symptomology from the Sport Concussion Assessment Tool, 5th edition, SCAT-5 (Group, 2017) which correlated to clinical outcomes. Their rationale was clear: to provide a unified evidence base toward individualized mTBI management and treatment to enhance recovery and reduce prolonged symptom burden.

Table 2.
Subtypes as described in (Langdon et al., 2020).
Table 2.
Subtypes as described in (Langdon et al., 2020).
| Subtype/ “Cluster” | Symptom | Association | Prevalence |
| Migraine | Headache, sensitivity to light/ noise, and nausea | Concomitant cognitive, balance, and vestibulo-ocular motor symptoms which correlated to prolonged recovery | 24% |
| Cognitiveemotional | Difficulty concentrating, remembering, ‘fogginess’, increased emotion, irritability, sadness, nervousness, and ‘feeling slowed down’. | Prolonged recovery, balance deficits, and greater total symptom severity scores | 19% |
| Sleepemotional | Trouble falling asleep, sleeping less (and more), increased emotion, irritiability, sadness, nervousness | Prolonged recovery, lower sleep quantity, cognitive impairment, balance impairment, and greater total symptom severity scores. | 21% |
| Neurological | Blurred vision, vomiting, neck pain, pressure in the head, visual problems, and double vision. | Associated with vestibulo-ocular motor screening symptoms. | 2% |
| Undefined feelings cluster | “Not feeling right” and confusion | No evidence to correlate this to a specific clinical outcome. | Not quantifiable in literature |
Prognosis and Risk Factors
Recovery from mTBI is variable depending on the population studied and the definition of mTBI. The two week recovery of patients with sport-related concussion has been reported to range from 50% to 90% (Institute of Medicine & Council, 2014). At two months, 4-12% of young adults will still be symptomatic (Barlow et al., 2015; Kara et al., 2020). Long-term outcomes are equally variable. In the primary care setting, half of patients experienced four or more post-mTBI symptoms at 12 months with 10.9% of participants suffering from low cognitive functioning and increased levels of mood disturbance (anxiety, depression) (Theadom et al., 2016). In this group, risk factors for prolonged recovery were a previous history of brain injury, living alone, ethnicity (non-caucasion), alcohol and medication use, and female gender. Even after adjusting for gender-related differences, evidence suggests an ongoing symptom burden in females of almost twice as long as males (Baker et al., 2016; Broshek et al., 2005), attributable to reduced neck-head segment mass (Tierney et al., 2005), differences in cerebral blood flow (Esposito et al., 1996), and reponse to injury on neurobehavioural assessments (Broshek et al., 2005; Covassin et al., 2006; Gioia et al., 2008). Other groups have also correlated severity of symptoms post-injury, repeat injury, subacute development of headaches and mood disturbances, age (children and adolescents), and pre-existing mental illness to prolonged symptom burden (Baker et al., 2016; Iverson et al., 2017; Kara et al., 2020; Slobounov et al., 2007).
In a cohort of 91 students, aged 13-19, a third of those who reported problems returning back to class following an mTBI had a history of previous head trauma. Similarly, a third of students struggled with return to school (57% of students took over 10 days recover and 29% took over 21 days to recover). Of these, vision problems were correlated to a 2.5x risk of difficulty at school (Baker et al., 2015). Likewise, a cohort of 247 patients aged 5-18 revealed a time to return-to-school of 12 days, but without accommodations (e.g. reduced hours, extra help) this was 35 days (Corwin et al., 2014). Adolescents were not symptom free until 64 days and were cleared for sport at 75 days. At 4 weeks post-injury, 73% of these patients were symptomatic and 61% showed a decline in grades that year.
There is a paucity of literature in the elderly population’s prognosis following mTBI, but studies in this area suggest poor outcomes on cognition and independence following an injury (Rapoport, 2000). A prospective study spanning two years post-injury found early cognitive decline more prevalent in the elderly population following head injury (mean age 69.9 +/- 11.5) even when controlling for pre-morbid cognitive status (Goldstein et al., 1999). Another study showed adults over 65 years at one year post injury had high rates of low mini-mental state examination score (62% scored < 24) with 32.6% reporting mild to moderate disability following their injury and 56.8% meeting the criteria for ‘postconcussion syndrome’ (Deb et al., 1998).
Postconcussion Syndrome (PCS)
This controversial clinical entity has been defined as a constellation of physical, cognitive, behavioral, and emotional symptoms that persist beyond 3 months postinjury. Symptoms include headache, fatigue, visual changes, balance issues, confusion, dizziness, insomnia, neuropsychiatric symptoms, and concentration deficits (Bazarian et al., 1999). Both the International Classification of Diseases, 10th revision (ICD-10), and the DSMIV (which refers to PCS as ‘postconcussional disorder’) have different clinical criteria (American Psychiatric Association, 1994; World Health Organization, 1993). When utilized on the same patient cohort, the incidence has been shown to vary depending on which criteria was used, despite no difference in outcome measures of psychiatric symptoms, quality of life, and community engagement (McCauley et al., 2005). It is unclear whether this is related to ongoing trauma-related neuropathological changes, a secondary phenomenon such as premorbid conditions (e.g. migraine, mental illness), or perhaps both (Leddy et al., 2012).
The pathophysiology of PCS remains poorly understood. Some theories suggest it has a psychogenic origin ((WHO), 1992; Fox et al., 1995; Iverson, 2006; Iverson & McCracken, 1997; Lees-Haley & Brown, 1993;
Lishman, 1988), while others suggest that it is related to persistent microstructural (Karlsen et al., 2019; Santhanam et al., 2019), autonomic (Clausen et al., 2016; La Fountaine et al., 2009; Metting et al., 2014) and metabolic alterations (Giza & Hovda, 2001) in the brain.
This evidence is supported by studies correlating diffusion MRI abnormalities (a surrogate for white matter tract damage) to ocular motor dysfunction in patients with ongoing symptom burden. Maruta and colleagues suggested that disrupted white matter integrity in the right anterior corona radiata, uncinate fasciculus, and genu of the corpus callosum correlated to decreased gaze accuracy in smooth pursuit eye tracking paradigms (Maruta et al., 2010). Taghdiri’s group found that diffusion measures of the left uncinate fasciculus mediated the relationship between time of last concussion to number of self-paced saccades (decreased numbers in postconcussion cohort) with a further correlation between the left cingulum to total symptom burden and number of self-paced saccades (Taghdiri et al., 2018). Tyler and colleagues’ small cohort of 12 mTBI patients (2 months to 35 years post-injury) suggested that their increased saccade latency, slower velocities, and reduced convergence and divergence velocities (compared to 11 agematched controls) were due to a 50% reduction in functional MRI signal (blood-oxygen-level-dependentcontrast) in brainstem nuclei responsible for eye movements (Tyler et al., 2015). There is clearly emerging evidence in this area to suggest ongoing pathophysiological change in these patients, but more studies are required with larger cohorts, more robust inclusion criteria (e.g. mTBI criteria, time since injury, recruitment), and standardized assessments of eye movement dysfunction in both methodology and analysis.
Arguments for premorbid mental illness or maladaptive psychological phenomena following mTBI cite significant overlap and similarities to somatization observed in psychiatric disorders such as depression, anxiety, and post-traumatic stress disorder (PTSD). The DSM-V classification for major depressive disorder includes difficulty concentrating, headaches, indecisiveness, loss of energy, anxiety, and insomina, all of which are mTBI symptoms (American Psychiatric Association, 2013). A study evaluating postconcussive symptoms in patients with depression showed that 78% experienced poor concentration, 86% fatigue, 59% headaches, 41% nausea, 66% nervous/ tense feelings, and 78% with disordered sleep (Iverson, 2006). In this group, 90% of patients with depression met self-reported criteria for postconcussion syndrome (Iverson, 2006). Symptom assessment alone may therefore prove ineffective in sufficiently classifying these patients. Additionally, the label of “postconcussion syndrome” may either validate a patients’ sick role to prevent further recovery attempts or provide false reassurance that their symptoms may spontaneously resolve, preventing further investigation or treatment (Sharp & Jenkins, 2015). “Ongoing (or prolonged) symptom burden” may serve as a more useful descriptor.
Overall, PCS remains a contentious diagnosis among health professionals despite an increase in literature rationalizing persistent neurological dysfunction. Individuals must continue to be treated on a case-by-case basis, accounting for multiple health-related factors.
Pathophysiology
Following an mTBI, a series of complex biochemical, metabolic, and microstructural changes occur. Mechanical trauma (from either acceleration/deceleration, blunt trauma, or rotational forces) causes shear and stress forces within the brain parenchyma which disrupts cellular membranes. This leads to extracellular shifts of potassium, intracellular shifts of sodium and calcium, glutamate release, and diffuse neuronal depolarization (Giza & Hovda, 2014; Katayama et al., 1990; Price, 2016). Global depression in neuronal function ensues which is attributed to hyperacute mTBI symptomology (e.g. loss of consciousness, amnesia, confusion, drowsiness). An ‘energy crisis’ follows where cellular membrane ion pumps use more adenosine triphosphate than is available to restore homeostasis leading to hyperglycolysis. Excessive free radical production from altered metabolism leads to a wellrecognized window of increased cerebral vulnerability where insufficient recovery time places the patient at risk of exponentially more serious damage from a second mTBI (Prins et al., 2013; Prins et al., 2010; Vagnozzi et al., 2008).
After the insult, disruption to the blood-brain barrier results from cerebral microvasculature trauma and loss of junctional adhesion proteins (Yeung et al., 2008), causing a downstream cascade of inflammation from blood-borne factors, initiating microglial activation and proinflammatory cytokines (Holmin et al., 1997; Kabadi et al., 2014; Katayama et al., 1990). The proinflammatory response is accelerated by glutamate release as the immune system mounts a response to oxidative stress (referred to as immune-excitotoxicity) (Blaylock & Maroon, 2011).
Microstructural changes to cytoskeletal architecture of dendrites, astrocytes, and axons (in particular microtubules and neurofilaments) leads to impaired neurotransmission. In particular, unmyelinated axons are at greater risk of damage which explains why younger brains may be at greater risk of long term sequelae (Reeves et al., 2012). In addition, repeat trauma also prevents synaptic plasticity (reorganization of axons) and neuronal recovery (Aungst et al., 2014; White et al., 2017). The degree of axonal disruption, as dictated by the severity of injury, positively correlates to the degree of neurobehavioural disruption (i.e. symptomology) which, in turn, leads to the degree of network disruption (Giza & Hovda, 2014; Kenzie et al., 2018). As recovery occurs, long fiber tracts and hubs (intersections of functional networks) continuously rearrange themselves which manifest as evolving symptomology (Kenzie et al., 2018).
For an in-depth review of this topic, refer to Romeu-Mejia et al. and Giza et al. (Giza & Hovda, 2014; Romeu-Mejia et al., 2019).
Conclusion
mTBI is a common injury which represents an unmet health need on a global scale. There are challenges in not only identifying and subsequently diagnosing these patients, but also grouping into sub-types for appropriate referral pathways. Further clarification of definitions and criteria in mTBI is required for unified efforts (both clinically and for researchers) in this area. In future, ocular motor characteristics may serve as a diagnostic aid given emerging evidence which positively correlates to white matter tract disruption in the brain. This is particularly relevant to a large group of mTBI patients suffering from visual symptoms. Measuring the scale of the epidemiological burden of mTBI in communities is difficult with large variation from hospital-based and primary care (general practice) surveys. This is compounded by underreporting in sports leagues and adolescent populations. Racial disparities in health literacy, health-seeking behaviours, and treatment outcomes reflect wider societal issues. Further studies exploring these issues in greater detail may elucidate contributing factors to facilitate policy and culture change. In addition, the lack of literature on mTBI in the elderly is alarming, considering the prevalence of falls in this population. Where symptoms may be difficult to evaluate in the cognitively impaired, biomarkers such as quantitative eye tracking may prove useful. When studying mTBI trajectories across populations, prognosis is varied as diagnosing a patient as ‘recovered’ results in discharge from services and may not reflect complete resolution of symptomology or physiological recovery. This is evident from primary care surveys at one year and outcomes in schools as mentioned above. The spectrum of ongoing symptom burden and label of postconcussion syndrome may not be helpful with notable overlaps in symptoms of mental illness. Unravelling the pathophysiology and identifying a biomarker in neural recovery is key to improved service provision. Overall, mTBI is a common injury with significant gaps in both our primary understanding of this condition and treatment modalities.
Ethics and Conflicts of Interest
The author(s) declare(s) that the contents of the article are in agreement with the ethics described in http://biblio.unibe.ch/portale/elibrary/BOP/jemr/ethics.ht ml and that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
This research was supported by the Health Research Council of New Zealand through the Clinical Research Training Fellowship. .
References
- (WHO), W. H. O. 1992. International Statistical Classification of Diseases and Related Health Problems, 10th ed. World Health Organization. [Google Scholar]
- Alosco, M. L., A. B. Kasimis, J. M. Stamm, A. S. Chua, C. M. Baugh, D. H. Daneshvar, C. A. Robbins, M. Mariani, J. Hayden, S. Conneely, R. Au, A. Torres, M. D. McClean, A. C. McKee, R. C. Cantu, J. Mez, C. J. Nowinski, B. M. Martin, C. E. Chaisson, Y. Tripodis, and R. A. Stern. 2017. Age of first exposure to American football and long-term neuropsychiatric and cognitive outcomes [Article]. Translational psychiatry 7, 9: e1236. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association, A. 1994. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, ed 4. [Google Scholar] [CrossRef]
- American Psychiatric Association, A. 2013. Diagnostic and statistical manual of mental disorders.
- Aungst, S. L., S. V. Kabadi, S. M. Thompson, B. A. Stoica, and A. I. Faden. 2014. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 34, 7: 1223–1232. [Google Scholar] [CrossRef]
- Baker, J. F., B. M. Devitt, J. Green, and C. McCarthy. 2013. Concussion among under 20 rugby union players in Ireland: incidence, attitudes and knowledge. Ir J Med Sci 182, 1: 121–125. [Google Scholar] [CrossRef]
- Baker, J. G., J. J. Leddy, S. R. Darling, B. P. Rieger, T. L. Mashtare, T. Sharma, and B. S. Willer. 2015. Factors Associated With Problems for Adolescents Returning to the Classroom After Sport-Related Concussion. Clin Pediatr (Phila) 54, 10: 961–968. [Google Scholar] [CrossRef] [PubMed]
- Baker, J. G., J. J. Leddy, S. R. Darling, J. Shucard, M. Makdissi, and B. S. Willer. 2016. Gender differences in recovery from sports-related concussion in adolescents. Clin Pediatr (Phila) 55, 8: 771–775. [Google Scholar] [CrossRef]
- Barlow, K. M., S. Crawford, B. L. Brooks, B. Turley, and A. Mikrogianakis. 2015. The Incidence of Postconcussion Syndrome Remains Stable Following Mild Traumatic Brain Injury in Children. Pediatric Neurology 53, 6: 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bazarian, J. J., T. Wong, M. Harris, N. Leahey, S. Mookerjee, and M. Dombovy. 1999. Epidemiology and predictors of post-concussive syndrome after minor head injury in an emergency population. Brain Inj 13, 3: 173–189. [Google Scholar] [CrossRef]
- Blaylock, R. L., and J. Maroon. 2011. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surgical neurology international 2: 107–107. [Google Scholar] [CrossRef]
- Bloodgood, B., D. Inokuchi, W. Shawver, K. Olson, R. Hoffman, E. Cohen, K. Sarmiento, and K. Muthuswamy. 2013. Exploration of awareness, knowledge, and perceptions of traumatic brain injury among American youth athletes and their parents. J Adolesc Health 53, 1: 34–39. [Google Scholar] [CrossRef]
- Bogduk, N., and J. Govind. 2009. Cervicogenic headache: an assessment of the evidence on clinical diagnosis, invasive tests, and treatment. The Lancet Neurology 8, 10: 959–968. [Google Scholar] [CrossRef] [PubMed]
- Borg, J., L. Holm, J. D. Cassidy, P. M. Peloso, L. J. Carroll, H. von Holst, and K. Ericson. 2004. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med, 61–75. [Google Scholar] [CrossRef]
- Broshek, D. K., T. Kaushik, J. R. Freeman, D. Erlanger, F. Webbe, and J. T. Barth. 2005. Sex differences in outcome following sports-related concussion. Journal of Neurosurgery 102, 5: 856–863. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L. J., J. D. Cassidy, L. Holm, J. Kraus, and V. G. Coronado. 2004. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med, 113–125. [Google Scholar] [CrossRef]
- Clausen, M., D. R. Pendergast, B. Willer, and J. Leddy. 2016. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J Head Trauma Rehabil 31, 3: 215–224. [Google Scholar] [CrossRef] [PubMed]
- Colvin, A. C., J. Mullen, M. R. Lovell, R. V. West, M. W. Collins, and M. Groh. 2009. The role of concussion history and gender in recovery from soccer-related concussion. The American journal of sports medicine 37, 9: 1699–1704. [Google Scholar] [CrossRef]
- Corwin, D. J., M. R. Zonfrillo, C. L. Master, K. B. Arbogast, M. F. Grady, R. L. Robinson, A. M. Goodman, and D. J. Wiebe. 2014. Characteristics of prolonged concussion recovery in a pediatric subspecialty referral population. J Pediatr 165, 6: 1207–1215. [Google Scholar] [CrossRef]
- Covassin, T., R. Moran, and R. J. Elbin. 2016. Sex Differences in Reported Concussion Injury Rates and Time Loss From Participation: An Update of the National Collegiate Athletic Association Injury Surveillance Program From 2004-2005 Through 2008-2009. Journal of Athletic Training 51, 3: 189–194. [Google Scholar] [CrossRef]
- Covassin, T., C. B. Swanik, M. Sachs, Z. Kendrick, P. Schatz, E. Zillmer, and C. Kaminaris. 2006. Sex differences in baseline neuropsychological function and concussion symptoms of collegiate athletes. Br J Sports Med 40, 11: 923–927; discussion 927. [Google Scholar] [CrossRef]
- Deb, S., I. Lyons, and C. Koutzoukis. 1998. Neuropsychiatric sequelae one year after a minor head injury. J Neurol Neurosurg Psychiatry 65, 6: 899–902. [Google Scholar] [CrossRef]
- Dewan, M. C., A. Rattani, S. Gupta, R. E. Baticulon, Y. C. Hung, M. Punchak, A. Agrawal, A. O. Adeleye, M. G. Shrime, A. M. Rubiano, J. V. Rosenfeld, and K. B. Park. 2018. Estimating the global incidence of traumatic brain injury. J Neurosurg, 1–18. [Google Scholar] [CrossRef]
- Dick, R. W. 2009. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med 43 Suppl 1: i46–50. [Google Scholar] [CrossRef] [PubMed]
- England, P. H. 2020. Falls: applying all our health. 1/11. Retrieved 1/11 from https://www.gov.uk/government/publications/falls-applying-all-our-health/falls-applying-all-ourhealth.
- Esposito, G., J. D. Van Horn, D. R. Weinberger, and K. F. Berman. 1996. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med 37, 4: 559–564. [Google Scholar] [PubMed]
- Feigin, V. L., A. Theadom, S. Barker-Collo, N. J. Starkey, K. McPherson, M. Kahan, A. Dowell, P. Brown, V. Parag, R. Kydd, K. Jones, A. Jones, and S. Ameratunga. 2013. Incidence of traumatic brain injury in New Zealand: a population-based study. Lancet Neurol 12, 1: 53–64. [Google Scholar] [CrossRef] [PubMed]
- Fox, D. D., P. R. Lees-haley, K. Earnest, and S. Dolezalwood. 1995. Post-Concussive symptoms: Base rates and etiology in psychiatric patients. The Clinical neuropsychologist 9, 1: 89–92. [Google Scholar] [CrossRef]
- Frost, R. B., T. J. Farrer, M. Primosch, and D. W. Hedges. 2013. Prevalence of Traumatic Brain Injury in the General Adult Population: A Meta-Analysis. Neuroepidemiology 40, 3: 154–159. [Google Scholar] [CrossRef]
- Gardner, A. J., K. L. Quarrie, and G. L. Iverson. 2019. The Epidemiology of Sport-Related Concussion: What the Rehabilitation Clinician Needs to Know. Journal of Orthopaedic & Sports Physical Therapy 49, 11: 768–778. [Google Scholar] [CrossRef]
- Gessel, L. M., S. K. Fields, C. L. Collins, R. W. Dick, and R. D. Comstock. 2007. Concussions among United States high school and collegiate athletes. Journal of Athletic Training 42, 4: 495–503. [Google Scholar] [PubMed] [PubMed Central]
- Gioia, G. A., M. Collins, and P. K. Isquith. 2008. Improving identification and diagnosis of mild traumatic brain injury with evidence.
- psychometric support for the acute concussion evaluation. J Head Trauma Rehabil 23, 4: 230–242. [CrossRef]
- Giza, C. C., and D. A. Hovda. 2001. The Neurometabolic Cascade of Concussion. Journal of Athletic Training 36, 3: 228–235. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC155411/. [CrossRef]
- Giza, C. C., and D. A. Hovda. 2014. The new neurometabolic cascade of concussion. Neurosurgery 75 Suppl 4: S24–33. [Google Scholar] [CrossRef]
- Goldstein, F. C., H. S. Levin, W. P. Goldman, A. D. Kalechstein, A. N. Clark, and T. Kenehan-Altonen. 1999. Cognitive and Behavioral Sequelae of Closed Head Injury in Older Adults According to Their Significant Others. The Journal of Neuropsychiatry and Clinical Neurosciences 11, 1: 38–44. [Google Scholar] [CrossRef] [PubMed]
- Group, T. C. I. S. 2017. Sport concussion assessment tool-5th edition. British Journal of Sports Medicine 51, 11: 851. [Google Scholar] [CrossRef]
- Hollander, K., S. Kluge, F. Glöer, H. Riepenhof, A. Zech, and A. Junge. 2020. Epidemiology of injuries during the Wheelchair Basketball World Championships 2018: A prospective cohort study. Scandinavian Journal of Medicine & Science in Sports 30, 1: 199–207. [Google Scholar] [CrossRef]
- Holmin, S., M. Schalling, B. Höjeberg, A.-C. S. Nordqvist, A.-K. Skeftruna, and T. Mathiesen. 1997. Delayed cytokine expression in rat brain following experimental contusion. Journal of Neurosurgery 86, 3: 493–504. [Google Scholar] [CrossRef]
- Hon, K. L., A. K. C. Leung, and A. R. Torres. 2019. Concussion: A Global Perspective. Seminars in Pediatric Neurology 30: 117–127. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine,, and Council, N. R. 2014. The National Academies Collection: Reports funded by National Institutes of Health. In Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. Edited by R. Graham, F. P. Rivara, M. A. Ford and C. M. Spicer. National Academies Press (US). [Google Scholar] [CrossRef]
- Iverson, G. L. 2006. Misdiagnosis of the persistent postconcussion syndrome in patients with depression. Archives of Clinical Neuropsychology 21, 4: 303–310. [Google Scholar] [CrossRef]
- Iverson, G. L., A. J. Gardner, D. P. Terry, J. L. Ponsford, A. K. Sills, D. K. Broshek, and G. S. Solomon. 2017. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med 51, 12: 941–948. [Google Scholar] [CrossRef]
- Iverson, G. L., and L. M. McCracken. 1997. ‘Postconcussive’ symptoms in persons with chronic pain. Brain Injury 11, 11: 783–790. [Google Scholar] [CrossRef]
- Kabadi, S. V., B. A. Stoica, D. J. Loane, T. Luo, and A. I. Faden. 2014. CR8, a Novel Inhibitor of CDK, Limits Microglial Activation.
- Astrocytosis, Neuronal Loss, and Neurologic Dysfunction after Experimental Traumatic Brain Injury. Journal of Cerebral Blood Flow & Metabolism 34, 3: 502–513. [CrossRef]
- Kara, S., H. Crosswell, K. Forch, A. Cavadino, J. McGeown, and M. Fulcher. 2020. Less Than Half of Patients Recover Within 2 Weeks of Injury After a Sports-Related Mild Traumatic Brain Injury: A 2-Year Prospective Study. Clin J Sport Med 30, 2: 96–101. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, R. H., C. Einarsen, H. K. Moe, A. K. Håberg, A. Vik, T. Skandsen, and L. Eikenes. 2019. Diffusion kurtosis imaging in mild traumatic brain injury and postconcussional syndrome. Journal of neuroscience research 97, 5: 568–581. [Google Scholar] [CrossRef]
- Katayama, Y., D. P. Becker, T. Tamura, and D. A. Hovda. 1990. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg 73, 6: 889–900. [Google Scholar] [CrossRef] [PubMed]
- Kenzie, E. S., E. L. Parks, E. D. Bigler, D. W. Wright, M. M. Lim, J. C. Chesnutt, G. W. J. Hawryluk, W. Gordon, and W. Wakeland. 2018. The dynamics of concussion: Mapping pathophysiology, persistence, and recovery with causal-loop diagramming. Frontiers in neurology 9: 203–203. [Google Scholar] [CrossRef]
- King, D., C. Gissane, M. Brughelli, P. Hume, and J. Harawira. 2014. Sport-related concussion in New Zealand: A Review of 10 Years of Accident Compensation Corporation Moderate to Severe Claims and Costs. J Sci Med Sport 17, 3: 250–255. [Google Scholar] [CrossRef]
- Kontos, A. P., R. J. Elbin, 3rd, T. Covassin, and E. Larson. 2010. Exploring differences in computerized neurocognitive concussion testing between African American and White athletes. Arch Clin Neuropsychol 25, 8: 734–744. [Google Scholar] [CrossRef] [PubMed]
- La Fountaine, M. F., K. S. Heffernan, J. D. Gossett, W. A. Bauman, and R. E. De Meersman. 2009. Transient suppression of heart rate complexity in concussed athletes. Auton Neurosci 148, 1–2: 101–103. [Google Scholar] [CrossRef]
- Langdon, S., M. Königs, E. A. M. C. Adang, E. Goedhart, and J. Oosterlaan. 2020. Subtypes of Sport-Related Concussion: a Systematic Review and Meta-cluster Analysis. Sports Medicine 50, 10: 1829–1842. [Google Scholar] [CrossRef]
- Langlois, J. A., W. Rutland-Brown, and K. E. Thomas. 2005. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil 20, 3: 229–238. [Google Scholar] [CrossRef]
- Leddy, J. J., H. Sandhu, V. Sodhi, J. G. Baker, and B. Willer. 2012. Rehabilitation of concussion and post-concussion syndrome. Sports Health 4, 2: 147–154. [Google Scholar] [CrossRef]
- Lees-Haley, P. R., and R. S. Brown. 1993. Neuropsychological complaint base rates of 170 personal injury claimants. Arch Clin Neuropsychol 8, 3: 203–209. [Google Scholar] [CrossRef]
- Lefevre-Dognin, C., M. Cogné, V. Perdrieau, A. Granger, C. Heslot, and P. Azouvi. 2021. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 67, 3: 218–221. [Google Scholar] [CrossRef]
- Leibson, C. L., A. W. Brown, J. E. Ransom, N. N. Diehl, P. K. Perkins, J. Mandrekar, and J. F. Malec. 2011. Incidence of traumatic brain injury across the full disease spectrum: a populationbased medical record review study. Epidemiology 22, 6: 836–844. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, A. E., S. V. Caswell, J. L. Almquist, R. E. Dunn, J. B. Norris, and R. Y. Hinton. 2011. Trends in concussion incidence in high school sports: a prospective 11-year study. The American journal of sports medicine 39, 5: 958–963. [Google Scholar] [CrossRef] [PubMed]
- Lishman, W. A. 1988. Physiogenesis and psychogenesis in the ’post-concussional syndrome’. Br J Psychiatry 153: 460–469. [Google Scholar] [CrossRef]
- Lumba-Brown, A., M. Teramoto, O. J. Bloom, D. Brody, J. Chesnutt, J. R. Clugston, M. Collins, G. Gioia, A. Kontos, A. Lal, A. Sills, and J. Ghajar. 2019. Concussion Guidelines Step 2: Evidence for Subtype Classification. Neurosurgery. [Google Scholar] [CrossRef]
- Lumba-Brown, A., K. O. Yeates, K. Sarmiento, M. J. Breiding, T. M. Haegerich, G. A. Gioia, M. Turner, E. C. Benzel, S. J. Suskauer, C. C. Giza, M. Joseph, C. Broomand, B. Weissman, W. Gordon, D. W. Wright, R. S. Moser, K. McAvoy, L. Ewing-Cobbs, A.-C. Duhaime, M. Putukian, and et al. 2018. Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatrics 172, 11: e182853–e182853. [Google Scholar] [CrossRef] [PubMed]
- Maas, A. I. R., D. K. Menon, P. D. Adelson, N. Andelic, M. J. Bell, A. Belli, P. Bragge, A. Brazinova, A. Büki, R. M. Chesnut, G. Citerio, M. Coburn, D. J. Cooper, A. T. Crowder, E. Czeiter, M. Czosnyka, R. Diaz-Arrastia, J. P. Dreier, A. C. Duhaime, A. Ercole, and et al. 2017. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 16, 12: 987–1048. [Google Scholar] [CrossRef]
- Marar, M., N. M. McIlvain, S. K. Fields, and R. D. Comstock. 2012. Epidemiology of concussions among United States high school athletes in 20 sports. The American journal of sports medicine 40, 4: 747–755. [Google Scholar] [CrossRef]
- Maruta, J., M. Suh, S. N. Niogi, P. Mukherjee, and J. Ghajar. 2010. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil 25, 4: 293–305. [Google Scholar] [CrossRef]
- Mauritz, W., A. Brazinova, M. Majdan, and J. Leitgeb. 2014. Epidemiology of traumatic brain injury in Austria. Wien Klin Wochenschr 126, 1–2: 42–52. [Google Scholar] [CrossRef]
- Mayer, A. R., D. K. Quinn, and C. L. Master. 2017. The spectrum of mild traumatic brain injury: A review. Neurology 89, 6: 623–632. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S. R., C. Boake, C. Pedroza, S. A. Brown, H. S. Levin, H. S. Goodman, and S. G. Merritt. 2005. Postconcussional disorder: Are the DSM-IV criteria an improvement over the ICD10? J Nerv Ment Dis 193, 8: 540–550. [Google Scholar] [CrossRef]
- McCrory, P., W. H. Meeuwisse, M. Aubry, B. Cantu, J. Dvořák, R. J. Echemendia, L. Engebretsen, K. Johnston, J. S. Kutcher, M. Raftery, A. Sills, B. W. Benson, G. A. Davis, R. G. Ellenbogen, K. Guskiewicz, S. A. Herring, G. L. Iverson,, B. D. Jordan, J. Kissick, M. McCrea, A. S. McIntosh, D. Maddocks, M. Makdissi, L. Purcell, M. Putukian, K. Schneider, C. H. Tator, and M. Turner. 2013. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. British Journal of Sports Medicine 47, 5: 250. [Google Scholar] [CrossRef] [PubMed]
- Meehan, W. P., 3rd, R. C. Mannix, M. J. O’Brien, and M. W. Collins. 2013. The prevalence of undiagnosed concussions in athletes. Clin J Sport Med 23, 5: 339–342. [Google Scholar] [CrossRef] [PubMed]
- Metting, Z., J. M. Spikman, L. A. Rödiger, and J. van der Naalt. 2014. Cerebral perfusion and neuropsychological follow up in mild traumatic brain injury: Acute versus chronic disturbances? Brain and Cognition 86: 24–31. [Google Scholar] [CrossRef]
- Mittl, R. L., R. I. Grossman, J. F. Hiehle, R. W. Hurst, D. R. Kauder, T. A. Gennarelli, and G. W. Alburger. 1994. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. American Journal of Neuroradiology 15, 8: 1583. Available online: http://www.ajnr.org/content/15/8/1583.abstract.
- Moreland, B, R. K., and A. Henry. 2020. Trends in Nonfatal Falls and Fall-Related Injuries Among Adults Aged ≥65 Years—United States, 2012–2018. MMWR Morb Mortal Wkly Rep 2020 69: 875881. [Google Scholar] [CrossRef]
- Preiss-Farzanegan, S. J., B. Chapman, T. M. Wong, J. Wu, and J. J. Bazarian. 2009. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. Pm r 1, 3: 245–253. [Google Scholar] [CrossRef]
- Prevention, C. F. D. C. a. 2019. Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States, 2014. Retrieved September 3 from https://stacks.cdc.gov/view/cdc/78062.
- Price, L. W. C., and C Grant. 2016. Blood-Brain Barrier Pathophysiology following Traumatic Brain Injury. Edited by G. G. Laskowitz D. In Translational Research in Traumatic Brain Injury. CRC Press/Taylor and Francis Group: https://www.ncbi.nlm.nih.gov/books/NBK326726/.
- Prins, M. L., D. Alexander, C. C. Giza, and D. A. Hovda. 2013. Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J Neurotrauma 30, 1: 30–38. [Google Scholar] [CrossRef]
- Prins, M. L., A. Hales, M. Reger, C. C. Giza, and D. A. Hovda. 2010. Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev Neurosci 32, 5–6: 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, M. J., and A. Feinstein. 2000. Outcome following traumatic brain injury in the elderly: a critical review. Brain Injury 14, 8: 749–761. [Google Scholar] [CrossRef]
- Reeves, T. M., T. L. Smith, J. C. Williamson, and L. L. Phillips. 2012. Unmyelinated Axons Show Selective Rostrocaudal Pathology in the Corpus Callosum After Traumatic Brain Injury. Journal of Neuropathology & Experimental Neurology 71, 3: 198–210. [Google Scholar] [CrossRef]
- Romeu-Mejia, R., C. C. Giza, and J. T. Goldman. 2019. Concussion Pathophysiology and Injury Biomechanics. Current Reviews in Musculoskeletal Medicine. [Google Scholar] [CrossRef]
- Rugg-Gunn, F. J., M. R. Symms, G. J. Barker, R. Greenwood, and J. S. Duncan. 2001. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. Journal of Neurology, Neurosurgery & Psychiatry 70, 4: 530. [Google Scholar] [CrossRef]
- Santhanam, P., T. Teslovich, S. H. Wilson, P. H. Yeh, T. R. Oakes, and L. K. Weaver. 2019. Decreases in White Matter Integrity of Ventro-Limbic Pathway Linked to Post-Traumatic Stress Disorder in Mild Traumatic Brain Injury. J Neurotrauma 36, 7: 1093–1098. [Google Scholar] [CrossRef]
- Selassie, A. W., D. A. Wilson, E. E. Pickelsimer, D. C. Voronca, N. R. Williams, and J. C. Edwards. 2013. Incidence of sport-related traumatic brain injury and risk factors of severity: a population-based epidemiologic study. Ann Epidemiol 23, 12: 750–756. [Google Scholar] [CrossRef]
- Sharp, D. J., and P. O. Jenkins. 2015. Concussion is confusing us all. Practical Neurology 15, 3: 172. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S., E. Slobounov, W. Sebastianelli, C. Cao, and K. Newell. 2007. Differential Rate of Recovery In Athletes After First and Second Concussion Episodes. Neurosurgery 61, 2: 338–344. [Google Scholar] [CrossRef]
- Sye, G., S. J. Sullivan, and P. McCrory. 2006. High school rugby players’ understanding of concussion and return to play guidelines. British Journal of Sports Medicine 40, 12: 1003–1005. [Google Scholar] [CrossRef]
- Taghdiri, F., J. Chung, S. Irwin, N. Multani, A. Tarazi, A. Ebraheem, M. Khodadadi, R. Goswami, R. Wennberg, D. Mikulis, R. Green, K. Davis, C. Tator, M. Eizenman, and M. C. Tartaglia. 2018. Decreased Number of Self-Paced Saccades in Post-Concussion Syndrome Associated with Higher Symptom Burden and Reduced White Matter Integrity. J Neurotrauma 35, 5: 719–729. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A., S. Mahon, P. Hume, N. Starkey, S. Barker-Collo, K. Jones, M. Majdan, and V. L. Feigin. 2020. Incidence of Sports-Related Traumatic Brain Injury of All Severities: A Systematic Review. Neuroepidemiology. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A., V. Parag, T. Dowell, K. McPherson, N. Starkey, S. Barker-Collo, K. Jones, S. Ameratunga, V. L. Feigin, and B. R. Group. 2016. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract 66, 642: e16–23. [Google Scholar] [CrossRef]
- Theadom, A., N. J. Starkey, T. Dowell, P. A. Hume, M. Kahan, K. McPherson, V. Feigin, and B. R. Group. 2014. Sports-related brain injury in the general population: an epidemiological study. J Sci Med Sport 17, 6: 591–596. [Google Scholar] [CrossRef] [PubMed]
- Tierney, R. T., M. R. Sitler, C. B. Swanik, K. A. Swanik, M. Higgins, and J. Torg. 2005. Gender differences in head-neck segment dynamic stabilization during head acceleration. Med Sci Sports Exerc 37, 2: 272–279. [Google Scholar] [CrossRef]
- Tyler, C. W., L. T. Likova, K. N. Mineff, and S. C. Nicholas. 2015. Deficits in the Activation of Human Oculomotor Nuclei in Chronic Traumatic Brain Injury. Frontiers in neurology 6: 173–173. [Google Scholar] [CrossRef]
- Vagnozzi, R., S. Signoretti, B. Tavazzi, R. Floris, A. Ludovici, S. Marziali, G. Tarascio, A. Amorini, V. Di Pietro, R. Delfini, and G. Lazzarino. 2008. Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectroscopic study in concussed athletes-Part III. Neurosurgery discussion 1295. 62: 1286–1295. [Google Scholar] [CrossRef]
- Ventura, R. E., J. M. Jancuska, L. J. Balcer, and S. L. Galetta. 2015. Diagnostic tests for concussion: is vision part of the puzzle? Journal of neuroophthalmology: the official journal of the North American Neuro-Ophthalmology Society 35, 1: 73–81. [Google Scholar] [CrossRef]
- Wallace, J. S., and R. C. Mannix. 2021. Racial Disparities in Diagnosis of Concussion and Minor Head Trauma and Mechanism of Injury in Pediatric Patients Visiting the Emergency Department. J Pediatr 233: 249–254.e241. [Google Scholar] [CrossRef]
- White, E. R., C. Pinar, C. A. Bostrom, A. Meconi, and B. R. Christie. 2017. Mild Traumatic Brain Injury Produces Long-Lasting Deficits in Synaptic Plasticity in the Female Juvenile Hippocampus. J Neurotrauma 34, 5: 1111–1123. [Google Scholar] [CrossRef]
- World Health Organization, and W. 1993. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines.
- Yeung, D., J. L. Manias, D. J. Stewart, and S. Nag. 2008. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol 115, 6: 635–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A. L., D. C. Sing, C. M. Rugg, B. T. Feeley, and C. Senter. 2016. The Rise of Concussions in the Adolescent Population. Orthopaedic journal of sports medicine 4, 8: 2325967116662458. [Google Scholar] [CrossRef] [PubMed]
Copyright © 2022. This article is licensed under a Creative Commons Attribution 4.0 International License.