Shorter Fixation Durations for Up-Directed Saccades During Saccadic Exploration: A Meta-Analysis
Abstract
Introduction
“…the model and good data go hand in hand in advancing the field.”(p. 7, Rayner, 2009)
Method
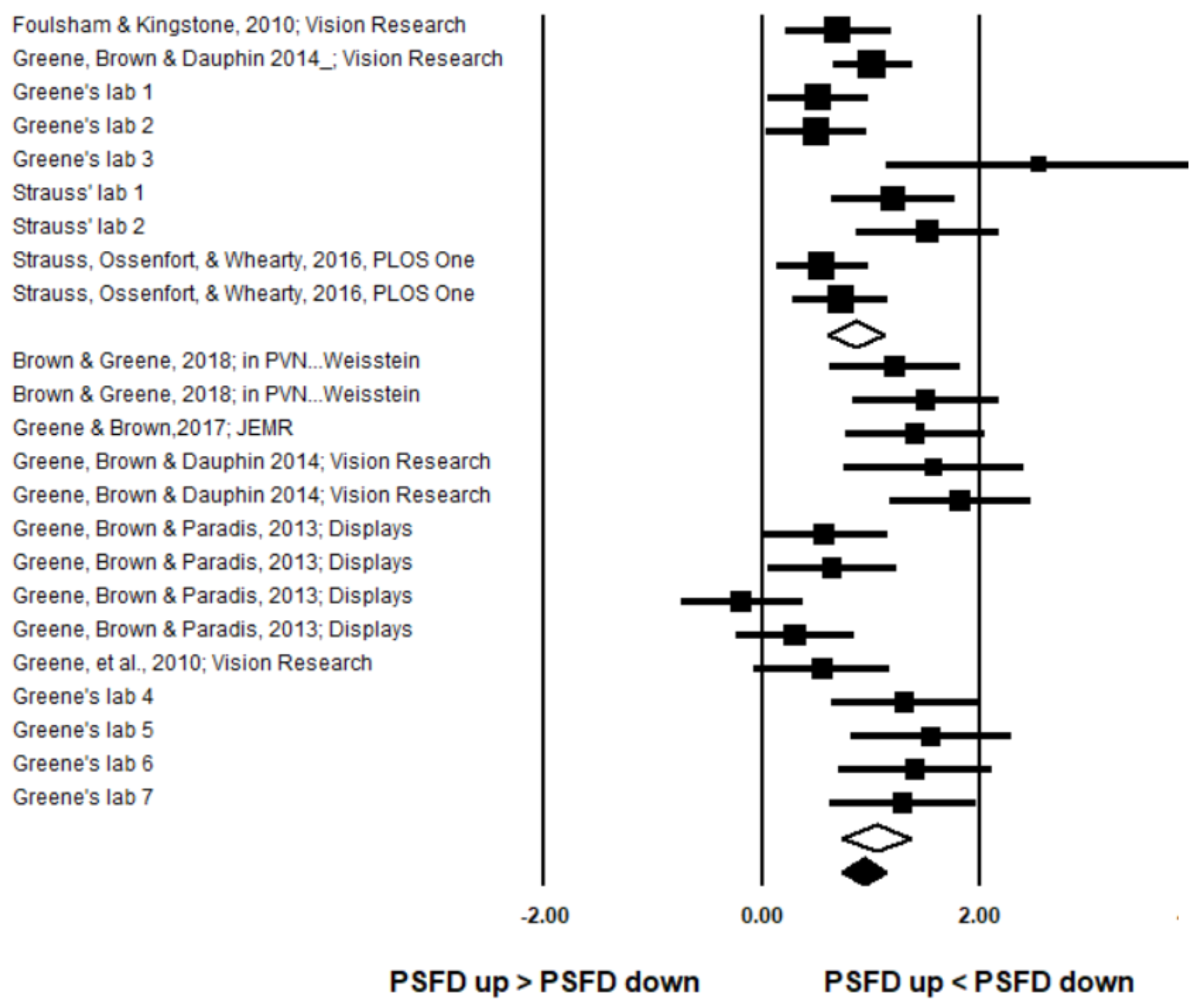
Data Selection Criteria
Description of Selected Datasets
Data Analysis
Results
Moderator: Task (Visual Search vs. Scene Viewing)
Moderator: Source (Published vs. Unpublished)
Moderator: Pre-Planned Test (Yes vs. No)
Moderator: Chinrest (Yes vs. No)
Discussion
Speculative Explanations
A Word on SRTs and PSFDs
Limitations
Conclusion
Ethics and Conflict of Interest
Acknowledgments
References
- Abegg, M.; Pianezzi, D.; Barton, J. A vertical asymmetry in saccades. Journal Of Eye Movement Research 2015, 8(5), 1–10. [Google Scholar] [CrossRef]
- Bell, A. H.; Everling, S.; Munoz, D. P. Influence of Stimulus Eccentricity and Direction on Characteristics of Proand Antisaccades in Non-Human Primates. Journal of Neurophysiology 2000, 84(5), 2595–2604. [Google Scholar] [CrossRef]
- Brown, J. M.; Greene, H. H. Brown, J. M., Ed.; We’re going to study the mind. In Pioneer Visual Neuroscience: A Festschrift for Naomi Weisstein; New York: Routledge, 2018; pp. 6–32. [Google Scholar]
- Castelhano, M. S.; Henderson, J. M. Stable individual differences across images in human saccadic eye movements. Canadian Journal of Experimental Psychology 2008, 62(1), 1–14. [Google Scholar] [CrossRef]
- Drager, U. C.; Hubel, D. H. Topography of visual and somatosensory projections to mouse superior colliculus. Journal of Neurophysiology 1976, 39(1), 91–101. [Google Scholar] [CrossRef] [PubMed]
- Foulsham, T.; Frost, E.; Sage, L. Stable individual differences predict eye movements to the left, but not handedness or line bisection. Vision Research 2018, 144, 38–46. [Google Scholar] [CrossRef]
- Foulsham, T.; Kingstone, A. Asymmetries in the direction of saccades during perception of scenes and fractals: Effects of image type and image features. Vision Research 2010, 50(8), 779–795. [Google Scholar] [CrossRef] [PubMed]
- Goldring, J.; Fischer, B. Reaction times of vertical prosaccades and antisaccades in gap and overlap tasks. Experimental Brain Research 1997, 113(1), 88–103. [Google Scholar] [CrossRef]
- Goldberg, M. E.; Walker, M. F. Hudspeth, A. J., Schwartz, J. H., Jessell, T. M., Siegelbaum, S. A., Kandel, E. R., Eds.; The control of gaze. In Principles of Neural Science; McGraw-Hill; New York, NY, USA, 2013; pp. 894–916. [Google Scholar]
- Greene, H. H.; Brown, J. M. Where did I come from? Where am I going? Functional differences in visual search fixation duration. Journal Of Eye Movement Research 2017, 10(1), 1–13. [Google Scholar] [CrossRef]
- Greene, H. H.; Brown, J. M.; Dauphin, B. When do you look where you look? A visual field asymmetry. Vision Research 2014, 102, 33. [Google Scholar] [CrossRef]
- Greene, H. H.; Brown, J. M.; Paradis, B. A. Luminance contrast and the visual span during visual target localization. Displays 2013, 34(1), 27–32. [Google Scholar] [CrossRef]
- Greene, H. H.; Pollatsek, A.; Masserang, K.; Lee, Y. J.; Rayner, K. Directional processing within the perceptual span during visual target localization. Vision Research 2010, 50(13), 1274–1282. [Google Scholar] [CrossRef][Green Version]
- Hackman, R. B. An experimental study of variability in ocular latency. Journal of Experimental Psychology 1940, 27(5), 546–558. [Google Scholar] [CrossRef]
- Hafed, Ziad M.; Chen, C.-Y. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Current Biology 2016, 26(13), 1647–1658. [Google Scholar] [CrossRef]
- Hagler, D. J., Jr. Visual field asymmetries in visual evoked responses. Journal of Vision 2014, 14(14), 13–13. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Ahmad, S.; Cui, Y. A theory of how columns in the neocortex enable learning the structure of the world. Frontiers in Neural Circuits 2017, 11(81), 1–17. [Google Scholar] [CrossRef]
- Hedges, L.; Olkin, I. Statistical Methods for Meta-Analysis; San Diego: Academic Press, 1985. [Google Scholar]
- Henderson, J. M.; Luke, S. G. Stable individual differences in saccadic eye movements during reading, pseudoreading, scene viewing, and scene search. Journal of Experimental Psychology: Human Perception and Performance 2014, 40(4), 1390–1400. [Google Scholar] [CrossRef]
- Heywood, S.; Churcher, J. Structure of the visual array and saccadic latency: implications for oculomotor control. Quarterly Journal of Experimental Psychology 1980, 32(2), 335–341. [Google Scholar] [CrossRef]
- Higgins, J. P. T.; Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002, 21(11), 1539–1558. [Google Scholar] [CrossRef]
- Holmes, G. M. Ferrier Lecture The organization of the visual cortex in man. Proceedings of the Royal Society of London. Series B Biological Sciences 1945, 132(869), 348–361. [Google Scholar] [CrossRef]
- Honda, H.; Findlay, J. M. Saccades to targets in three-dimensional space: Dependence of saccadic latency on target location. Perception & Psychophysics 1992, 52(2), 167–174. [Google Scholar] [CrossRef][Green Version]
- Itti, L.; Koch, C. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Research 2000, 40(10), 1489–1506. [Google Scholar] [CrossRef] [PubMed]
- Kremlácek, J.; Kuba, M.; Chlubnová, J.; Kubová, Z. Effect of stimulus localisation on motion-onset VEP. Vision Research 2004, 44(26), 2989–3000. [Google Scholar] [CrossRef]
- Lasker, A. G.; Zee, D. S. Ocular motor abnormalities in Huntington's disease. Vision Research 1997, 37(24), 3639–3645. [Google Scholar] [CrossRef]
- Laubrock, J.; Cajar, A.; Engbert, R. Control of fixation duration during scene viewing by interaction of foveal and peripheral processing. Journal of Vision 2013, 13(12). [Google Scholar] [CrossRef]
- Maxwell, S. E.; Lau, M. Y.; Howard, G. S. Is psychology suffering from a replication crisis? What does “failure to replicate” really mean? American Psychologist 2015, 70(6), 487–498. [Google Scholar] [CrossRef] [PubMed]
- Miles, W. R. The reaction time of the eye. Psychological Monographs 1936, 47(2), 268–293. [Google Scholar] [CrossRef]
- Najemnik, J.; Geisler, W. S. Simple summation rule for optimal fixation selection in visual search. Vision Research 2009, 49(10), 1286–1294. [Google Scholar] [CrossRef]
- Nuthmann, A. Fixation durations in scene viewing: Modeling the effects of local image features, oculomotor parameters, and task. Psychonomic Bulletin & Review 2017, 24(2), 370–392. [Google Scholar] [CrossRef]
- Nuthmann, A.; Henderson, J. M. Object-based attentional selection in scene viewing. Journal of Vision 2010, 10(8). [Google Scholar] [CrossRef]
- Nuthmann, A.; Smith, T. J.; Engbert, R.; Henderson, J. M. CRISP: a computational model of fixation durations in scene viewing. Psychological Review 2010, 117(2), 382. [Google Scholar] [CrossRef]
- Parkhurst, D.; Law, K.; Niebur, E. Modeling the role of salience in the allocation of overt visual attention. Vision Research 2002, 42(1), 107–123. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. S.; Jankovic, J.; Hood, A. J.; Jeter, C. B.; Sereno, A. B. Reflexive and volitional saccades: Biomarkers of Huntington disease severity and progression. Journal of the Neurological Sciences 2012, 313(1), 35–41. [Google Scholar] [CrossRef]
- Pitzalis, S.; Di Russo, F. Spatial anisotropy of saccadic latency in normal subjects and braindamaged patients. Cortex; a journal devoted to the study of the nervous system and behavior 2001, 37(4), 475–492. [Google Scholar] [CrossRef] [PubMed]
- Portin, K.; Vanni, S.; Virsu, V.; Hari, R. Stronger occipital cortical activation to lower than upper visual field stimuli Neuromagnetic recordings. Experimental Brain Research 1999, 124(3), 287–294. [Google Scholar] [CrossRef] [PubMed]
- Previc, F. H. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behavioral and Brain Sciences 1990, 13(3), 519–575. [Google Scholar] [CrossRef]
- Rao, R. P. N.; Zelinsky, G. J.; Hayhoe, M. M.; Ballard, D. H. Eye movements in iconic visual search. Vision Research 2002, 42(11), 1447–1463. [Google Scholar] [CrossRef]
- Rayner, K. Eye movements in reading and information processing: 20 years of research. Psychological Bulletin 1998, 124(3), 372. [Google Scholar] [CrossRef]
- Rayner, K. The 35th Sir Frederick Bartlett Lecture: Eye movements and attention in reading, scene perception, and visual search. Quarterly Journal of Experimental Psychology 2009, 62(8), 1457–1506. [Google Scholar] [CrossRef]
- Schlykowa, L.; Hoffmann, K.-P.; Bremmer, F.; Thiele, A. Monkey saccadic latency and pursuit velocity show a preference for upward directions of target motion. Neuroreport: An International Journal for the Rapid Communication of Research in Neuroscience 1996, 7(2), 409–412. [Google Scholar] [CrossRef]
- Sheth, B. R.; Young, R. Two Visual Pathways in Primates Based on Sampling of Space: Exploitation and Exploration of Visual Information. Frontiers in Integrative Neuroscience 2016, 10(37). [Google Scholar] [CrossRef]
- Shrout, P. E.; Rodgers, J. Psychology, Science, and Knowledge Construction: Broadening Perspectives from the Replication Crisis. Annual Review of Psychology 69 2018, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Silva, M. F.; Brascamp, J. W.; Ferreira, S.; CasteloBranco, M.; Dumoulin, S. O.; Harvey, B. M. Radial asymmetries in population receptive field size and cortical magnification factor in early visual cortex. NeuroImage 167 2018, 41–52. [Google Scholar] [CrossRef]
- Skrandies, W. The upper and lower visual field of man: Electrophysiological and functional differences. Progress in sensory physiology 8 1987, 2–93. [Google Scholar]
- Strauss, G. P.; Ossenfort, K. L.; Whearty, K. M. Reappraisal and Distraction Emotion Regulation Strategies Are Associated with Distinct Patterns of Visual Attention and Differing Levels of Cognitive Demand. PLoS One 2016, 11(11), e0162290. [Google Scholar] [CrossRef]
- Tatler, B. W.; Vincent, B. T. The prominence of behavioural biases in eye guidance. Visual Cognition 2009, 17(6-7), 1029–1054. [Google Scholar] [CrossRef]
- Termsarasab, P.; Thammongkolchai, T.; Rucker, J. C.; Frucht, S. J. The diagnostic value of saccades in movement disorder patients: a practical guide and review. Journal of Clinical Movement Disorders 2015, 2(1), 14. [Google Scholar] [CrossRef]
- Trukenbrod, H.; Engbert, R. ICAT: a computational model for the adaptive control of fixation durations. Psychonomic Bulletin & Review 2014, 1–28. [Google Scholar] [CrossRef]
- Tzelepi, A.; Laskaris, N.; Amditis, A.; Kapoula, Z. Cortical activity preceding vertical saccades: A MEG study. Brain Research 1321 2010, 105–116. [Google Scholar] [CrossRef]
- Tzelepi, A.; Yang, Q.; Kapoula, Z. The effect of transcranial magnetic stimulation on the latencies of vertical saccades. Experimental Brain Research 2005, 164(1), 67–77. [Google Scholar]
- Woodworth, R. S. Experimental psychology; New York: H. Holt and Company, 1938. [Google Scholar]
- Zelinsky, G. J. A theory of eye movements during target acquisition. Psychological Review 2008, 115(4), 787. [Google Scholar]
- Zhou, W.; King, W. M. Attentional sensitivity and asymmetries of vertical saccade generation in monkey. Vision Research 2002, 42(6), 771–779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, G.; Xuefei, Y.; Wu, S.; Zhang, M. Asymmetric representations of upper and lower visual fields in egocentric and allocentric references. Journal of Vision 2017, 17(1), 111. [Google Scholar] [CrossRef] [PubMed]

| Study | Condition & stimuli in study | Sample Size | PSFD up- saccades (ms) | PSFD downsaccade (ms) | Difference in means (ms) | Hedge’s g | Std Err |
| Foulsham & Kingstone (2010) | 2-second viewing of natural scenes and fractals (encoding phase) | 20 | 243.55 | 269.17 | 25.62 | 0.702 | 0.242 |
| Greene et al., (2014) | Engaged viewing of ambiguous (Rorschach) inkblots. | 44 | 327.28 | 355.25 | 27.97 | 1.015 | 0.183 |
| Greene’s lab ~ | 15-second viewing to rate the attractiveness of 9 urban scenes. | 20 | 252.40 | 270.71 | 18.31 | 0.515 | 0.230 |
| Greene’s lab ~ | 15-second viewing to rate the secureness of 9 urban scenes. | 19 | 252.98 | 278.63 | 25.65 | 0.499 | 0.234 |
| Greene’s lab ~ | Engaged viewing of university webpages. | 8 | 185.91 | 227.49 | 41.58 | 2.539 | 0.708 |
| Strauss’ lab ~ | Passive viewing of IAPS unpleasant scenes. | 20 | 272.89 | 300.2 | 27.31 | 1.204 | 0.287 |
| Strauss’ lab ~ | Passive viewing of IAPS unpleasant scenes. | 19 | 265.02 | 302.36 | 37.34 | 1.527 | 0.331 |
| Strauss et al., (2016) | 5-second viewing of unpleasant IAPS scenes, while distracted by thoughts of unrelated neutral objects. | 25 | 291.77 | 314.82 | 23.05 | 0.552 | 0.209 |
| Strauss et al., (2016) | 5-second viewing of unpleasant IAPS scenes, while reappraising them to be neutral. | 25 | 282.27 | 304.66 | 22.39 | 0.726 | 0.219 |
| Brown & Greene, (2018) | Visual search for low contrast square in a random gray-dot display. | 18 | 268.08 | 297.77 | 29.69 | 1.218 | 0.303 |
| Brown & Greene, (2018) Greene & | Visual search for low contrast square in a random red-dot display. Visual search for low contrast | 18 18 | 280.39 256.14 | 313.86 278.71 | 33.47 22.57 | 1.506 1.409 | 0.337 0.325 |
| Brown, (2017) | square in a random gray-dot display. | ||||||
| Greene et al., (2014) | Visual search for low contrast checkerboard in a random gray-dot display. | 12 | 213.84 | 255.27 | 41.43 | 1.579 | 0.420 |
| Greene et al., (2014) | Visual search for low contrast square in a random gray-dot display. | 24 | 243.18 | 286.52 | 43.34 | 1.822 | 0.329 |
| Greene et al., (2013) | Monocular visual search for high contrast square in a random gray-dot display. | 12 | 218.29 | 227.82 | 9.53# | 0.577 | 0.293 |
| Greene et al., (2013) | Monocular visual search for low contrast square in a random gray-dot display. | 12 | 341.6 | 366 | 24.40 | 0.647 | 0.299 |
| Greene et al., (2013) | Visual search for high contrast square in a random gray-dot display. | 11 | 206.92 | 201.69 | -5.23# | -0.186 | 0.281 |
| Greene et al., (2013) | Visual search for low contrast square in a random gray-dot display. | 12 | 305.56 | 317.6 | 12.04# | 0.306 | 0.276 |
| Greene et al., (2010) | Visual search of roadmaps. | 10 | 242.13 | 257.91 | 15.78# | 0.552 | 0.314 |
| Greene’s lab ~ | 3-second visual search-white circles on blue background. | 15 | 201.52 | 220.41 | 18.89 | 1.313 | 0.342 |
| Greene’s lab ~ | 3-second visual search-white circles on green background. | 15 | 198.24 | 224.16 | 25.92 | 1.553 | 0.374 |
| Greene’s lab ~ | 3-second visual search-white circles on red background. | 15 | 200.31 | 220.96 | 20.65 | 1.411 | 0.355 |
| Greene’s lab ~ | Visual search for low contrast square in a random gray-dot display. | 15 | 206.02 | 234.46 | 28.44 | 1.296 | 0.340 |
| Means | 247.07 | 272.25 | 25.18 |
Copyright © 2020. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Greene, H.H.; Brown, J.M.; Strauss, G.P. Shorter Fixation Durations for Up-Directed Saccades During Saccadic Exploration: A Meta-Analysis. J. Eye Mov. Res. 2019, 12, 1-12. https://doi.org/10.16910/jemr.12.8.5
Greene HH, Brown JM, Strauss GP. Shorter Fixation Durations for Up-Directed Saccades During Saccadic Exploration: A Meta-Analysis. Journal of Eye Movement Research. 2019; 12(8):1-12. https://doi.org/10.16910/jemr.12.8.5
Chicago/Turabian StyleGreene, Harold H., James M. Brown, and Gregory P. Strauss. 2019. "Shorter Fixation Durations for Up-Directed Saccades During Saccadic Exploration: A Meta-Analysis" Journal of Eye Movement Research 12, no. 8: 1-12. https://doi.org/10.16910/jemr.12.8.5
APA StyleGreene, H. H., Brown, J. M., & Strauss, G. P. (2019). Shorter Fixation Durations for Up-Directed Saccades During Saccadic Exploration: A Meta-Analysis. Journal of Eye Movement Research, 12(8), 1-12. https://doi.org/10.16910/jemr.12.8.5



