Microsaccades Reflect the Dynamics of Misdirected Attention in Magic
Abstract
Introduction
Microsaccades as an index of covert attention
Method
Participants
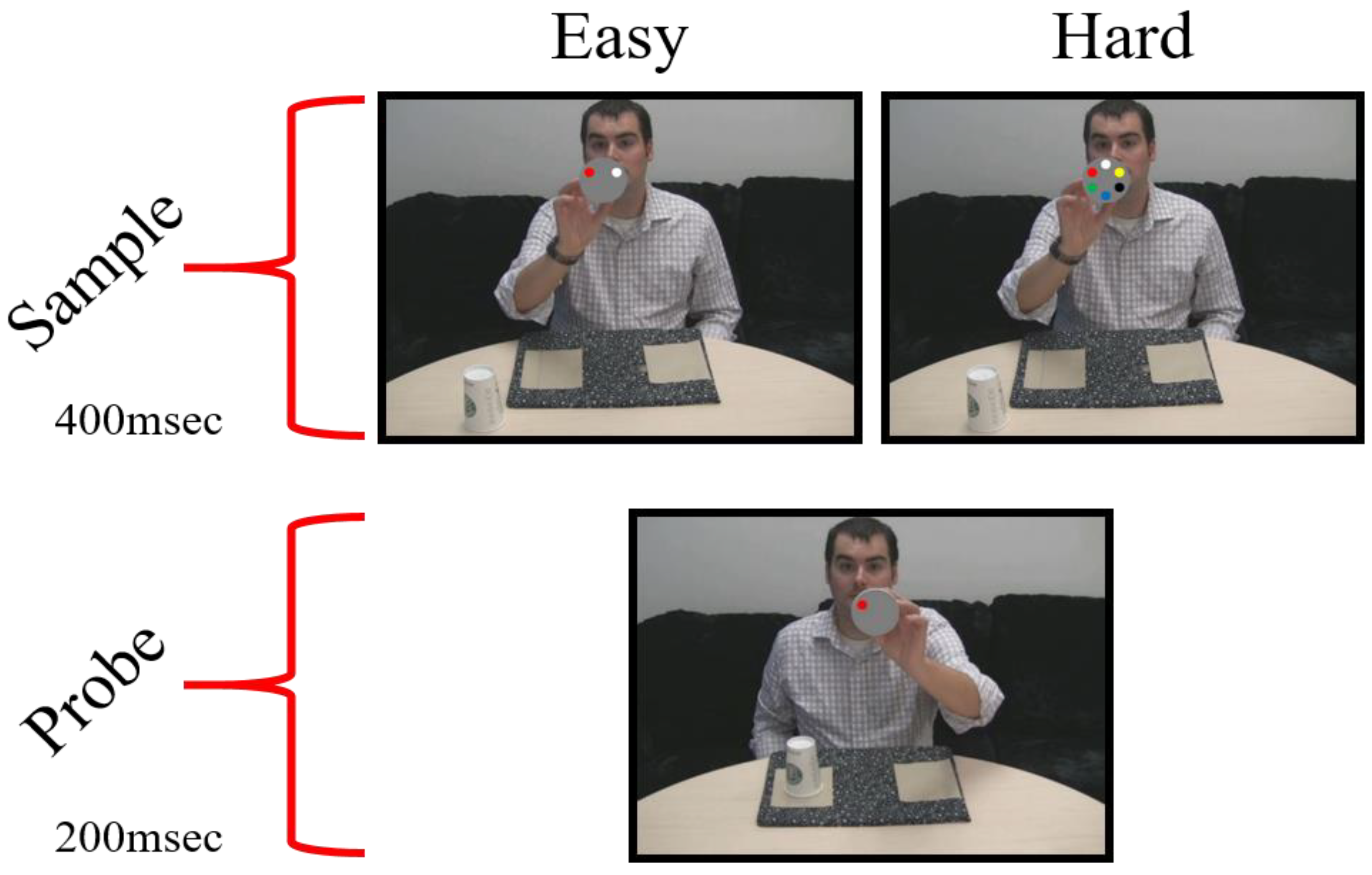
Stimuli
Apparatus
Procedure
Eye Movement Analyses
Results
Task Accuracy
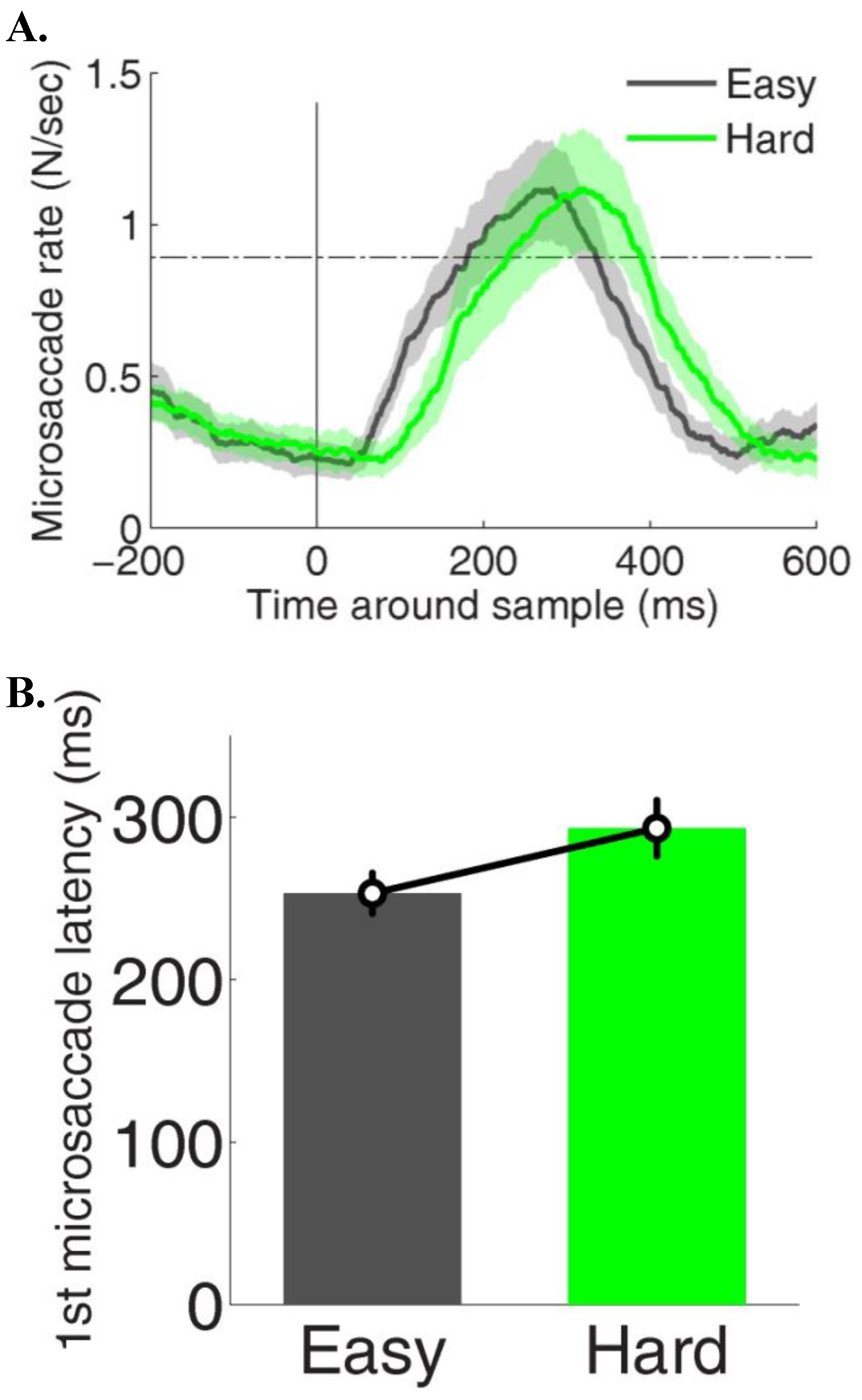
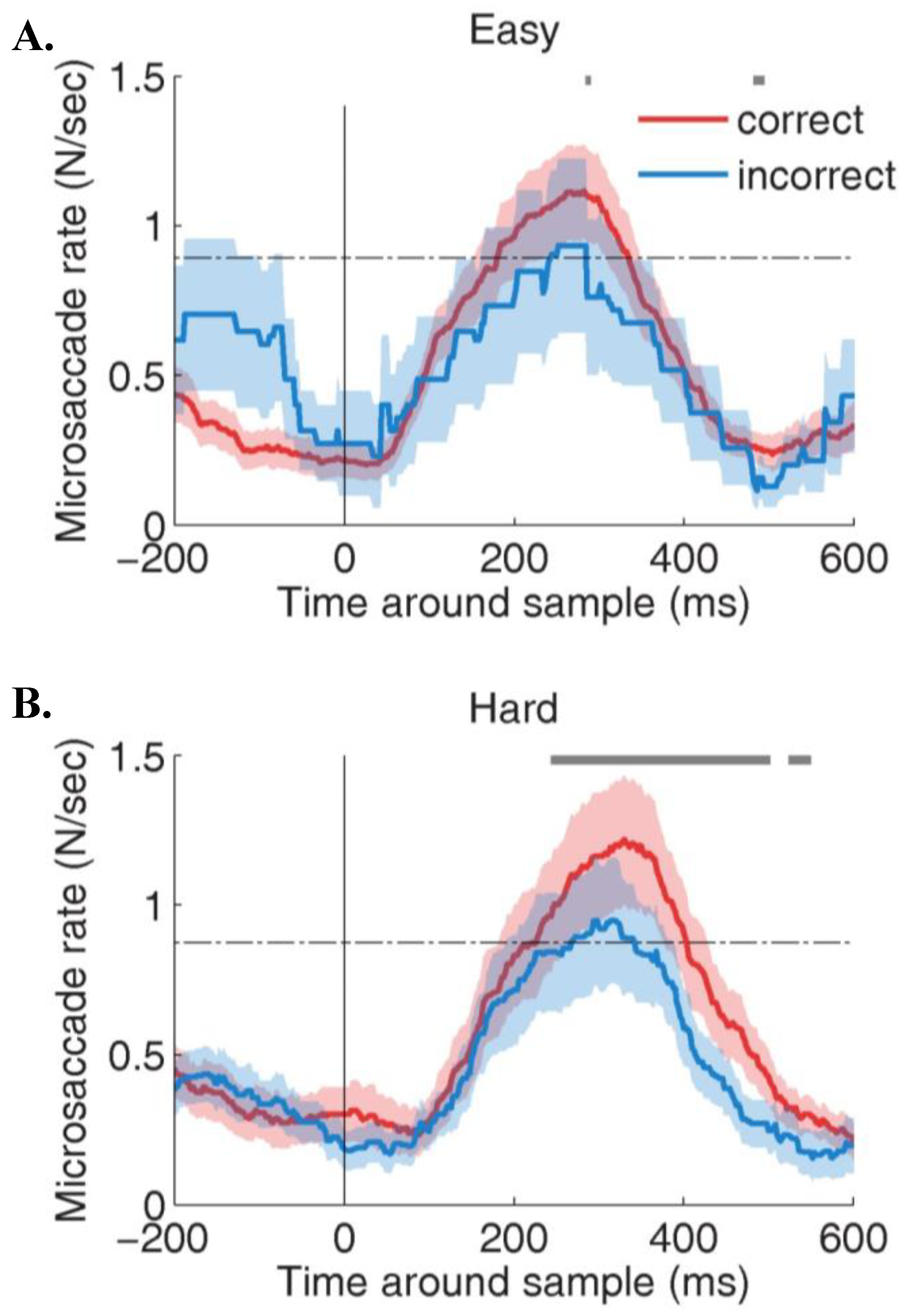
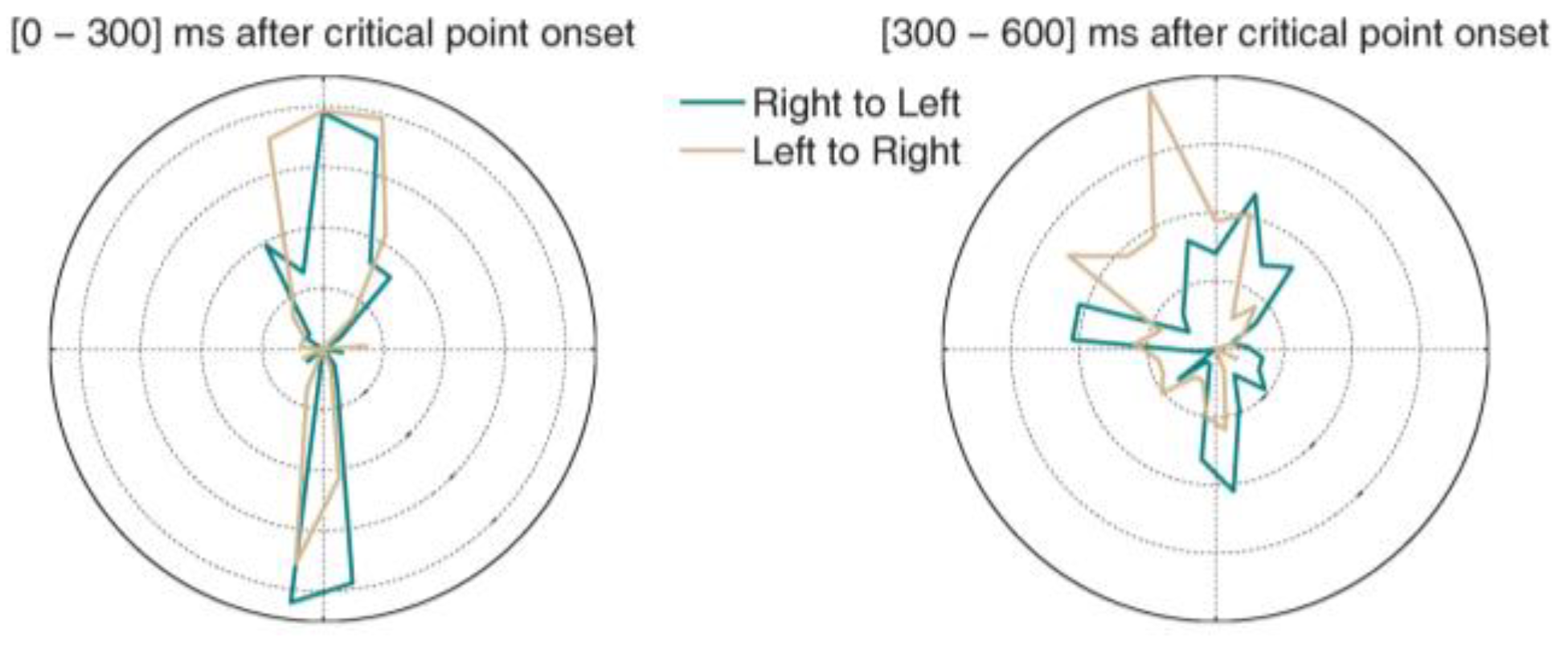
Microsaccades during constrained viewing
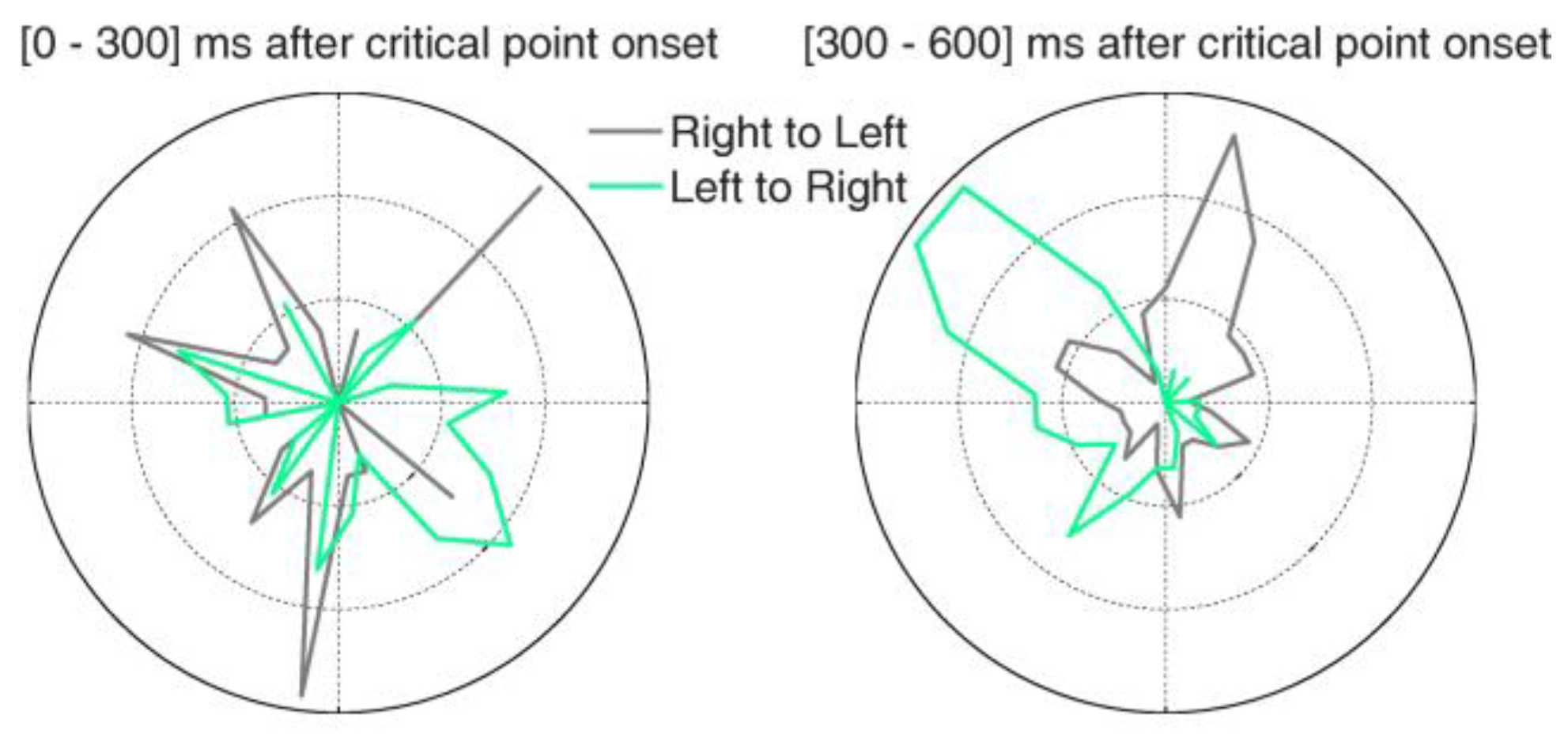
Microsaccades during free-viewing
Discussion
Acknowledgments
Conflicts of Interest
References
- Barnhart, A. S., and S. D. Goldinger. 2014. Blinded by magic: Eye-movements reveal the misdirection of attention. Frontiers in Psychology 5: 1461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barnhart, A. S., M. J. Ehlert, S. D. Goldinger, and A. D. Mackey. 2018. Cross-modal attentional entrainment: Insights from magicians. In Attention, Perception, & Psychophysics. Advance online publication. [Google Scholar] [CrossRef]
- Beanland, V., and K. Pammer. 2010. Looking without seeing or seeing without looking? Eye movements in sustained inattentional blindness. Vision Research 50: 977–988. [Google Scholar] [CrossRef] [PubMed]
- Boyer, T. W., and M. Wang. 2018. Direct gaze, eye movements, and covert and overt social attention processes. Attention, Perception, & Psychophysics 80: 1654–1659. [Google Scholar] [CrossRef]
- Betta, E., and M. Turatto. 2006. Are you ready? I can tell by looking at your microsaccades. Neuroreport 17: 1001–1004. [Google Scholar] [CrossRef]
- Cavina-Pratesi, C., G. Kuhn, M. Ietswaart, and A. D. Milner. 2011. The magic grasp: Motor expertise in deception. PLoS ONE 6: e16568. [Google Scholar] [CrossRef]
- Cornsweet, T. N. 1956. Determination of the stimuli for involuntary drifts and saccadic eye movements. Journal of the Optical Society of America 46: 987–993. [Google Scholar] [CrossRef]
- Costela, F. M., J. Otero-Millan, M. B. McCamy, S. L. Macknik, X. G. Troncoso, A. N. Jazi, S. M. Crook, and S. Martinez-Conde. 2014. Fixational eye movement correction of blink-induced gaze position errors. PLoS One 9: e110889. [Google Scholar] [CrossRef]
- Cui, J., J. Otero-Millan, S. L. Macknik, M. King, and S. Martinez-Conde. 2011. Social misdirection fails to enhance a magic illusion. Frontiers in Human Neuroscience 5: 103. [Google Scholar] [CrossRef][Green Version]
- Danek, A. H., T. Fraps, A. von Müller, B. Grothe, and M. Öllinger. 2014. It’s a kind of magic--What self-reports can reveal about the phenomenology of insight problem solving. Frontiers in Psychology 5: 1408. [Google Scholar] [CrossRef]
- Ditchburn, R. W., and B. L. Ginsborg. 1952. Vision with a stabilized retinal image. Nature 170: 36–37. [Google Scholar] [CrossRef]
- Eayrs, J., and N. Lavie. 2018. Establishing individual differences in perceptual capacity. Journal of Experimental Psychology: Human Perception and Performance 44: 1240–1257. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R. 2006. Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research 154: 177–192. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R., and R. Kliegl. 2003. Microsaccades uncover the orientation of covert attention. Vision Research 43: 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R., and K. Mergenthaler. 2006. Microsaccades are triggered by low retinal image slip. Proceedings of the National Academy of Sciences 103: 7192–7197. [Google Scholar] [CrossRef]
- Hafed, Z. M., and J. J. Clark. 2002. Microsaccades as an overt measure of covert attention shifts. Vision Research 42: 2533–2545. [Google Scholar] [CrossRef]
- Hafed, Z. M., L. P. Lovejoy, and R. J. Krauzlis. 2011. Modulation of microsaccades in monkey during a covert visual attention task. The Journal of Neuroscience 31: 15219–15230. [Google Scholar] [CrossRef]
- Hall, L., T. Strandberg, P. Pärnamets, A. Lind, B. Tärning, and P. Johansson. 2013. How the polls can be both spot on and dead wrong: Using choice blindness to shift political attitudes and voter intentions. PLoS ONE 8: e60554. [Google Scholar] [CrossRef]
- Hergovich, A., K. Gröbl, and C.-C. Carbon. 2011. The paddle move commonly used in magic tricks as a ameans for analysing the perceptual limits of combined motion trajectories. Perception 40: 358–366. [Google Scholar] [CrossRef]
- Horowitz, T. S., E. M. Fine, D. E. Fencsik, S. Yurgenson, and J. M. Wolfe. 2007. Fixational eye movements are not an index of covert attention. Psychological Science 18: 356–363. [Google Scholar] [CrossRef]
- Johansson, P., L. Hall, S. Sikström, and A. Olsson. 2005. Failure to detect mismatches between intention and outcome in a simple decision task. Science 310: 116–119. [Google Scholar] [CrossRef]
- Kuhn, G., A. A. Amlani, and R. A. Rensink. 2008. Towards a science of magic. Trends in Cognitive Sciences 12: 349–354. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G., and L. M. Martinez. 2012. Misdirection-Past, present, and the future. Frontiers in Human Neuroscience 5: 172. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G., and B. W. Tatler. 2005. Magic and fixation: Now you don’t see it, now you do. Perception 34: 1155–1161. [Google Scholar] [CrossRef]
- Kuhn, G., B. W. Tatler, J. M. Findlay, and G. G. Cole. 2008. Misdirection in magic: Implications for the relationship between eye gaze and attention. Visual Cognition 16: 391–405. [Google Scholar] [CrossRef]
- Lamont, P., and J. M. Henderson. 2008. More attention and greater awareness in the scientific study of magic. Nature Reviews Neuroscience 10: 871–879. [Google Scholar] [CrossRef]
- Lamont, P., J. M. Henderson, and T. J. Smith. 2010. Where science and magic meet: The illusion of a "science of magic". Review of General Psychology 14: 16–21. [Google Scholar] [CrossRef]
- Laubrock, J., R. Engbert, and R. Kliegl. 2005. Microsaccade dynamics during covert attention. Vision Research 45: 721–730. [Google Scholar] [CrossRef]
- Laubrock, J., R. Engbert, M. Rolfs, and R. Kliegl. 2007. Microsaccades are an index of covert attention: Commentary on Horowitz, Fine, Fencsik, Yurgenson, and Wolfe (2007). Psychological Science 18: 364–366. [Google Scholar] [CrossRef]
- Laubrock, J., R. Kliegl, M. Rolfs, and R. Engbert. 2010. When do microsaccades follow spatial attention? Attention. Perception, & Psychophysics 72: 683–694. [Google Scholar] [CrossRef]
- Macknik, S. L., M. King, J. Randi, A. Robbins, Teller, Thompson J., and S. Martinez-Conde. 2008. Attention and awareness in stage magic: Turning tricks into research. Nature Reviews Neuroscience 9: 871–879. [Google Scholar] [CrossRef]
- Macknik, S. L., S. Martinez-Conde, and S. Blakeslee. 2010. Sleights of mind: What the neuroscience of magic reveals about our everyday deceptions. New York: Henry Holt & Co. [Google Scholar]
- Martinez-Conde, S., and S. L. Macknik. 2007. Mind tricks. Nature 448: 414. [Google Scholar] [CrossRef]
- Martinez-Conde, S., and S. L. Macknik. 2008. Magic and the brain. Scientific American 299: 72–79. [Google Scholar] [CrossRef]
- Martinez-Conde, S., S. L. Macknik, X. G. Troncoso, and T. A. Dyar. 2006. Microsaccades counteract visual fading during fixation. Neuron 49: 297–305. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S., S. L. Macknik, X. G. Troncoso, and D. H. Hubel. 2009. Microsaccades: A neurophysiological analysis. Trends in Neurosciences 32: 463–475. [Google Scholar] [CrossRef]
- Martinez-Conde, S., J. Otero-Millan, and S. L. Macknik. 2013. The impact of microsaccades on vision: Towards a unified theory of saccadic function. Nature Reviews Neuroscience 14: 83–96. [Google Scholar] [CrossRef]
- McCamy, M. B., Collins, N., Otero-Millan, J., Al-Kalbani, M., Macknik, S. L., Coakley, D.,... Martinez-Conde, S. 2013. Simultaneous recordings of ocular microtremor and microsaccades with a piezoelectric sensor and a video-oculography system. PeerJ 1: e14. [Google Scholar] [CrossRef]
- McCamy, M. B., A. N. Jazi, J. Otero-Millan, S. L. Macknik, and S. Martinez-Conde. 2013. The effects of fixation target size and luminance on microsaccades and square-wave jerks. PeerJ 1: e9. [Google Scholar] [CrossRef]
- McCamy, M. B., S. L. Macknik, and S. Martinez-Conde. 2014. Different fixational eye movements mediate the prevention and the reversal of visual fading. Journal of Physiology 592: 4381–4394. [Google Scholar] [CrossRef]
- McCamy, M. B., J. Otero-Millan, L. L. Di Stasi, S. L. Macknik, and S. Martinez-Conde. 2014. Highly informative natural scene regions increase microsaccade production during visual scanning. Journal of Neuroscience 34: 2956–2966. [Google Scholar] [CrossRef]
- Melloni, L., C. M. Schwiedrzik, E. Rodriguez, and W. Singer. 2009. (Micro)Saccades, corollary activity and cortical oscillations. Trends in Cognitive Sciences 13: 239–245. [Google Scholar] [CrossRef]
- Memmert, D. 2006. The effects of eye movements, age, and expertise on inattentional blindness. Consciousness and Cognition 15: 620–627. [Google Scholar] [CrossRef] [PubMed]
- Olson, J. A., A. A. Amlani, A. Raz, and R. A. Rensink. 2015. Influencing choice without awareness. Consciousness and Cognition 37: 225–236. [Google Scholar] [CrossRef] [PubMed]
- Olson, J. A., M. Landry, K. Appourchaux, and A. Raz. 2016. Simulated thought insertion: Influencing the sense of agency using deception and magic. Consciousness and Cognition 43: 11–26. [Google Scholar] [CrossRef] [PubMed]
- Otero-Millan, J., S. L. Macknik, R. E. Langston, and S. Martinez-Conde. 2013. An oculomotor continuum from exploration to fixation. Proceedings of the National Academy of Sciences 110: 6175–6180. [Google Scholar] [CrossRef]
- Otero-Millan, J., S. L. Macknik, A. Robbins, M. McCamy, and S. Martinez-Conde. 2011. Stronger misdirection in curved than in straight motion. Frontiers in Human Neuroscience 5: 133. [Google Scholar] [CrossRef]
- Pastukhov, A., and J. Braun. 2010. Rare but precious: Microsaccades are highly informative about attentional allocation. Vision Research 50: 1173–1184. [Google Scholar] [CrossRef]
- Phillips, F., M. B. Natter, and E. J. Egan. 2015. Magically deceptive biological motion--the French Drop sleight. Frontiers in Psychology 6: 371. [Google Scholar] [CrossRef][Green Version]
- Quian Quiroga, R. 2016. Magic and cognitive neuroscience. Current Biology 26: R390–R394. [Google Scholar] [CrossRef]
- Rieiro, H., S. Martinez-Conde, and S. L. Macknik. 2013. Perceptual elements in Penn & Teller’s "Cups and Balls "magic trick. PeerJ 1: e19. [Google Scholar] [CrossRef][Green Version]
- Rensink, R. A., and G. Kuhn. 2015. The possibility of a science of magic. Frontiers in Psychology 6: 1576. [Google Scholar] [CrossRef]
- Rolfs, M., J. Laubrock, and R. Kliegl. 2006. Shortening and prolongation of saccade latencies following microsaccades. Experimental Brain Research 169: 369–376. [Google Scholar] [CrossRef] [PubMed]
- Rucci, M., R. Iovin, M. Poletti, and F. Santini. 2007. Miniature eye movements enhance fine spatial detail. Nature 447: 851–855. [Google Scholar] [CrossRef] [PubMed]
- Shalom, D. E., M. G. de Sousa Serro, M. Giaconia, L. M. Martinez, A. Rieznik, and M. Sigman. 2013. Choosing in freedom or forced to choose? Introspective blindness to psychological forcing in stage-magic. PLoS ONE 8: e58254. [Google Scholar] [CrossRef]
- Siegenthaler, E., Costela, F. M., McCamy, M. B., Di Stasi, L. L., Otero-Millan, J., Sonderegger, A.,…Martinez-Conde, S. 2014. Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. European Journal of Neuroscience 39: 287–294. [Google Scholar] [CrossRef]
- Smith, T. J. 2015. The role of audience participation and task relevance on change detection during a card trick. Frontiers in Psychology 6: 13. [Google Scholar] [CrossRef]
- Smith, T. J., P. Lamont, and J. M. Henderson. 2012. The penny drops: Change blindness at fixation. Perception 41: 489–492. [Google Scholar] [CrossRef]
- Thomas, C., and A. Didierjean. 2016. Magicians fix your mind: How unlikely solutions block obvious ones. Cognition 154: 169–173. [Google Scholar] [CrossRef]
- Thomas, C., A. Didierjean, F. Maquestiaux, and P. Gygax. 2015. Does magic offer a cryptozoology ground for psychology? Review of General Psychology 19: 117–128. [Google Scholar] [CrossRef]
- Troncoso, X. G., S. L. Macknik, J. Otero-Millan, and S. Martinez-Conde. 2008. Microsaccades drive illusory motion in the Enigma illusion. Proceedings of the National Academy of Sciences 105: 16033–16038. [Google Scholar] [CrossRef]
- Valsecchi, M., E. Betta, and M. Turatto. 2007. Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research 177: 196–208. [Google Scholar] [CrossRef]
- van Dam, L. C., and R. van Ee. 2005. The role of (micro)saccades and blinks in perceptual bistability from slant rivalry. Vision Research 45: 2417–2435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, K., and C. C. French. 2014. Magic and memory: Using conjuring to explore the effects of suggestion, social influence, and paranormal belief on eyewitness testimony for an ostensibly paranormal event. Frontiers in Psychology 5: 1289. [Google Scholar] [CrossRef] [PubMed]
- Yuval-Greenberg, S., E. P. Merriam, and D. J. Heeger. 2014. Spontaneous microsaccades reflect shifts in covert attention. Journal of Neuroscience 34: 13693–13700. [Google Scholar] [CrossRef] [PubMed]




Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Barnhart, A.S.; Costela, F.M.; Martinez-Conde, S.; Macknik, S.L.; Goldinger, S.D. Microsaccades Reflect the Dynamics of Misdirected Attention in Magic. J. Eye Mov. Res. 2019, 12, 1-14. https://doi.org/10.16910/jemr.12.6.7
Barnhart AS, Costela FM, Martinez-Conde S, Macknik SL, Goldinger SD. Microsaccades Reflect the Dynamics of Misdirected Attention in Magic. Journal of Eye Movement Research. 2019; 12(6):1-14. https://doi.org/10.16910/jemr.12.6.7
Chicago/Turabian StyleBarnhart, Anthony S., Francisco M. Costela, Susana Martinez-Conde, Stephen L. Macknik, and Stephen D. Goldinger. 2019. "Microsaccades Reflect the Dynamics of Misdirected Attention in Magic" Journal of Eye Movement Research 12, no. 6: 1-14. https://doi.org/10.16910/jemr.12.6.7
APA StyleBarnhart, A. S., Costela, F. M., Martinez-Conde, S., Macknik, S. L., & Goldinger, S. D. (2019). Microsaccades Reflect the Dynamics of Misdirected Attention in Magic. Journal of Eye Movement Research, 12(6), 1-14. https://doi.org/10.16910/jemr.12.6.7


