Saccadic Intrusions in Amyotrophic Lateral Sclerosis (ALS)
Abstract
:Introduction
Methods
Participants
Equipment
Procedures
Data Analysis

Statistics
Results
Qualitative observations
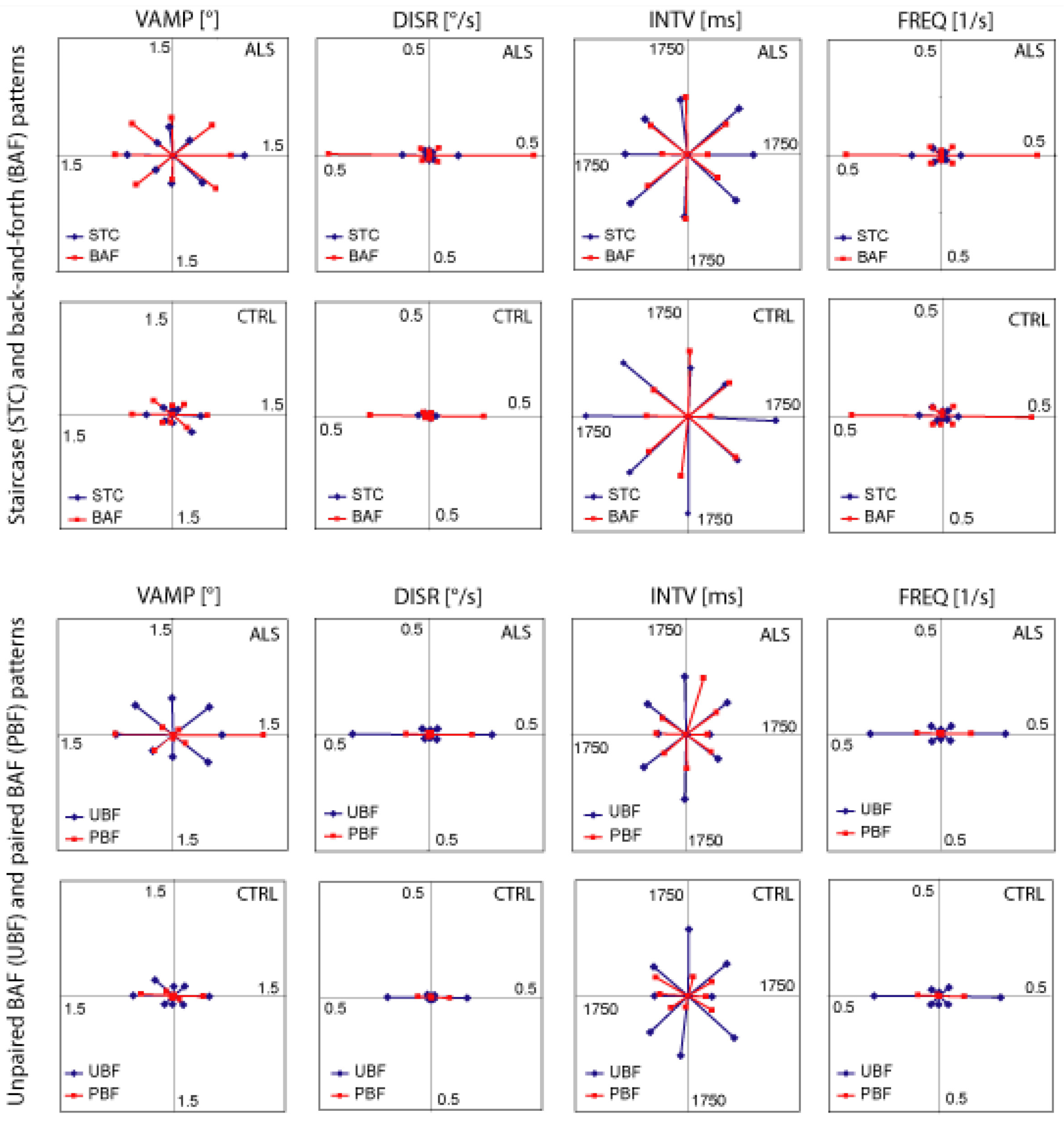
- (1)
- the majority of intrusions was confined to the horizontal plane (ALS 70%, Controls 67%),
- (2)
- the percentages of intrusions in the vertical (ALS 5%, controls 6%) and each of the oblique planes (ALS 12 and 13%, controls 13 and 14%) were virtually identical in both groups, and
- (3)
- BAF intrusions occurred more frequently than STC intrusions (both groups p < 10−5).
- (1)
- in both groups the displacement rates of non-horizontal BAF and STC were negligible although within each pattern the amplitudes often were of roughly the same order of magnitude in all directions. Thus, the displacement rate (which reflects the product of amplitude and frequency) was essentially determined by the pattern frequency. By the same token,
- (2)
- the displacement rates of STC were much smaller than those of BAF (both groups p < 10−5).
- (1)
- UBF intrusions occurred more frequently than PBF (controls 80 vs. 20%, ALS 78 vs. 22%; both groups p < 10−5) and produced larger displacement rates, therefore (both groups p < 10−5).
- (2)
- The intervals of horizontal UBF and PBF were nearly identical.
- (3)
- Paired BAF intrusions occurred almost exclusively in the horizontal plane.
Quantitative comparison between ALS and controls
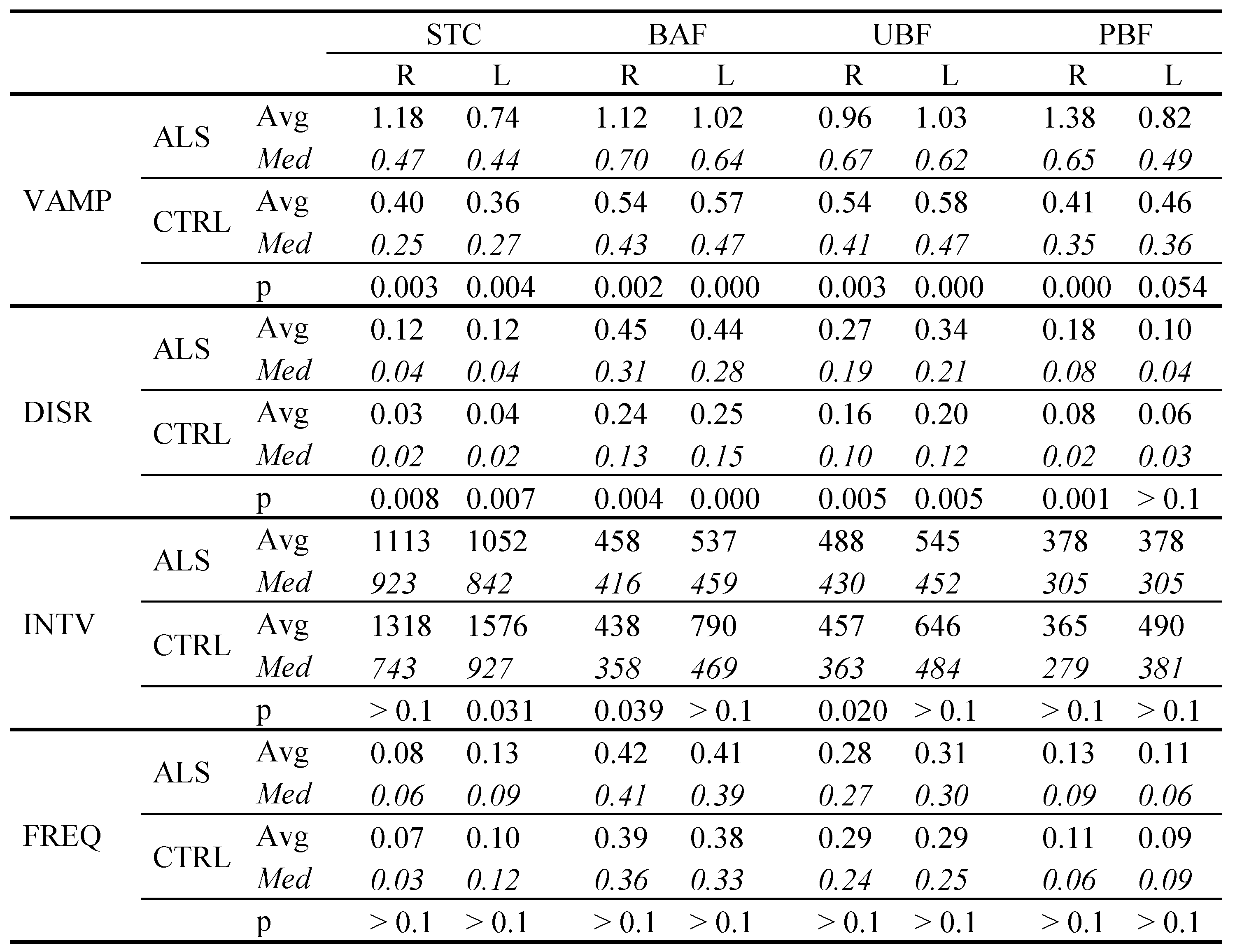 |
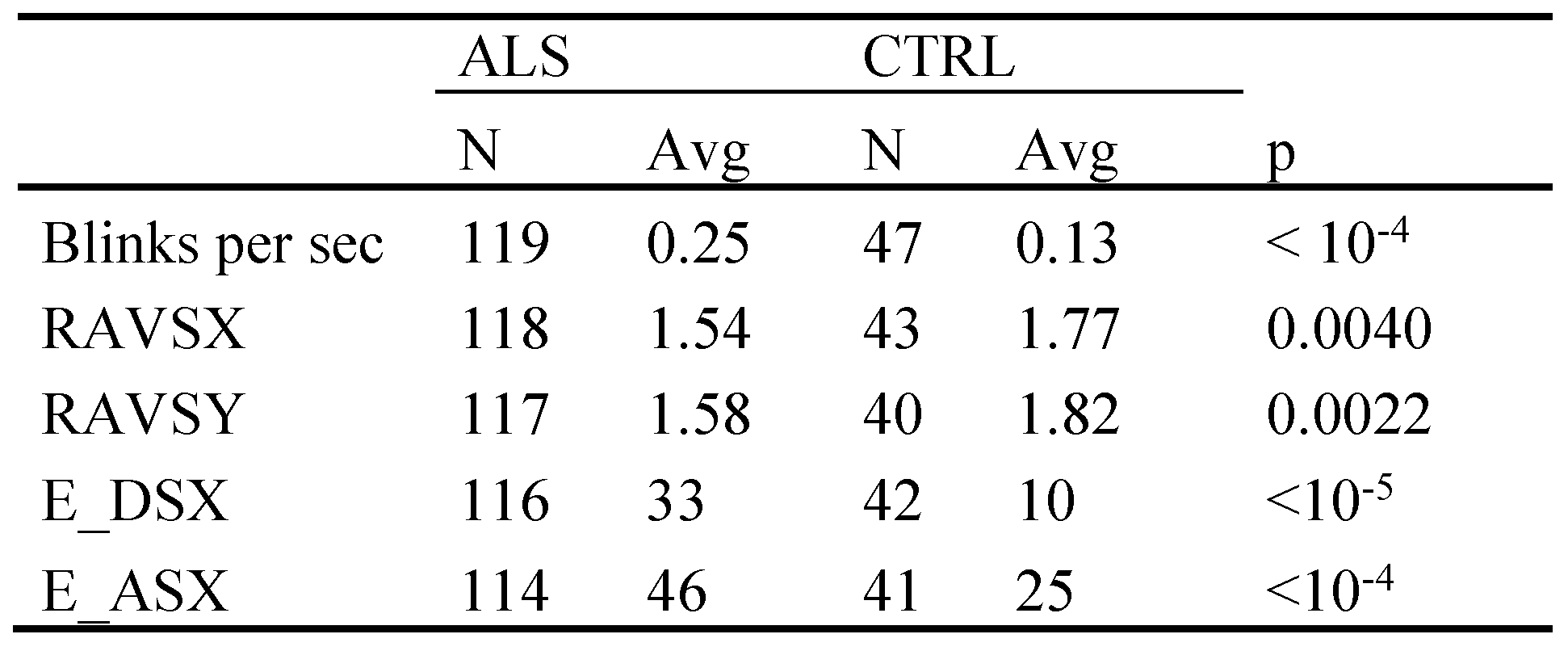
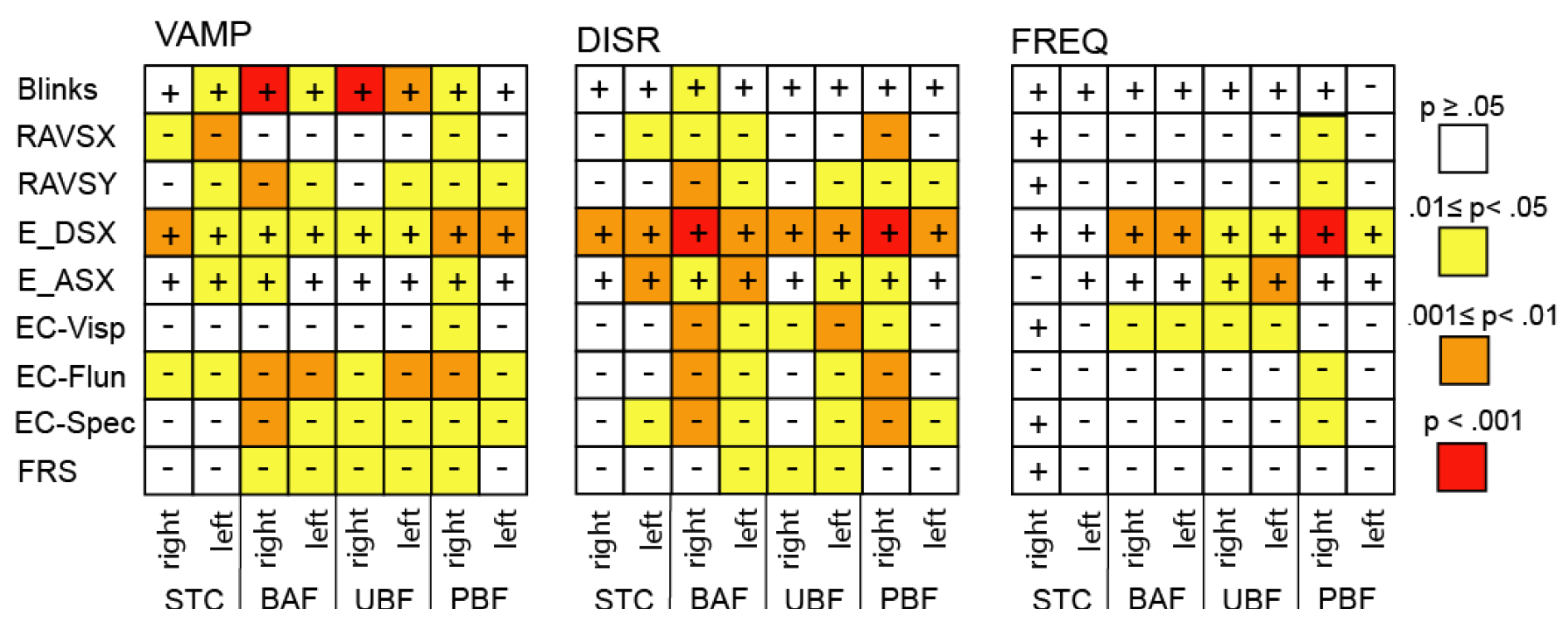
Correlation with executive functions
- (1)
- In all cases of an error probability of less than 0.05 (coloured cells), the signs of the co-variations in a given row were identical in all three matrices.
- (2)
- In all cases of an error probability of less than 0.05, the correlation coefficients indicated an increase of VAMP, DISR, and FREQ when the executive parameters reflected a functional worsening. For example, a decrease of the number of rapidly alternating voluntary saccades, an increase of erroneous responses in the delayed saccade task and a reduced ECAS fluency score (EC_Flun) all reflect an impaired performance. Accordingly, there was a negative co-variation of all SI parameters with RAVS and EC_Flun, and a positive one with the delayed saccade error rate (E_DSX).
- (3)
- Across SI parameters the closest co-variation occurred with the delayed saccade error rate which also was the only score to exhibit a possibly trustworthy relationship with the frequency of intrusions.
- (4)
- Among the ECAS scores, verbal fluency (EC_Flun) and the compound score EC_Spec were the best related ones to SI parameters, whereas the visuospatial score (EC_Visp) exhibited a conspicuous lack of co-variation with VAMP in contrast to all other parameters.
- (5)
- For PBF intrusions (the analogues of SWJ) the direction appears to matter since the number correlations with low error probability was clearly larger for rightward as compared to leftward SI, paralleling the right dominance of PBF amplitudes and displacement rates in ALS.
- (6)
- The eye blink rate correlated positively with SI amplitude and in particular so with that of BAF intrusions
- (7)
- The functional ALS rating scale (ALSFRS-R, FRS in Figure 4) exhibited little co-variation with SI parameters.
 |
Discussion
Pattern definition
Saccadic intrusions in controls
Qualitatively common features of ALS and controls
Differences between ALS and controls: an effect of impaired inhibitory control?
Correlation with ECAS scores and oculomotor executive scores
Conclusion
Acknowledgments
Conflicts of Interest
References
- Abadi, R., and E. Gowen. 2004. Characteristics of saccadic intrusions. Vision Research 44: 2675–2690. [Google Scholar] [CrossRef] [PubMed]
- Abadi, R., C. J. Scallan, and R. A. Clement. 2000. The characteristics of dynamic overshoot in square-wave jerks, and in congenital and manifest latent nystagmus. Vision Research 40: 2813–2829. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, S., P. N. Leigh, A. Harvey, G. N. Vythelingum, D. Grise, and L. H. Goldstein. 2000. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38: 734–747. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A., L. H. van den Berg, and J. Veldink. 2017. Gene discovery in amyotrophic lateral sclerosis: implications for clinical management. Nat.Rev.Neurol. 13: 96–104. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R. G., S. L. Macknik, and S. Martinez-Conde. 2018. Microsaccade Characteristics in Neurological and Ophthalmic Disease. Front Neurol 9: 144. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E., I. Kouzi, S. Vassilopoulou, G. P. Paraskevas, and K. Spengos. 2012. Spontaneous eyeblink rate in focal cerebrovascular lesions. European Neurology 67: 39–44. [Google Scholar] [CrossRef] [PubMed]
- Averbuch-Heller, L., J. S. Stahl, M. L. Hlavin, and R. J. Leigh. 1999. Square-wave jerks induced by pallidotomy in parkinsonian patients. Neurology (Cleveland OH) 52: 185–188. [Google Scholar] [CrossRef] [PubMed]
- Bahill, A. T., M. R. Clark, and L. Stark. 1975. The main sequence, a tool for studying human eye movements. Mathematical Biosciences 24: 191–204. [Google Scholar] [CrossRef]
- Barlow, H. B. 1952. Eye movements during fixation. Journal of Physiology 116: 290–306. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A. L., Garbutt, S., Seeley, W. W., Jafari, A., Heuer, H. W., Mirsky, J. et al. 2012. Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer disease. Archives of Neurology (Chicago) 69: 509–517. [Google Scholar] [CrossRef] [PubMed]
- Braak, H., J. Brettschneider, A. C. Ludolph, V. M. Lee, J. Q. Trojanowski, and T. K. Del. 2013. Amyotrophic lateral sclerosis--a model of corticofugal axonal spread. Nat.Rev.Neurol. 9: 708–714. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J., Del, T. K., Irwin, D. J., Grossman, M., Robinson, J. L., Toledo, J. B. et al. 2014. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 127: 423–439. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S., F. Pradhan, D. S. Ni, M. Treacy, L. Cassidy, and O. Hardiman. 2013. Blink rate in ALS. Amyotroph.Lateral.Scler.Frontotemporal.Degener. 14: 291–293. [Google Scholar] [CrossRef] [PubMed]
- Cedarbaum, J. M., Stambler, N., Malta, E., Fuller, C., Hilt, D., Thurmond, B. et al. 1999. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol.Sci. 169: 13–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, A. L., Riley, D. E., King, S. A., Joshi, A. C., Serra, A., Liao, K. et al. 2010. The disturbance of gaze in progressive supranuclear palsy: implications for pathogenesis. Front.Neurol. 1: 147. [Google Scholar] [CrossRef] [PubMed]
- Collewijn, H., and E. Kowler. 2008. The significance of microsaccades for vision and oculomotor control. J.Vis. 8: 20–21. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L. S., W. P. van den Wildenberg, N. C. van Wouwe, M. M. Pannebakker, and B. Hommel. 2009. Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp Brain Res 196: 467–474. [Google Scholar] [CrossRef] [PubMed]
- Daroff, R. B. 1977. Ocular oscillations. Annals of Otology, Rhinology and Laryngology 86: 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ditchburn, R. W. 1980. Letter to the editors The function of small saccades. Vision Research 20: 271–272. [Google Scholar] [CrossRef] [PubMed]
- Ditchburn, R. W., D. H. Fender, and S. Mayne. 1959. Vision with controlled movements of the retinal image. Journal of Physiology 145: 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ditchburn, R. W., and B. L. Ginsborg. 1953. Involuntary eye movements during fixation. Journal of Physiology 119: 1–17. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, C., Pinnock, R., Abrahams, S., Cardwell, C., Hardiman, O., Patterson, V. et al. 2009. Ocular fixation instabilities in motor neurone disease. A marker of frontal lobe dysfunction? J Neurol 256: 420–426. [Google Scholar] [CrossRef] [PubMed]
- Doslak, M. J., L. F. Dell’Osso, and R. B. Daroff. 1983. Multiple double saccadic pulses occurring with other saccadic intrusions and oscillations. Neuro-ophthalmology 3: 109–116. [Google Scholar] [CrossRef] [PubMed]
- Engbert, R. 2006. Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Prog.Brain Res 154: 177–192. [Google Scholar] [PubMed]
- Engbert, R., and R. Kliegl. 2003. Microsaccades uncover the orientation of covert attention. Vision Res 43: 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, S., Matlin, A., Hellmuth, J., Schenk, A. K., Johnson, J. K., Rosen, H. et al. 2008. Oculomotor function in frontotemporal lobar degeneration, related disorders and Alzheimer’s disease. Brain (Oxford) 131: 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Goffart, L., Z. M. Hafed, and R. J. Krauzlis. 2012. Visual fixation as equilibrium: evidence from superior colliculus inactivation. J Neurosci 32: 10627–10636. [Google Scholar] [CrossRef] [PubMed]
- Gorges, M., Müller, H. P., Lulé, D., Del, T. K., Brettschneider, J., Keller, J. et al. 2015. Eye Movement Deficits Are Consistent with a Staging Model of pTDP-43 Pathology in Amyotrophic Lateral Sclerosis. PLoS.One 10: e0142546. [Google Scholar] [CrossRef] [PubMed]
- Gowen, E., R. V. Abadi, and E. Poliakoff. 2005. Paying attention to saccadic intrusions. Brain Res Cogn Brain Res 25: 810–825. [Google Scholar] [CrossRef] [PubMed]
- Guerrasio, L., J. Quinet, U. Büttner, and L. Goffart. 2010. Fastigial oculomotor region and the control of foveation during fixation. Journal of Neurophysiology 103: 1988–2001. [Google Scholar] [CrossRef] [PubMed]
- Guitton, D., H. A. Buchtel, and R. M. Douglas. 1985. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Experimental Brain Research 58: 455–472. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z. M., and J. J. Clark. 2002. Microsaccades as an overt measure of covert attention shifts. Vision Res 42: 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z. M., L. Goffart, and R. J. Krauzlis. 2009. A neural mechanism for microsaccade generation in the primate superior colliculus. Science (Washington) 323: 940–943. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y., and J. A. Sharpe. 1981. Normal square wave jerks. Investigative Ophthalmology and Visual Science (St.Louis)T 20: 268–272. [Google Scholar] [PubMed]
- Jung, R., and H. H. Kornhuber. 1964. Results of electronystagmography in man: the value of optokinetic, vestibular and spontaneous nystagmus for neurologic diagnosis and research. In The Oculomotor System. Edited by M. B. Bender. New York: Hoeber Medical Division, Harper & Row: pp. 428–482. [Google Scholar] [CrossRef]
- inal Kapoula, Z., Yang, Q., Otero-Millan, J., Xiao, S., Macknik, S. L., Lang, A. et al. 2014. Distinctive features of microsaccades in Alzheimer’s disease and in mild cognitive impairment. Age (Dordr.) 36: 535–543. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O. et al. 2011. Amyotrophic lateral sclerosis. Lancet 377: 942–955. [Google Scholar] [CrossRef] [PubMed]
- Kowler, E., and R. M. Steinman. 1980. Small saccades serve no useful purpose: Reply to a letter by R.W. Ditchburn. Vision Research 20: 273–276. [Google Scholar] [CrossRef] [PubMed]
- Krauzlis, R. J., L. Goffart, and Z. M. Hafed. 2017. Neuronal control of fixation and fixational eye movements. Philos.Trans.R.Soc.Lond B Biol.Sci. 372. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, J., and M. Tanaka. 2012. Alteration of the timing of self-initiated but not reactive saccades by electrical stimulation in the supplementary eye field. Eur.J Neurosci 36: 3258–3268. [Google Scholar] [CrossRef] [PubMed]
- Lulé, D., Burkhardt, C., Abdulla, S., Böhm, S., Kollewe, K., Uttner, I. et al. 2015. The Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph.Lateral.Scler.Frontotemporal.Degener. 16: 16–23. [Google Scholar] [CrossRef] [PubMed]
- Machado, L., and R. D. Rafal. 2004. Control of fixation and saccades during an anti-saccade task: an investigation in humans with chronic lesions of oculomotor cortex. Experimental Brain Research 156: 55–63. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S., S. L. Macknik, and D. H. Hubel. 2004. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience 5: 229–240. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S., S. L. Macknik, X. G. Troncoso, and D. H. Hubel. 2009. Microsaccades: a neurophysiological analysis. Trends Neurosci 32: 463–475. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S., J. Otero-Millan, and S. L. Macknik. 2013. The impact of microsaccades on vision: towards a unified theory of saccadic function. Nat.Rev.Neurosci 14: 83–96. [Google Scholar] [CrossRef] [PubMed]
- McCamy, M. B., J. A. Najafian, J. Otero-Millan, S. L. Macknik, and S. Martinez-Conde. 2013. The effects of fixation target size and luminance on microsaccades and square-wave jerks. PeerJ 1: e9. [Google Scholar] [CrossRef] [PubMed]
- McGivern, R. C., and J. M. Gibson. 2006. Characterisation of ocular fixation in humans by analysis of saccadic intrusions and fixation periods: A pragmatic approach. Vision Research 46: 3741–3747. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, K., K. Mukuno, K. Ukai, and S. Ishikawa. 1986. The origin of square wave jerks: conditions of fixation and microsaccades. Jpn.J Ophthalmol. 30: 209–215. [Google Scholar] [PubMed]
- Otero-Millan, J., R. Schneider, R. J. Leigh, S. L. Macknik, and S. Martinez-Conde. 2013. Saccades during Attempted Fixation in Parkinsonian Disorders and Recessive Ataxia: From Microsaccades to Square-Wave Jerks. PLoS.One 8: e58535. [Google Scholar] [CrossRef] [PubMed]
- Otero-Millan, J., A. Serra, R. J. Leigh, X. G. Troncoso, S. L. Macknik, and S. Martinez-Conde. 2011. Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy. J Neurosci 31: 4379–4387. [Google Scholar] [CrossRef] [PubMed]
- Peel, T. R., Z. M. Hafed, S. Dash, S. G. Lomber, and B. D. Corneil. 2016. A Causal Role for the Cortical Frontal Eye Fields in Microsaccade Deployment. PLoS.Biol. 14: e1002531. [Google Scholar] [CrossRef] [PubMed]
- Perneger, T. V. 1998. What’s wrong with Bonferroni adjustements. British Medical Journal 316: 1236–1238. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, C., R. M. Müri, C. J. Ploner, B. Gaymard, and S. Rivaud-Pechoux. 2003. Edited by C. Prablanc, D. Pélisson and Y. Rossetti. Cortical control of ocular saccades in humans: A model for motricity. In Progress in Brain research. Elsevier Science B.V.: Vol. 142, pp. 2–16. [Google Scholar] [CrossRef] [PubMed]
- Pinnock, R. A., R. C. McGivern, R. Forbes, and J. M. Gibson. 2010. An exploration of ocular fixation in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. Journal of Neurology 257: 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ploner, C. J., B. M. Gaymard, S. Rivaud-Pechoux, and C. Pierrot-Deseilligny. 2005. The prefrontal substrate of reflexive saccade inhibition in humans. Biological Psychiatry 57: 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Poletti, M., and M. Rucci. 2010. Eye movements under various conditions of image fading. J Vis 10: 6–18. [Google Scholar] [CrossRef] [PubMed]
- Rascol, O., U. Sabatini, M. Simonetta-Moreau, J.-L. Montastruc, and M. Clanet. 1991. Square wave jerks in Parkinsonian syndromes. Journal of Neurology, Neurosurgery and Psychiatry (London) 54: 599–602. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, F., and L. Riggs. 1950. Involuntary motions of the eye during monocular fixation. Journal of Experimental Psychology 40: 687–701. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A. G., M. Xu-Wilson, S. Grill, and D. S. Zee. 2011. ‘Staircase’ square-wave jerks in early Parkinson’s disease. Br.J Ophthalmol. 95: 705–709. [Google Scholar] [CrossRef] [PubMed]
- Shallo-Hoffmann, J., J. Petersen, and H. Mühlendyck. 1989. How normal are “normal” square wave jerks? Investigative Ophthalmology and Visual Science (St.Louis)T 30: 1009–1011. [Google Scholar] [PubMed]
- Original Shaunak, S., Orrell, R. W., O’Sullivan, E., Hawken, M. B., Lane, R. J. M., Henderson, L. et al. 1995. Oculomotor function in amyotrophic lateral sclerosis: Evidence for frontal impairment. Annals of Neurology (Boston) 38: 38–44. [Google Scholar] [CrossRef] [PubMed]
- Yarbus, A. L. 1967. Eye Movements and Vision. (English Translation). New York: Plenum Press. [Google Scholar]
- Zuber, B. L., and L. Stark. 1965. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science (Washington) 150: 1459–1460. [Google Scholar] [CrossRef] [PubMed]

Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Becker, W.; Gorges, M.; Lulé, D.; Pinkhardt, E.; Ludolph, A.C.; Kassubek, J. Saccadic Intrusions in Amyotrophic Lateral Sclerosis (ALS). J. Eye Mov. Res. 2019, 12, 1-17. https://doi.org/10.16910/jemr.12.6.8
Becker W, Gorges M, Lulé D, Pinkhardt E, Ludolph AC, Kassubek J. Saccadic Intrusions in Amyotrophic Lateral Sclerosis (ALS). Journal of Eye Movement Research. 2019; 12(6):1-17. https://doi.org/10.16910/jemr.12.6.8
Chicago/Turabian StyleBecker, Wolfgang, Martin Gorges, Dorothée Lulé, Elmar Pinkhardt, Albert C. Ludolph, and Jan Kassubek. 2019. "Saccadic Intrusions in Amyotrophic Lateral Sclerosis (ALS)" Journal of Eye Movement Research 12, no. 6: 1-17. https://doi.org/10.16910/jemr.12.6.8
APA StyleBecker, W., Gorges, M., Lulé, D., Pinkhardt, E., Ludolph, A. C., & Kassubek, J. (2019). Saccadic Intrusions in Amyotrophic Lateral Sclerosis (ALS). Journal of Eye Movement Research, 12(6), 1-17. https://doi.org/10.16910/jemr.12.6.8



