Microsaccades and Covert Attention: Evidence from a Continuous, Divided Attention Task
Abstract
:Introduction
Methods
Participants
Apparatus
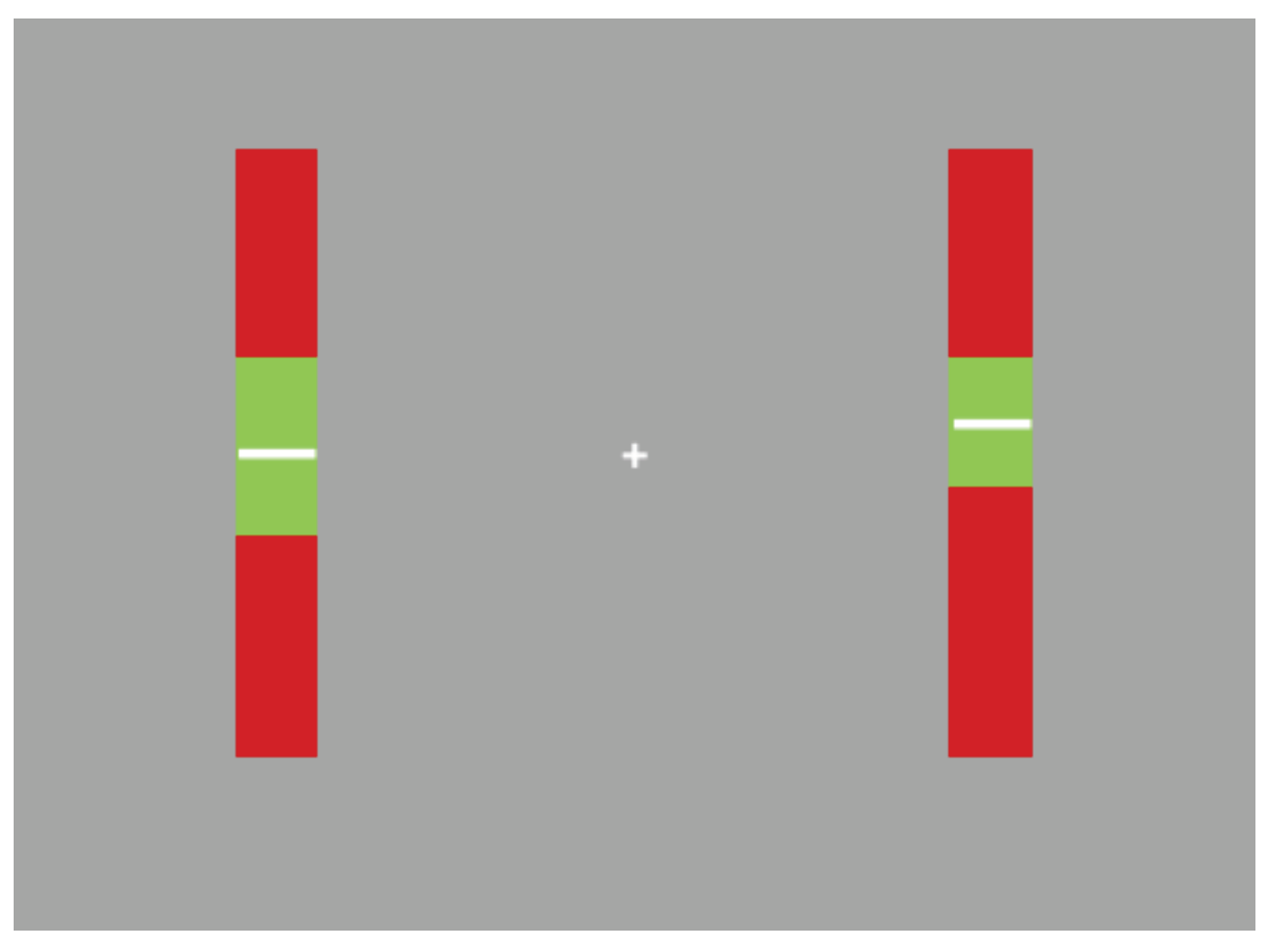
Task
Measures
Results
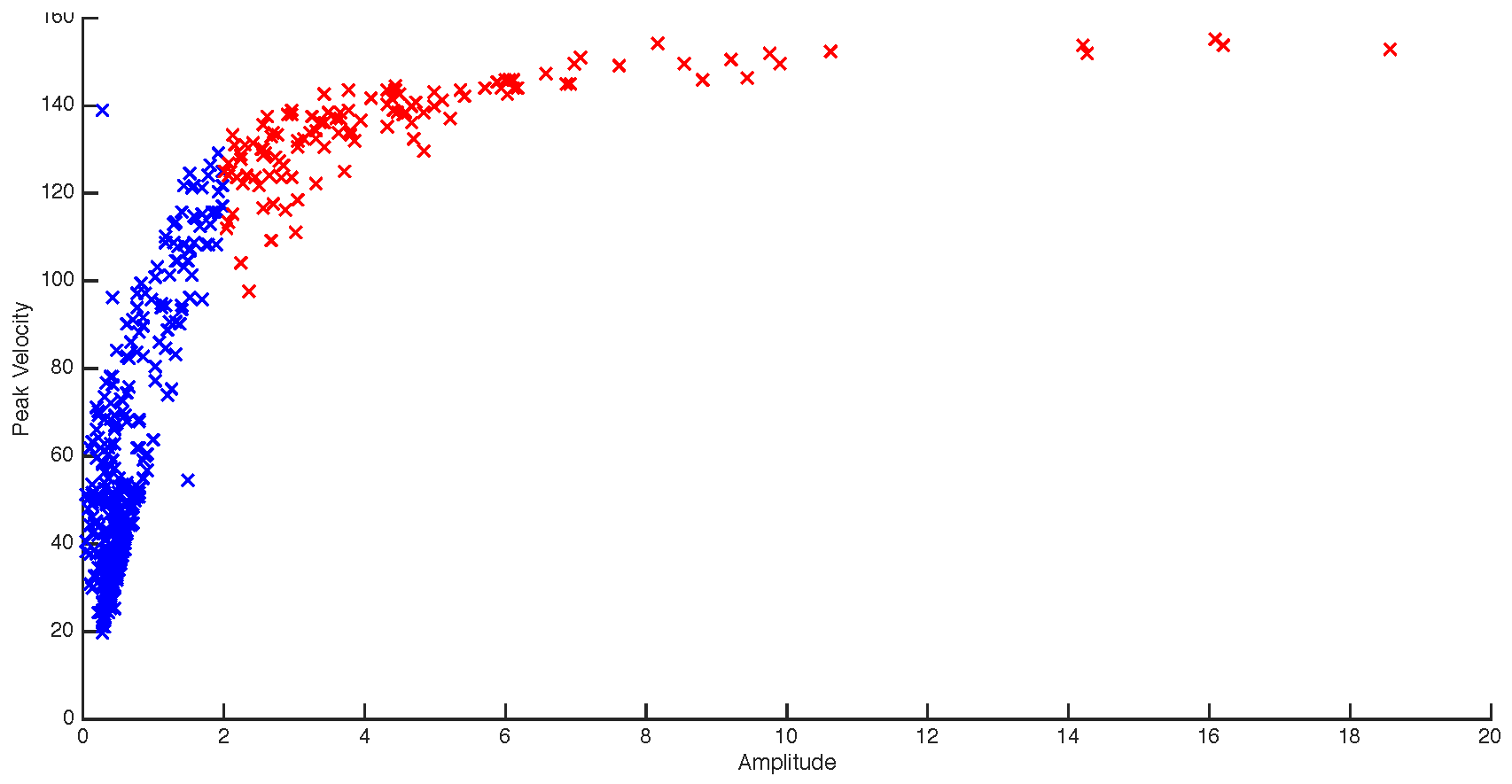
Drift Correction
Characterising responses
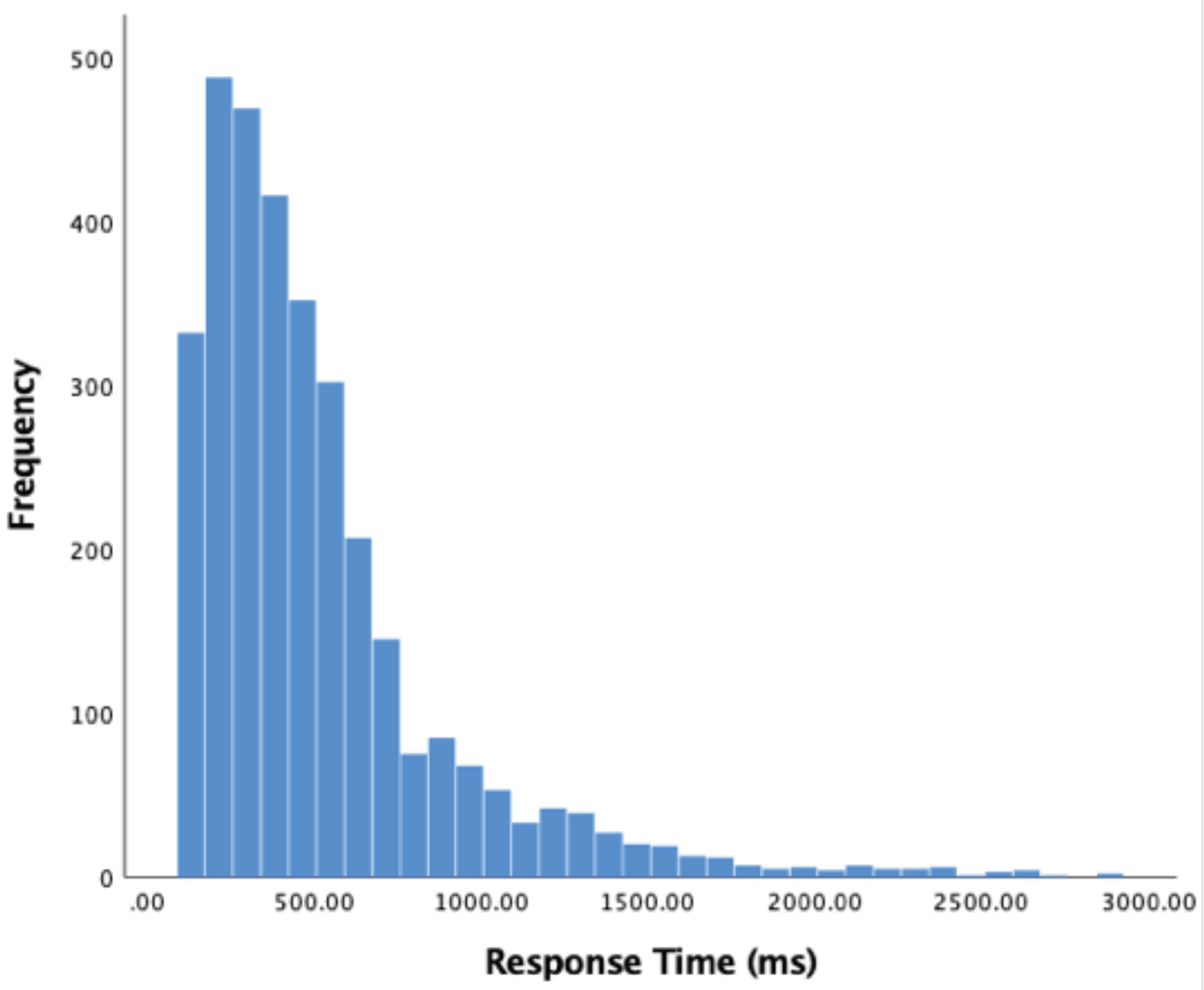
Response time
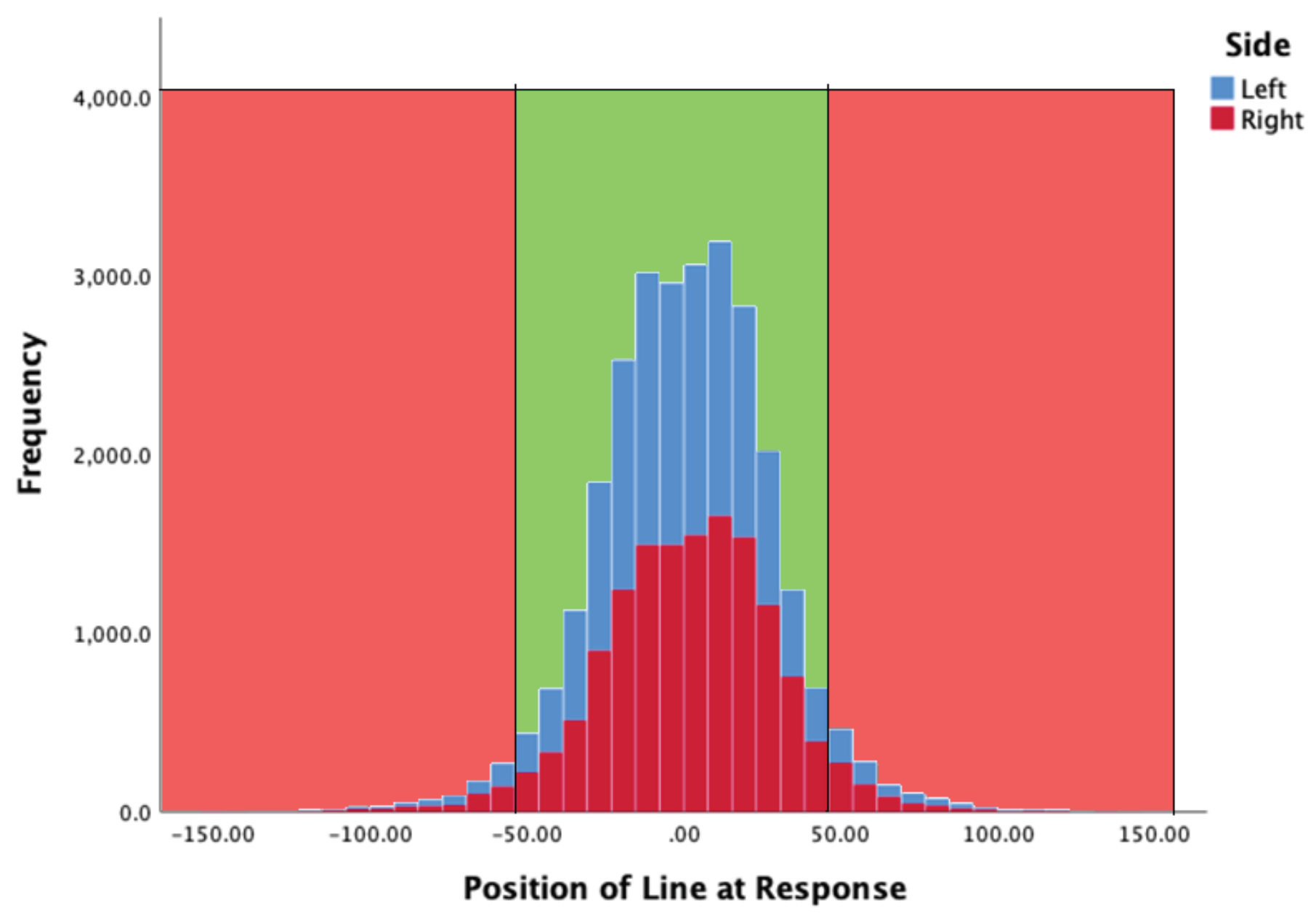
Line locus at moment of response
Microsaccades and Attention
Response accuracy and the direction of the preceding microsaccade
Correct Responses

Incorrect Responses
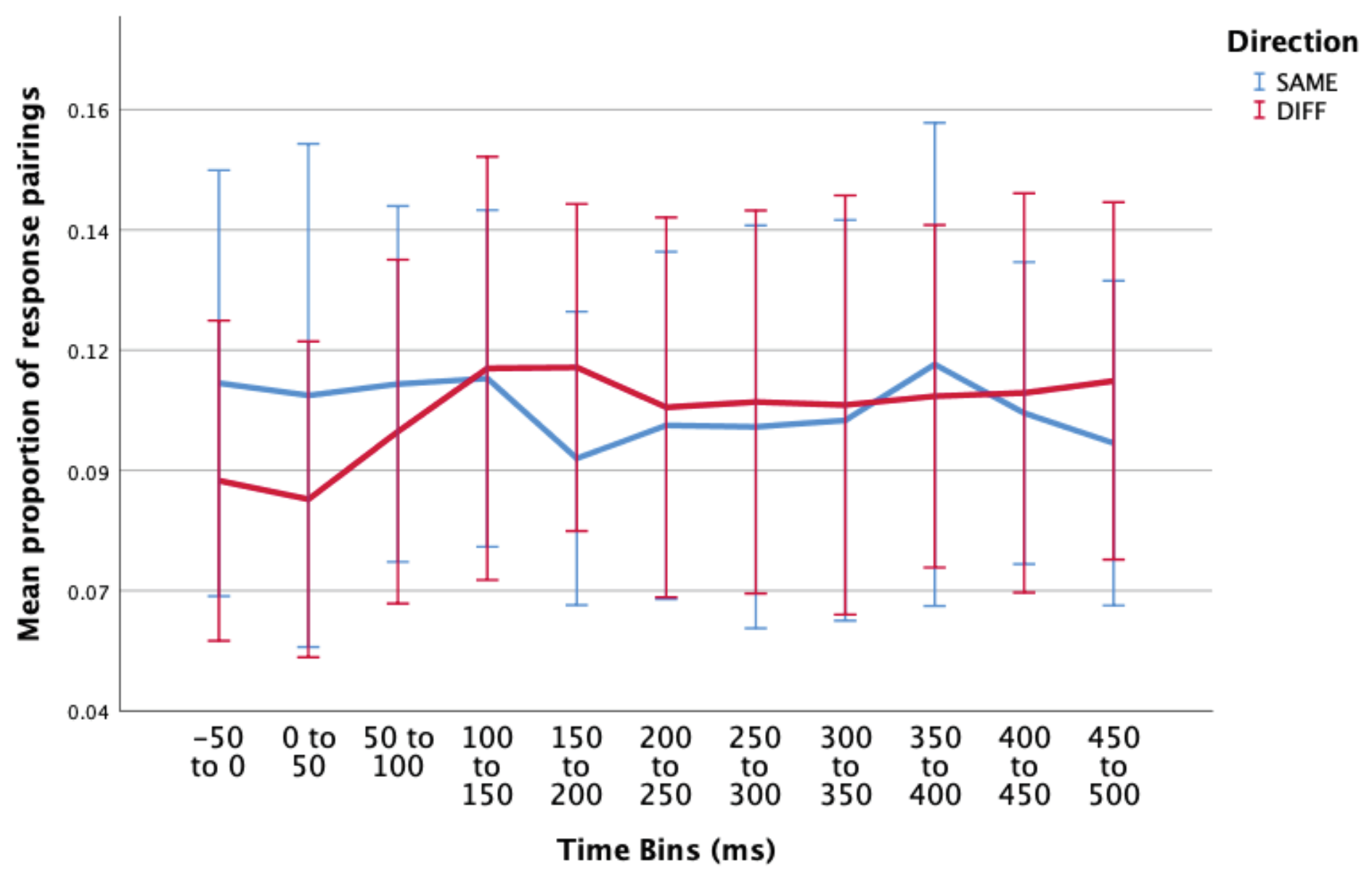
Laterality of response following a microsaccade, irrespective of accuracy
- 1)
- Pre-MS (-200 to 0ms): we suggest that any responses that occur pre-microsaccade are more likely to be related to a previous shift of attention.
- 2)
- Immediate Post-MS (0 to 200ms): again, it could be suggested that any responses that occur within this time period are more likely to be related to previous attention shift, as in general, people require > 120ms for motor response.
- 3)
- Delayed Post-MS (200ms to 450ms): we suggest that any responses that occur within this time period are most likely to be related to the current microsaccade/attention shift. This time bin includes responses that occur between 200ms and 450ms post-microsaccade (median response time ~400ms).
Discussion
Acknowledgments
Conflicts of Interest
References
- Bridgeman, B., and J. Palca. 1980. The role of microsaccades in high acuity observational tasks. Vision Research 20, 9: 813–817. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M. 2006. Covert attention increases contrast sensitivity: psychophysical, neurophysiological and neuroimaging studies. Prog. Brain Res. 154: 33–70. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M., and B. McElree. 2001. Covert attention accelerates the rate of visual information processing. Proceedings of the National Academy of Sciences of the United States of America 98, 9: 5363–5367. [Google Scholar] [CrossRef]
- Carrasco, M., P. E. Williams, and Y. Yeshurun. 2002. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision 2, 6: 467–479. [Google Scholar] [CrossRef]
- Chen, C.-Y., A. Ignashchenkova, P. Thier, and Ziad M. Hafed. 2015. Neuronal Response Gain Enhancement prior to Microsaccades. Current Biology 25, 16: 2065–2074. [Google Scholar] [CrossRef]
- Cornsweet, T. N. 1956. Determination of the stimuli for involuntary drifts and saccadic eye movements. Journal of the Optical Society of America 46, 11: 987–993. [Google Scholar] [CrossRef]
- Denison, R. N., S. Yuval-Greenberg, and M. Carrasco. 2019. Directing Voluntary Temporal Attention Increases Fixational Stability. J Neurosci 39, 2: 353–363. [Google Scholar] [CrossRef]
- Engbert, R., and R. Kliegl. 2003. Microsaccades uncover the orientation of covert attention. Vision Research 43, 9: 1035–1045. [Google Scholar] [CrossRef]
- Gao, X., H. Yan, and H. J. Sun. 2015. Modulation of microsaccade rate by task difficulty revealed through between-and within-trial comparisons. J Vis 15, 3. [Google Scholar] [CrossRef]
- Haddad, G. M., and R. M. Steinman. 1973. Smallest voluntary saccade: implications for fixation. Vision Res 13: 1075–1086. [Google Scholar] [CrossRef]
- Hafed, Z. M. 2011. Mechanisms for generating and compensating for the smallest possible saccades. Eur J Neurosci 33, 11: 2101–2113. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z. M. 2013. Alteration of visual perception prior to microsaccades. Neuron 77, 4: 775–786. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z. M., and J. J. Clark. 2002. Microsaccades as an overt measure of covert attention shifts. Vision Research 42, 22: 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z. M., L. Goffart, and R. J. Krauzlis. 2009. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323, 5916: 940–943. [Google Scholar] [CrossRef]
- Horowitz, T. S., E. M. Fine, D. E. Fencsik, S. Yurgenson, and J. M. Wolfe. 2007. Fixational eye movements are not an index of covert attention. Psychological science 18, 4: 356–363. [Google Scholar] [CrossRef]
- Just, M. A., and P. A. Carpenter. 1980. A theory of reading: from eye fixations to comprehension. Psychological review 87, 4: 329–354. [Google Scholar] [CrossRef]
- Ko, H. K., D. M. Snodderly, and M. Poletti. 2016. Eye movements between saccades: Measuring ocular drift and tremor. Vision Res 122: 93–104. [Google Scholar] [CrossRef]
- Kowler, E., E. Anderson, B. Dosher, and E. Blaser. 1995. The role of attention in the programming of saccades. Vision Res 35, 13: 1897–1916. [Google Scholar] [CrossRef]
- Laubrock, J., R. Engbert, and R. Kliegl. 2005. Microsaccade dynamics during covert attention. Vision Research 45, 6: 721–730. [Google Scholar] [CrossRef]
- Laubrock, J., R. Engbert, M. Rolfs, and R. Kliegl. 2007. Microsaccades are an index of covet attention: Commentary on Horowitz, Fine, Fencsik, Yurgenson, and Wolfe (2007). Psychological science 18, 4: 364–366. [Google Scholar] [CrossRef]
- Laubrock, J., R. Kliegl, M. Rolfs, and R. Engbert. 2010. When do microsaccades follow spatial attention? Attention, perception & psychophysics 72, 3: 683–694. [Google Scholar]
- Lou, L. 1999. Selective Peripheral Fading: Evidence for Inhibitory Sensory Effect of Attention. Perception 28, 4: 519–526. [Google Scholar] [CrossRef] [PubMed]
- Lowet, E., B. Gomes, K. Srinivasan, H. Zhou, R. J. Schafer, and R. Desimone. 2018. Enhanced Neural Processing by Covert Attention only during Microsaccades Directed toward the Attended Stimulus. Neuron 99, 1: 207–214.e203. [Google Scholar] [CrossRef] [PubMed]
- Mack, A., and L. Rock. 1998. Inattentional Blindness. Cambridge, MA: MIT Press. [Google Scholar]
- Martinez-Conde, S., S. L. Macknik, and D. H. Hubel. 2004. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience 5, 3: 229–240. [Google Scholar] [CrossRef]
- Martinez-Conde, S., S. L. Macknik, X. G. Troncoso, and T. A. Dyar. 2006. Microsaccades Counteract Visual Fading during Fixation. Neuron 49, 2: 297–305. [Google Scholar] [CrossRef]
- Maxwell, S. E., and H. D. Delany. 2004. Designing experiments and analyzing data: A model comparison perspective, 2nd ed. New York, NY: Psychology Press. [Google Scholar]
- McCamy, M. B., Otero-Millan, J., Macknik, S. L., Yang, Y., Troncoso, X. G., Baer, S. M.,... Martinez-Conde, S. 2012. Microsaccadic Efficacy and Contribution to Foveal and Peripheral Vision. Journal of Neuroscience 32, 27: 9194–9204. [Google Scholar] [CrossRef]
- Meyberg, S., P. Sinn, R. Engbert, and W. Sommer. 2017. Revising the link between microsaccades and the spatial cueing of voluntary attention. Vision Research 133: 47–60. [Google Scholar] [CrossRef]
- Moran, J., and R. Desimone. 1985. Selective attention gates visual processing in the extrastriate cortex. Science 229, 4715: 782–784. [Google Scholar] [CrossRef]
- Pastukhov, A., and J. Braun. 2010. Rare but precious: microsaccades are highly informative about attentional allocation. Vision Res 50, 12: 1173–1184. [Google Scholar] [CrossRef]
- Poletti, M., and M. Rucci. 2016. A compact field guide to the study of microsaccades: Challenges and functions. Vision Res 118: 83–97. [Google Scholar] [CrossRef]
- Poletti, M., M. Rucci, and M. Carrasco. 2017. Selective attention within the foveola. Nature Neuroscience 20, 10: 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Posner, M. I. 1980. Orienting of attention. The Quarterly journal of experimental psychology 32, 1: 3–25. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G., L. Riggio, I. Dascola, and C. Umilta. 1987. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia 25, 1A: 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, M. 2009. Microsaccades: small steps on a long way. Vision Res 49, 20: 2415–2441. [Google Scholar] [CrossRef]
- Rucci, M., R. Iovin, M. Poletti, and F. Santini. 2007. Miniature eye movements enhance fine spatial detail. Nature 447, 7146: 851–854. [Google Scholar] [CrossRef]
- Shapiro, S. S., and M. B. Wilk. 1965. An analysis of variance test for normailty (complete samples). Biometrika 52, 3/4: 591–611. [Google Scholar] [CrossRef]
- Sheliga, B. M., L. Craighero, L. Riggio, and G. Rizzolatti. 1997. Effects of spatial attention on directional manual and ocular responses. Experimental Brain Research 114: 339–351. [Google Scholar] [CrossRef]
- Willeke, K.-F., X. Tian, A. Buonocore, J. Bellet, A. Ramirez-Cardenas, and Z. M. Hafed. 2019. Memory-guided microsaccades. bioRxiv. [Google Scholar] [CrossRef]
- Yeshurun, Y., and M. Carrasco. 1998. Attention improves or impairs visual performance by enhancing spatial resolution. Nature 396, 6706: 72–75. [Google Scholar] [CrossRef]
- Yuval-Greenberg, S., E. P. Merriam, and D. J. Heeger. 2014. Spontaneous Microsaccades Reflect Shifts in Covert Attention. The Journal of Neuroscience 34, 41: 13693–13700. [Google Scholar] [CrossRef]

 |
Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Ryan, A.E.; Keane, B.; Wallis, G. Microsaccades and Covert Attention: Evidence from a Continuous, Divided Attention Task. J. Eye Mov. Res. 2019, 12, 1-11. https://doi.org/10.16910/jemr.12.6.6
Ryan AE, Keane B, Wallis G. Microsaccades and Covert Attention: Evidence from a Continuous, Divided Attention Task. Journal of Eye Movement Research. 2019; 12(6):1-11. https://doi.org/10.16910/jemr.12.6.6
Chicago/Turabian StyleRyan, Aimee E., Brendan Keane, and Guy Wallis. 2019. "Microsaccades and Covert Attention: Evidence from a Continuous, Divided Attention Task" Journal of Eye Movement Research 12, no. 6: 1-11. https://doi.org/10.16910/jemr.12.6.6
APA StyleRyan, A. E., Keane, B., & Wallis, G. (2019). Microsaccades and Covert Attention: Evidence from a Continuous, Divided Attention Task. Journal of Eye Movement Research, 12(6), 1-11. https://doi.org/10.16910/jemr.12.6.6



