Effects of Aligning Prisms on the Objective and Subjective Fixation Disparity in Far Distance
Abstract
Introduction
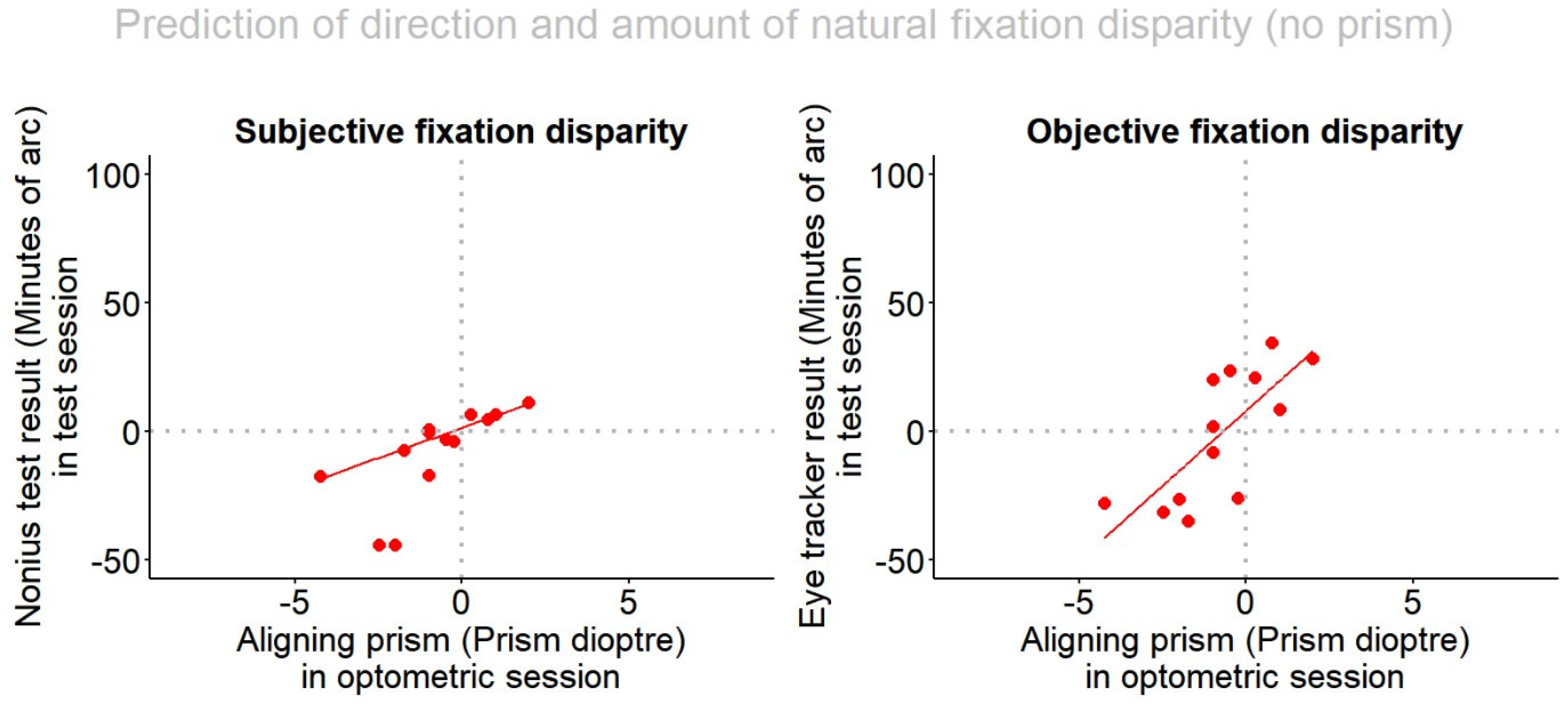
- In natural vision (i. e. no prisms) the regression is tested how the individual aligning prism is able to predict
- (a)
- The subjective fixation disparity; given that both these measures are subjective and rely on the same test target, a regression should be expected, but the quantitative relation was not yet investigated for the present Cross test.
- (b)
- The objective fixation disparity, since users of the Cross test assume that the corresponding aligning prism should reflect the oculomotor vergence error (oFD). But this has not yet been confirmed experimentally.
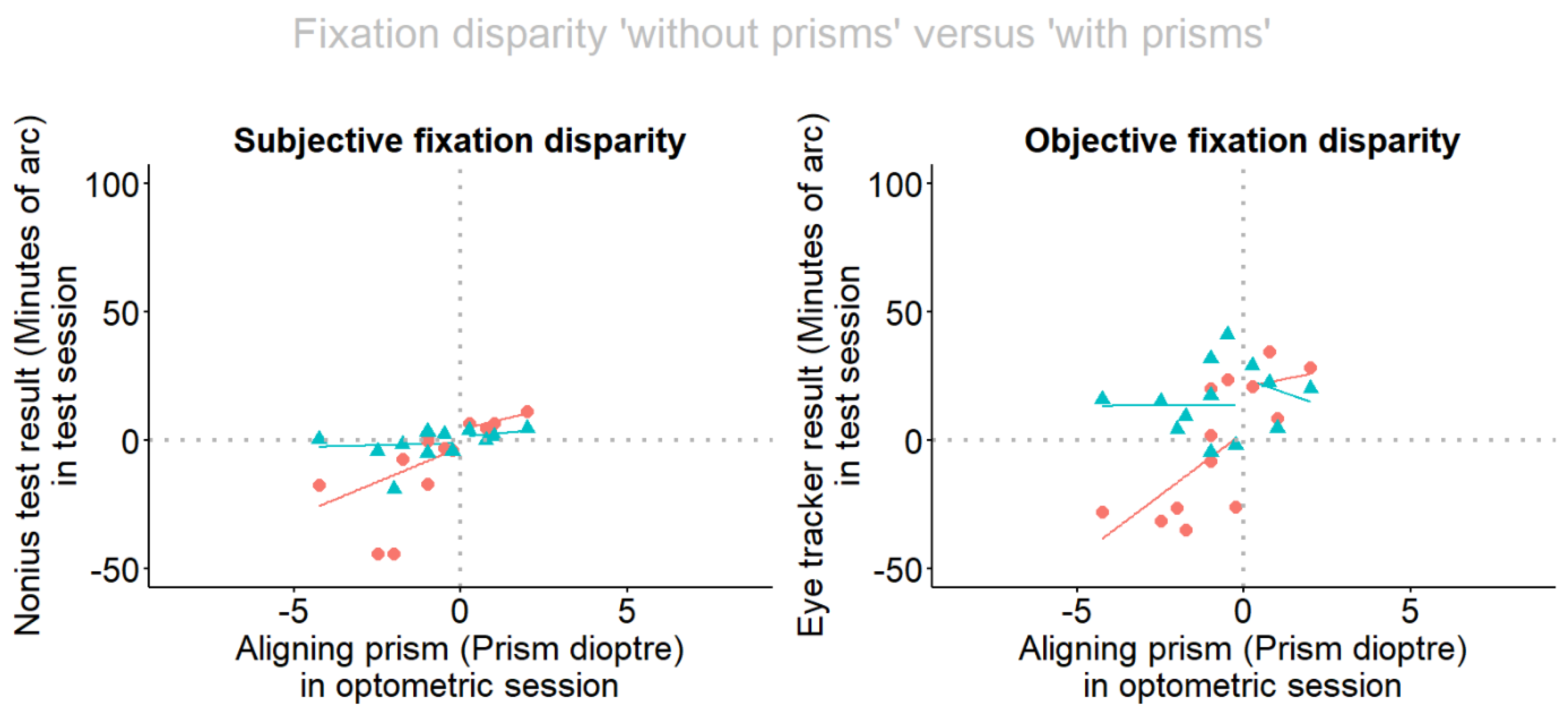
- The conditions without and with prisms are compared regarding how the individual amount of the aligning prism is able to correct the subjective and objective fixation disparity.
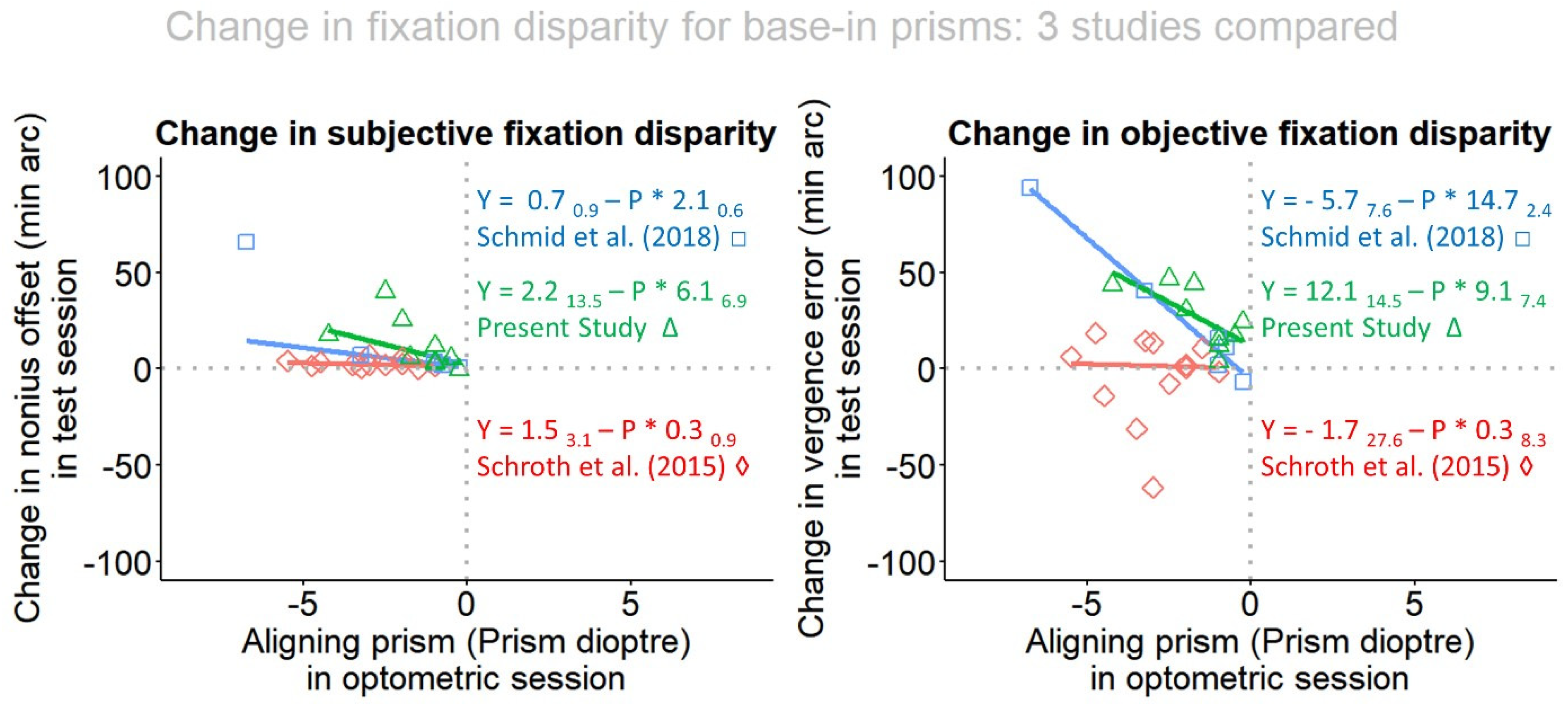
- The individual aligning prism is tested to see how it is able to predict the change both in subjective and in objective fixation disparity.
Methods
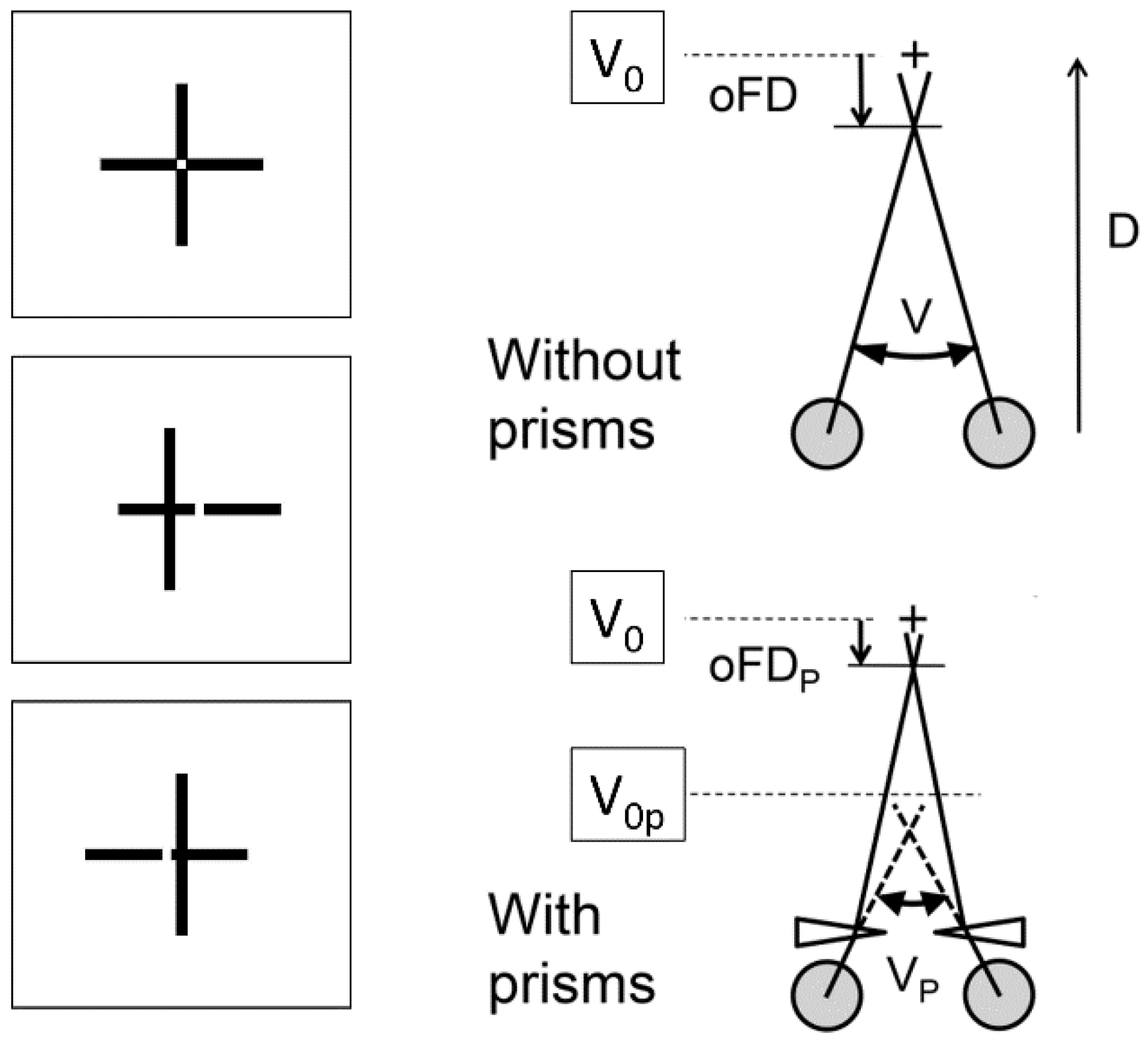
Stimuli and apparatus
Eye movements recordings
Design of the study
Participants
Data analysis and statistics
Results
Discussion
- The direction and amount of a subjective aligning prism test such as the Cross test allows for a prediction of the direction and amount the objective FD with an adjusted R-squared of 0.5; for subjective fixation disparity the adjusted R-squared was 0.8.
- Wearing the aligning prism for 60 sec induced effects in the expected direction and the amount of the changes was linearly related to the amount of the prism, in the objective FD. Only a trend was found for subjective FD. This result refers to base-in prisms in the present study.
- In terms of prisms adaptation, we conclude that wearing base-in prisms for 2 - 5 sec in Schmid et al. [30] and for 60 sec in the present study showed very similar effects on objective fixation disparity; this suggests that – at least in the present test conditions - no substantial vergence adaptation occurs within one minute. However, there is the caveat that this interpretation is based on the comparison of different groups in two studies. Further research may vary the prism exposure duration as a parameter in an intra-individual experimental design.
Ethics and Conflict of Interest
Acknowledgments
References
- Howard, I.P. Perceiving in Depth, Volume 1: Basic Mechanisms; Oxford University Press, USA: New York, 2012. [Google Scholar]
- Howard, I.P.; Rogers, B.J. Perceiving in Depth, Volume 2: Stereoscopic Vision; Oxford University Press, USA: New York, 2012. [Google Scholar]
- Howard, I.P. Perceiving in Depth, Volume 3: Other Mechanisms of Depth Perception; Oxford University Press, USA: New York, 2012. [Google Scholar]
- Maddox, E.E. The wing test for heterophoria. Trans Ophthal Soc UK 1913, 33, 22–227. [Google Scholar]
- Babinsky, E.; Sreenivasan, V.; Candy, T.R. Near heterophoria in early childhood. Invest Ophthalmol Vis Sci. 2015, 56, 1406–1415, Epub 2015/01/31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, S.J.; Guo, Y.; Granger-Donetti, B.; Vicci, V.R.; Alvarez, T.L. Quantification of heterophoria and phoria adaptation using an automated objective system compared to clinical methods. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2010, 30, 95–107, Epub 2009/08/18. [Google Scholar] [CrossRef] [PubMed]
- Jaschinski, W.; Jainta, S.; Kloke, W.B. Objective vs subjective measures of fixation disparity for short and long fixation periods. Ophthalmic Physiol Opt. 2010, 30, 379–390, Epub 2010/07/16. [Google Scholar] [CrossRef] [PubMed]
- Mestre, C.; Otero, C.; Diaz-Douton, F.; Gautier, J.; Pujol, J. An automated and objective cover test to measure heterophoria. PLoS ONE 2018, 13, e0206674, Epub 2018/11/02. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.W. Pickwell’s binocular vision anomalies, 5th ed.; Elsevier Butterworth Heinemann: Edinburgh; New York, 2007. [Google Scholar]
- Mallett, R. Fixation disparity-its genesis and relation to asthenopia. The Ophthalmic Optician 1974, 1159–1168. [Google Scholar]
- Ogle, K.; Martens, T.; Dyer, J. Oculomotor Imbalance in Binocular Vision and Fixation Disparity; Lea and Febiger: Philadelphia, 1967. [Google Scholar]
- Jaschinski, W. The proximity-fixationdisparity curve and the preferred viewing distance at a visual display as an indicator of near vision fatigue. Optom Vis Sci. 2002, 79, 158–169, Epub 2002/03/27. [Google Scholar] [CrossRef] [PubMed]
- Pickwell, D.; Jenkins, T.; Yekta, A.A. The effect on fixation disparity and associated heterophoria of reading at an abnormally close distance. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 1987, 7, 345–347, Epub 1987/01/01. [Google Scholar] [PubMed]
- Sheedy, J.; Saladin, J. Validity of diagnostic criteria and case analysis in binocular vision disorders. In Vergence Eye Movements: Basic and Clinical Aspects; Schor, C., Ciuffreda, K., Eds.; Butterworths: Boston, 1983; pp. 517–540. [Google Scholar]
- Mallett, R. The investigation of heterophoria at near and a new fixation disparity technique. The Optician 1964, 148, 547–551, 574–581. [Google Scholar]
- Haase, H.-J. Binocular testing and distance correction with the Berlin Polatest (Transl W. Baldwin). Journal of the American Optometric Association 1962, 34, 115–124. [Google Scholar]
- Jaschinski, W. Individual objective versus subjective fixation disparity as a function of forced vergence. PLoS ONE 2018, 13, e0199958, Epub 2018/07/07. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pickwell, L.D.; Yekta, A.A.; Jenkins, T.C. Effect of reading in low illumination on fixation disparity. American journal of optometry and physiological optics 1987, 64, 513–518, Epub 1987/07/01. [Google Scholar] [PubMed]
- Kertesz, A.E.; Lee, H.J. Comparison of simultaneously obtained objective and subjective measurements of fixation disparity. American journal of optometry and physiological optics 1987, 64, 734–738, Epub 1987/10/01. [Google Scholar] [CrossRef] [PubMed]
- Fogt, N.; Jones, R. Comparison of fixation disparities obtained by objective and subjective methods. Vision Res. 1998, 38, 411–421, Epub 1998/04/16. [Google Scholar] [CrossRef] [PubMed]
- Brautaset, R.L.; Jennings, J.A. Measurements of objective and subjective fixation disparity with and without a central fusion stimulus. Medical science monitor: international medical journal of experimental and clinical research 2006, 12, Mt1–Mt4, Epub 2006/02/02. [Google Scholar] [PubMed]
- Wick, B. Stability of retinal correspondence in normal binocular vision. Optom Vis Sci. 1991, 68, 146–158, Epub 1991/02/01. [Google Scholar] [CrossRef] [PubMed]
- Yekta, A.A.; Jenkins, T.; Pickwell, D. The clinical assessment of binocular vision before and after a working day. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 1987, 7, 349–352, Epub 1987/01/01. [Google Scholar] [PubMed]
- Lie, I.; Opheim, A. Long-term acceptance of prisms by heterophorics. J Am Optom Assoc. 1985, 56, 272–278, Epub 1985/04/01. [Google Scholar] [PubMed]
- Lie, I.; Opheim, A. Long-term stability of prism correction of heterophorics and heterotropics; a 5 year follow-up. Part I: Heterophorics. J Am Optom Assoc. 1990, 61, 491–498, Epub 1990/06/01. [Google Scholar] [PubMed]
- Jaschinski, W. Fixation disparity and accommodation as a function of viewing distance and prism load. Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists) 1997, 17, 324–339. [Google Scholar] [PubMed]
- Jenkins, T.C.; Pickwell, L.D.; Yekta, A.A. Criteria for decompensation in binocular vision. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 1989, 9, 121–125, Epub 1989/04/01. [Google Scholar] [PubMed]
- Schroth, V. Binocular Correction. Aligning prisms according to the Haase approach; Zijdar Book: Heemskerk, The Netherlands, 2012. [Google Scholar]
- Schroth, V.; Joos, R.; Jaschinski, W. Effects of Prism Eyeglasses on Objective and Subjective Fixation Disparity. PLoS ONE 2015, 10, e0138871, Epub 2015/10/03. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmid, L.; von Handorff, C.; Jaschinski, W. Messung der Augenbewegungen bei MKHKreuztest-Prismen. Teil 2 Messung der Vergenzstellung mit und ohne Prisma. DOZ, Deutsche Optikerzeitung 2018, 73, 198–205. [Google Scholar]
- Graf, E.W.; Maxwell, J.S.; Schor, C.M. Comparison of the time courses of concomitant and nonconcomitant vertical phoria adaptation. Vision Res. 2003, 43, 567–576, Epub 2003/02/22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schor, C.M.; Ciuffreda, K.J. Vergence Eye Movements: Basic and Clinical Aspects; Butterworth-Heinemann Ltd., 1983; 760p. [Google Scholar]
- Przekoracka-Krawczyk, A.; Michalak, K.P.; Pyzalska, P. Deficient vergence prism adaptation in subjects with decompensated heterophoria. PLoS ONE 2019, 14, e0211039, Epub 2019/01/19. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.M.; Yaramothu, C.; Alvarez, T.L. Comparison of symmetrical prism adaptation to asymmetrical prism adaptation in those with normal binocular vision. Vision Research 2018, 149, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.B. Fixation disparity and heterophoria following prolonged wearing of prisms. American journal of optometry and archives of American Academy of Optometry 1965, 42, 141–152, Epub 1965/03/01. [Google Scholar] [PubMed]
- Mitchell, A.M.; Ellerbrock, V.J. Fixational disparity and the maintainence of fusion in the horizontal meridian. American journal of optometry and archives of American Academy of Optometry 1955, 32, 520–534, Epub 1955/10/01. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.B. Parameters of fixation disparity. American journal of optometry and physiological optics 1980, 57, 610–617, Epub 1980/09/01. [Google Scholar] [PubMed]
- Sheedy, J.E.; Saladin, J.J. Association of symptoms with measures of oculomotor deficiencies. American journal of optometry and physiological optics 1978, 55, 670–676, Epub 1978/10/01. [Google Scholar] [PubMed]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision; Wolters Kluwer. Lippincott Williams & Williams: Philadelphia, 2014. [Google Scholar]
- Schor, C.M. The influence of rapid prism adaptation upon fixation disparity. Vision Res. 1979, 19, 757–765, Epub 1979/01/01. [Google Scholar] [CrossRef] [PubMed]
- Schor, C.M. The relationship between fusional vergence eye movements and fixation disparity. Vision Research 1979, 19, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, M.; Hovis, J.K.; Chou, R.B. Repeatability of Associated Phoria Tests. Optom Vis Sci. 2015, 92, 900–907, Epub 2015/06/23. [Google Scholar] [CrossRef] [PubMed]
- Methling, D.; Jaschinski, W. Contrast sensitivity after wearing prisms to correct for heterophoria. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 1996, 16, 211–215, Epub 1996/05/01. [Google Scholar] [PubMed]
- London, R.; Crelier, R.S. Fixation disparity analysis: sensory and motor approaches. Optometry (St Louis, Mo) 2006, 77, 590–608, Epub 2006/12/13. [Google Scholar] [CrossRef] [PubMed]
- Jaschinski, W. Individual Objective and Subjective Fixation Disparity in Near Vision. PLoS ONE 2017. [Google Scholar] [CrossRef]
- IVBS IAoBV. Guidelines for the Application of MCH. In: IVBS IAoBV, editor. Available online: http://www.ivbs.org/information-in-english/2012.
- Heritier, S.; Cantoni, E.; Copt, S.; VictoriaFeser, M.-P. Robust methods in biostatistics; John Wiley & Sons, 2009. [Google Scholar]
- Jaschinski, W. Pupil size affects measures of eye position in video eye tracking: implications for recording vergence accuracy. 2016, 9. Available online: https://bop.unibe.ch/JEMR/article/view/2523. [CrossRef]
- Sethi, B. Heterophoria: a vergence adaptive position. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists) 1986, 6, 151–156, Epub 1986/01/01. [Google Scholar] [PubMed]
- Sethi, B. Vergence adaptation: a review. Documenta ophthalmologica Advances in ophthalmology 1986, 63, 247–263, Epub 1986/09/30. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.; North, R.V. Vergence adaptive changes with varying magnitudes of prism-induced disparities and fusional amplitudes. American journal of optometry and physiological optics 1987, 64, 263–268, Epub 1987/04/01. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. Clinical implications of vergence adaptation. Optom Vis Sci. 1992, 69, 300–307, Epub 1992/04/01. [Google Scholar] [PubMed]
- McDaniel, C.; Fogt, N. Vergence adaptation in clinical vergence testing. Optometry (St Louis, Mo) 2010, 81, 469–475, Epub 2010/07/27. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, M. Tonic vergence and vergence adaptation. Optom Vis Sci. 1997, 74, 303–328, Epub 1997/05/01. [Google Scholar] [CrossRef] [PubMed]
- Jaschinski-Kruza, W. Eyestrain in VDU users: viewing distance and the resting position of ocular muscles. Hum Factors 1991, 33, 69–83, Epub 1991/02/01. [Google Scholar] [CrossRef] [PubMed]
- Hooge, I.T.C.; Hessels, R.S.; Nystrom, M. Do pupil-based binocular video eye trackers reliably measure vergence? Vision Res. 2019, 156, 1–9, Epub 2019/01/15. [Google Scholar] [CrossRef] [PubMed]

Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Schroth, V.; Joos, R.; Alshuth, E.; Jaschinski, W. Effects of Aligning Prisms on the Objective and Subjective Fixation Disparity in Far Distance. J. Eye Mov. Res. 2019, 12, 1-11. https://doi.org/10.16910/jemr.12.4.8
Schroth V, Joos R, Alshuth E, Jaschinski W. Effects of Aligning Prisms on the Objective and Subjective Fixation Disparity in Far Distance. Journal of Eye Movement Research. 2019; 12(4):1-11. https://doi.org/10.16910/jemr.12.4.8
Chicago/Turabian StyleSchroth, Volkhard, Roland Joos, Ewald Alshuth, and Wolfgang Jaschinski. 2019. "Effects of Aligning Prisms on the Objective and Subjective Fixation Disparity in Far Distance" Journal of Eye Movement Research 12, no. 4: 1-11. https://doi.org/10.16910/jemr.12.4.8
APA StyleSchroth, V., Joos, R., Alshuth, E., & Jaschinski, W. (2019). Effects of Aligning Prisms on the Objective and Subjective Fixation Disparity in Far Distance. Journal of Eye Movement Research, 12(4), 1-11. https://doi.org/10.16910/jemr.12.4.8


