Changes in the Disparity Vergence Main Sequence After Treatment of Symptomatic Convergence Insufficiency in Children
Abstract
Introduction
Methods
Patient Selection
Study Design
Clinical Testing
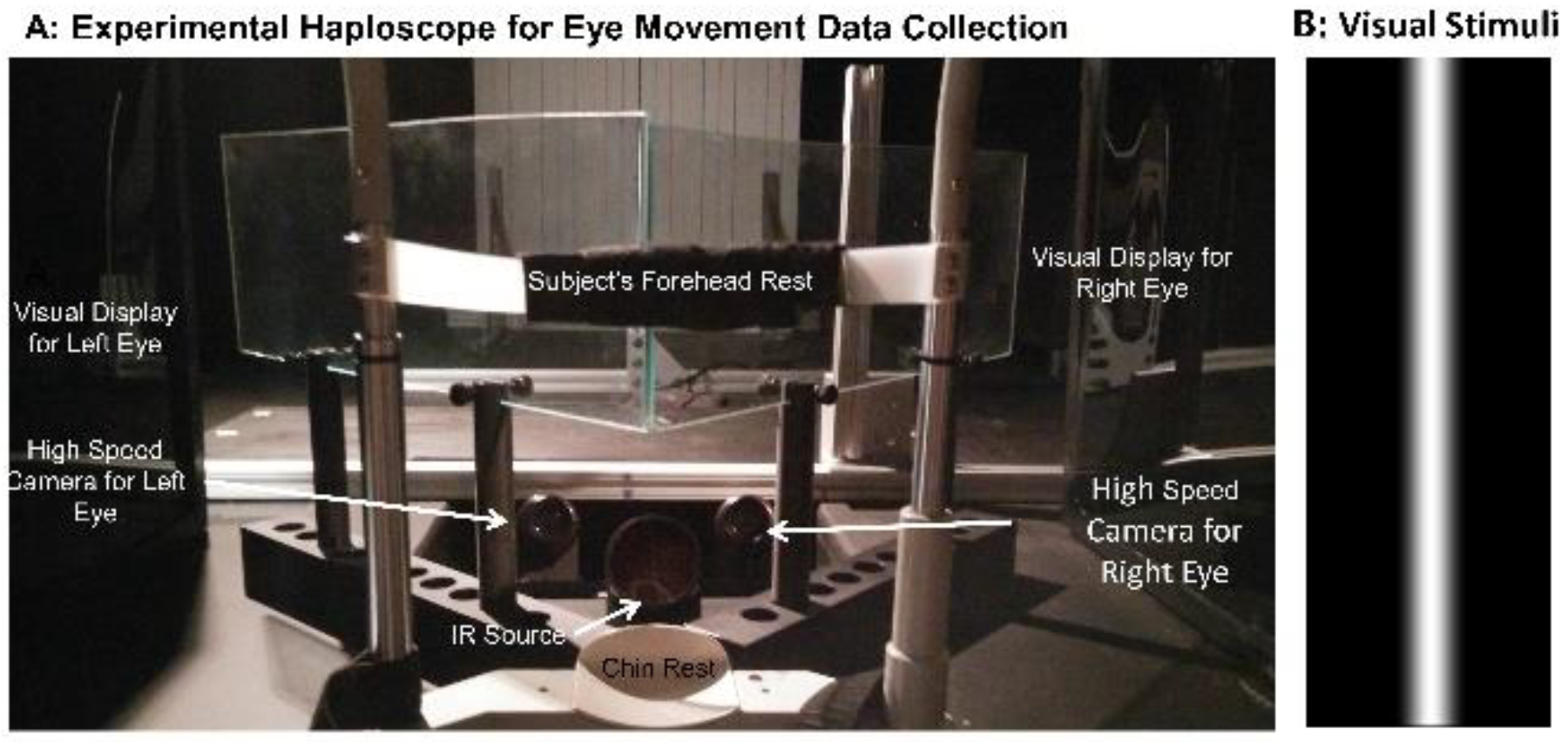
Objective Outcome Measures of Disparity Vergence: Instrumentation
Stimuli Presentation and Data Collection
Calibration
Eye Movement Analyses
Treatment: Office-based Vergence and Accommodative Therapy with Home Reinforcement (OBVAT)
Follow-up Visit
Statistical Analysis
Results
Discussion
Ethics and Conflict of Interest
Acknowledgments
References
- Rouse, M.W.; Borsting, E.; Hyman, L.; Hussein, M.; Cotter, S.; Flynn, M.; et al. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999, 76, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Wick, B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders, 5th ed.; Wolters-Kluwer: Philadelphia, 2020. [Google Scholar]
- Borsting, E.; Rouse, M.W.; De Land, P.N.; Convergence Insufficiency and Reading Study (CIRS) group. Prospective comparison of convergence insufficiency and normal binocular children on CIRS symptom surveys. Optom Vis Sci. 1999, 76, 221–228. [Google Scholar] [CrossRef]
- Rouse, M.W.; Borsting, E.; Mitchell, G.L.; Scheiman, M.; Cotter, S.; Cooper, J.; et al. Validity and reliability of the revised convergence insufficiency symptom survey in adults. Ophthal Physiol Opt. 2004, 24, 384–390. [Google Scholar] [CrossRef]
- Birnbaum, M.H.; Soden, R.; Cohen, A.H. Efficacy of vision therapy for convergence insufficiency in an adult male population. J Am Optom Assoc. 1999, 70, 225–232. [Google Scholar]
- Scheiman, M.; Gwiazda, J.; Li, T. Non-surgical interventions for convergence insufficiency. Cochrane Database Syst Rev. 2011, CD006768, Epub 2011/03/18. [Google Scholar] [CrossRef] [PubMed]
- Convergence Insufficiency Treatment Trial Investigator Group. A randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008, 126, 1336–1349. [Google Scholar]
- Scheiman, M.; Mitchell, G.L.; Cotter, S.; Cooper, J.C.; Kulp, M.T.; Rouse, M.W.; et al. A randomized trial of the effectiveness of treatments for convergence insufficiency in children. Arch Ophthalmol. 2005, 123, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Mitchell, G.L.; Cotter, S.; Kulp, M.T.; Cooper, J.; Rouse, M.; et al. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005, 82, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Convergence Insufficency Investigator Group. A Randomized Clinical Trial of Treatment for Symptomatic Convergence Insufficiency in Children (CITT-ART). Optom Vis Sci. under review.
- CITT-ART Investigator Group. Treatment of Symptomatic Convergence Insufficiency in Children Enrolled in the Convergence Insufficiency Treatment Trial–Attention & Reading Trial: A Randomized Clinical Trial. Optom Vis Sci. 2019, 96, 825–835. [Google Scholar]
- Scheiman, M.; Talasan, H.; Alvarez, T.L. Objective Assessment of Disparity Vergence after Treatment of Symptomatic Convergence Insufficiency in Children. Optom Vis Sci. 2019, 96, 3–16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, T.L. A pilot study of disparity vergence and near dissociated phoria in convergence insufficiency patients before vs. after vergence therapy. Front Hum Neurosci. 2015, 9, 419, Epub 2015/08/19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Bahill, A.T.; Clark, M.R.; Stark, L. The main sequence, a tool for stdying human eye movements. Mathematical Bioscience. 1975, 24, 191–204. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Chen, T.; Alvarez, T.L. Quantitative assessment of divergence eye movements. J Vis. 2008, 8, 5, Epub 2008/10/04. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, T.L.; Semmlow, J.L.; Yuan, W.; Munoz, P. Dynamic details of disparity convergence eye movements. Ann Biomed Eng. 1999, 27, 380–390, Epub 1999/06/22. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Semmlow, J.L.; Yuan, W.; Alvarez, T.L. Short term modification of disparity vergence eye movements. Vision Res. 1999, 39, 1695–1705, Epub 1999/05/27. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, T.L.; Semmlow, J.L.; Yuan, W. Closely spaced, fast dynamic movements in disparity vergence. J Neurophysiol. 1998, 79, 37–44, Epub 1998/02/21. [Google Scholar] [CrossRef] [PubMed]
- Jones, R. Fusional vergence: sustained and transient components. Am J Optom Physiol Opt. 1980, 57, 640–644, Epub 1980/09/01. [Google Scholar] [CrossRef] [PubMed]
- Semmlow, J.L.; Hung, G.K.; Horng, J.L.; Ciuffreda, K. Initial control component in disparity vergence eye movements. Ophthalmic Physiol Opt. 1993, 13, 48–55, Epub 1993/01/01. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Vicci, V.R.; Han, S.J.; Alvarez, T.L. Sustained fixation induced changes in phoria and convergence peak velocity. PLoS ONE 2011, 6, e20883, Epub 2011/06/24. [Google Scholar] [CrossRef]
- Kim, E.H.; Vicci, V.R.; Granger-Donetti, B.; Alvarez, T.L. Short-term adaptations of the dynamic disparity vergence and phoria systems. Exp Brain Res. 2011, 212, 267–278, Epub 2011/05/20. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.K.; Bahill, A.T.; Stark, L. Parametric sensitivity analysis of a homeomorphic model for saccadic and vergence eye movements. Comput Programs Biomed. 1976, 6, 108–116, Epub 1976/07/01. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.M.; Talasan, H.; Mitchell, G.L.; Alvarez, T.L. Objective Assessment of Vergence after Treatment of Concussion-Related CI: A Pilot Study. Optom Vis Sci. 2017, 94, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Talasan, H.; Scheiman, M.; Li, X.; Alvarez, T.L. Disparity vergence responses before versus after repetitive vergence therapy in binocularly normal controls. J Vis. 2016, 16, 1–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, T.L.; Vicci, V.R.; Alkan, Y.; Kim, E.H.; Gohel, S.; Barrett, A.M.; et al. Vision therapy in adults with convergence insufficiency: clinical and functional magnetic resonance imaging measures. Optom Vis Sci. 2010, 87, 985–1002, Epub 2010/11/09. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rouse, M.; Borsting, E.; Mitchell, G.L.; Cotter, S.A.; Kulp, M.; Scheiman, M.; et al. Validity of the convergence insufficiency symptom survey: A confirmatory study. Optom Vis Sci. 2009, 86, 357–363. [Google Scholar] [CrossRef]
- Ranjbaran, M.; Galiana, H.L. Hybrid model of the context dependent vestibulo-ocular reflex: implications for vergence-version interactions. Front Comput Neurosci. 2015, 9, 6, Epub 2015/02/25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alvarez, T.L.; Kim, E.H. Analysis of saccades and peak velocity to symmetrical convergence stimuli: binocularly normal controls compared to convergence insufficiency patients. Invest Ophthalmol Vis Sci. 2013, 54, 4122–4135, Epub 2013/05/16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, E.H.; Alvarez, T.L. The frequency of horizontal saccades in near and far symmetrical disparity vergence. Vision Res. 2012, 63, 9–19, Epub 2012/05/12. [Google Scholar] [CrossRef] [PubMed]
- Zee, D.S.; Fitzgibbon, E.J.; Optican, L.M. Saccade-vergence interactions in humans. J Neurophysiol. 1992, 68, 1624–1641, Epub 1992/11/01. [Google Scholar] [CrossRef] [PubMed]
- Mays, L.E.; Porter, J.D.; Gamlin, P.D.; Tello, C.A. Neural control of vergence eye movements: neurons encoding vergence velocity. J Neurophysiol. 1986, 56, 1007–1021, Epub 1986/10/01. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Lee, Y.-Y.; Chen, T.; Semmlow, J.L.; Alvarez, T.L. Behaviors, Models and Clinical Applications of Vergence Eye Movements. J of Med and Biological Engineering 2010, 1, 1–15. [Google Scholar]
- Semmlow, J.L.; Yuan, W.; Alvarez, T.L. Short-term adaptive control processes in vergence eye movement. Curr Psychol Cognition. 2002, 21, 343–375. [Google Scholar]
- Daum, K.M. A comparison of the results of tonic and phasic vergence training. Am J Optom Physiol Opt. 1983, 60, 769–775. [Google Scholar] [CrossRef]
- Yuan, W.; Semmlow, J.L.; Alvarez, T.L.; Munoz, P. Dynamics of the disparity vergence step response: a model-based analysis. IEEE Trans Biomed Eng. 1999, 46, 1191–1198, Epub 1999/10/08. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Cotter, S.; Kulp, M.T.; Mitchell, G.L.; Cooper, J.; Gallaway, M.; et al. Treatment of accommodative dysfunction in children: results from a randomized clinical trial. Optom Vis Sci. 2011, 88, 1343–1352, Epub 2011/08/30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jainta, S.; Bucci, M.P.; Wiener-Vacher, S.; Kapoula, Z. Changes in vergence dynamics due to repetition. Vision Res. 2011, 51, 1845–1852, Epub 2011/07/13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Semmlow, J.L. The influence of repetitive eye movements on vergence performance. Vision Res. 2000, 40, 3089–3098, Epub 2000/09/21. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, T.L.; Gayed, B.A.; Semmlow, J.L. (Eds.) Neural control of divergence eye movements. Proc. (Vol. 2). The 10th World Multi-Conference on Systemics, Cybernetics and Informatics, Jointly with the 12th International Conference on Information Systems Analysis and Synthesis ISAS 2006, 2006. [Google Scholar]
- Yaramothu, C.; Alvarez, T.L. (Eds.) Short-term modification of vergence ramp eye movements in the convergent direction. Proceedings of the IEEE Annual Northeast Bioengineering Conference, NEBEC; 2014: NEBEC (Vol. 2014-Decem).
- Alvarez, T.L.; Semmlow, J.L.; Pedrono, C. Dynamic assessment of disparity vergence ramps. Comput Biol Med. 2007, 37, 903–909, Epub 2006/06/02. [Google Scholar] [CrossRef] [PubMed]
- Lestrange, S.; Talasan, H.; Alvarez, T.L. (Eds.) Repetitive vergence training improves precision. Proceedings of the IEEE Annual Northeast Bioengineering Conference, NEBEC, 2014. [Google Scholar]
- Brautaset, R.L.; Jennings, J.A. Horizontal and vertical prism adaptation are different mechanisms. Ophthalmic Physiol Opt. 2005, 25, 215–218, Epub 2005/04/28. [Google Scholar] [CrossRef] [PubMed]
- Schor, C. Fixation of disparity: a steady state error of disparity-induced vergence. Am J Optom Physiol Opt. 1980, 57, 618–631, Epub 1980/09/01. [Google Scholar] [CrossRef] [PubMed]
| Function | Mean Pre-VT | Mean Post VT | Mean Change | Sig | Cohen’s d Effect Size | Confidence Interval |
|---|---|---|---|---|---|---|
| CISS | 35.8 | 11.2 | 24.6 | P=0.002 | 2.84 | 17.9 to 31.4 |
| Cover Test (Distance) (Δ)* | -0.8 | -0.5 | 0.3 | P=0.16 | 0.16 | -0.8 to 0.2 |
| Cover Test (Near) (Δ)* | -9.2 | -7.3 | 1.8 | P=0.03 | 0.43 | -3.2 to -0.4 |
| Base-in Break (Δ) | 14.8 | 19.6 | 4.8 | P=0.02 | 0.74 | -8.1 to -1.4 |
| Base-out Break (Δ) | 11.8 | 38.3 | 26.6 | P=0.002 | 3.59 | -32.9 to -20.2 |
| Near point of convergence Break (cm) | 16.5 | 3.6 | 12.9 | P=0.002 | 4.03 | 10.2 to 15.6 |
| Accommodation Amplitude Right Eye (cm) | 14.8 | 8.5 | 6.3 | P=0.003 | 1.94 | 3.7 to 8.9 |
| Vergence Facility (FPM) | 8.7 | 34.8 | 22.4 | P=0.003 | 2.56 | 11.6 to 30.8 |
| Monocular Accommodative Facility (FPM) | 7.4 | 28.3 | 20.9 | P=0.001 | 2.89 | 15.4 to 26.3 |
| 4° Symmetrical Convergence | ||||||
| Function | Mean Pre OBVAT | Mean Post OBVAT | Mean Change | Sig | Cohen’s d Effect Size | Confidence Interval |
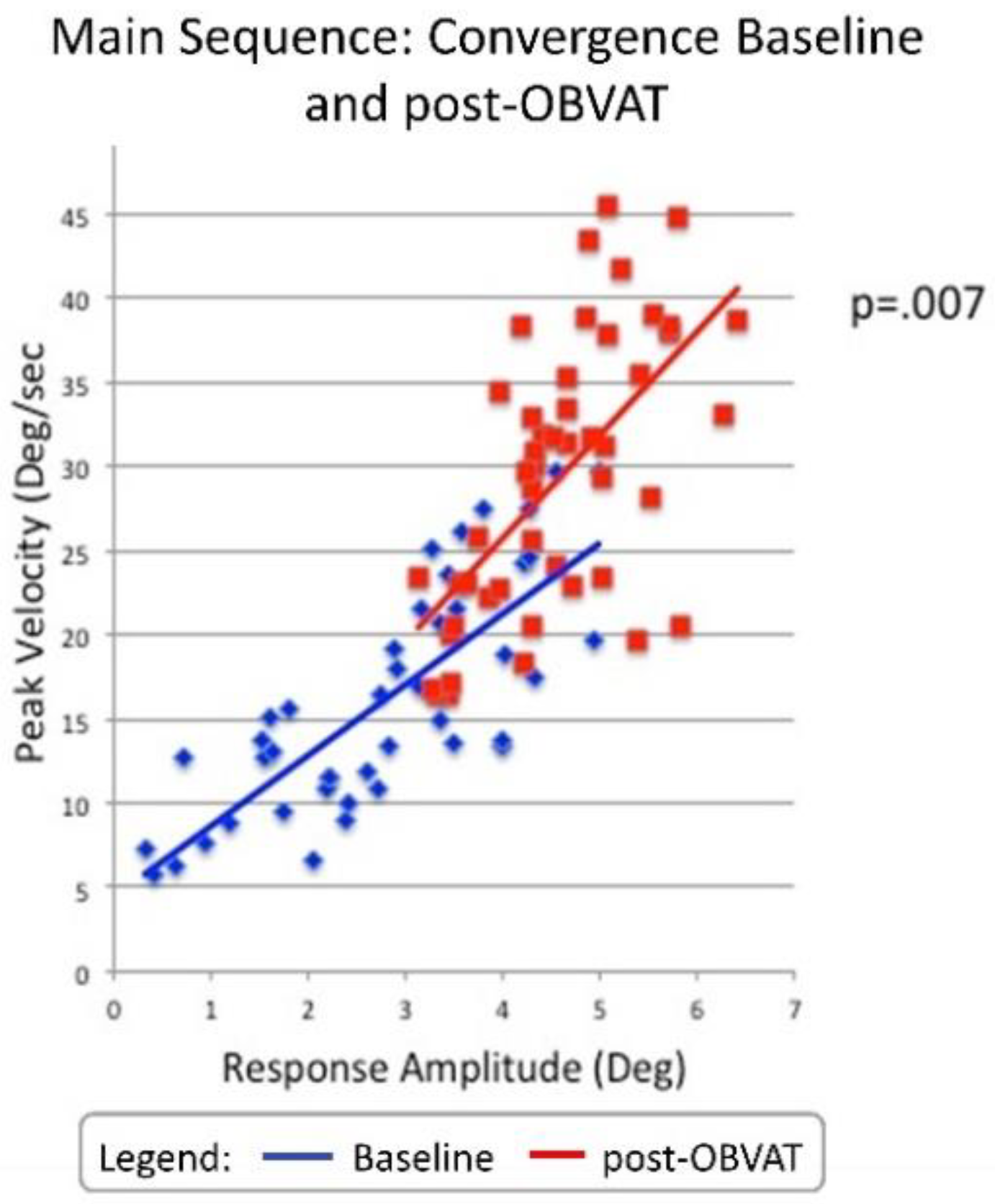
| Peak Velocity (°/sec) | 14.7 | 26.1 | 11.4 | P=<0.001 | 1.48 | 8.3 to 15.0 |
| Time to Peak Velocity(sec) | 0.50 | 0.40 | 0.10 | P=<0.001 | 0.99 | .07 to .17 |
| Response Amplitude (°) | 2.6 | 4.1 | 1.5 | P=<0.001 | 1.31 | 1.0 to 1.9 |
| Latency (sec) | 0.25 | 0.22 | 0.03 | P=0.13 | 0.31 | -01 to .07 |
| 6° Symmetrical Convergence | ||||||
| Peak Velocity (°/sec) | 14.7 | 26.1 | 11.4 | P=<0.001 | 1.46 | 8.0 to 14.6 |
| Time to Peak Velocity(sec) | 0.50 | 0.40 | 0.10 | P=0.01 | 0.51 | 0.05 to 0.15 |
| Response Amplitude (°) | 2.6 | 4.1 | 1.5 | P=<0.001 | 1.15 | 1.0 to 1.9 |
| Latency (sec) | 0.23 | 0.25 | 0.02 | P=0.31 | 0.12 | -0.08 to 0.02 |
| 4° Symmetrical Divergence | ||||||
| Function | Mean Pre OBVAT | Mean Post OBVAT | Mean Change | Sig | Cohen’s d Effect Size | Confidence Interval |
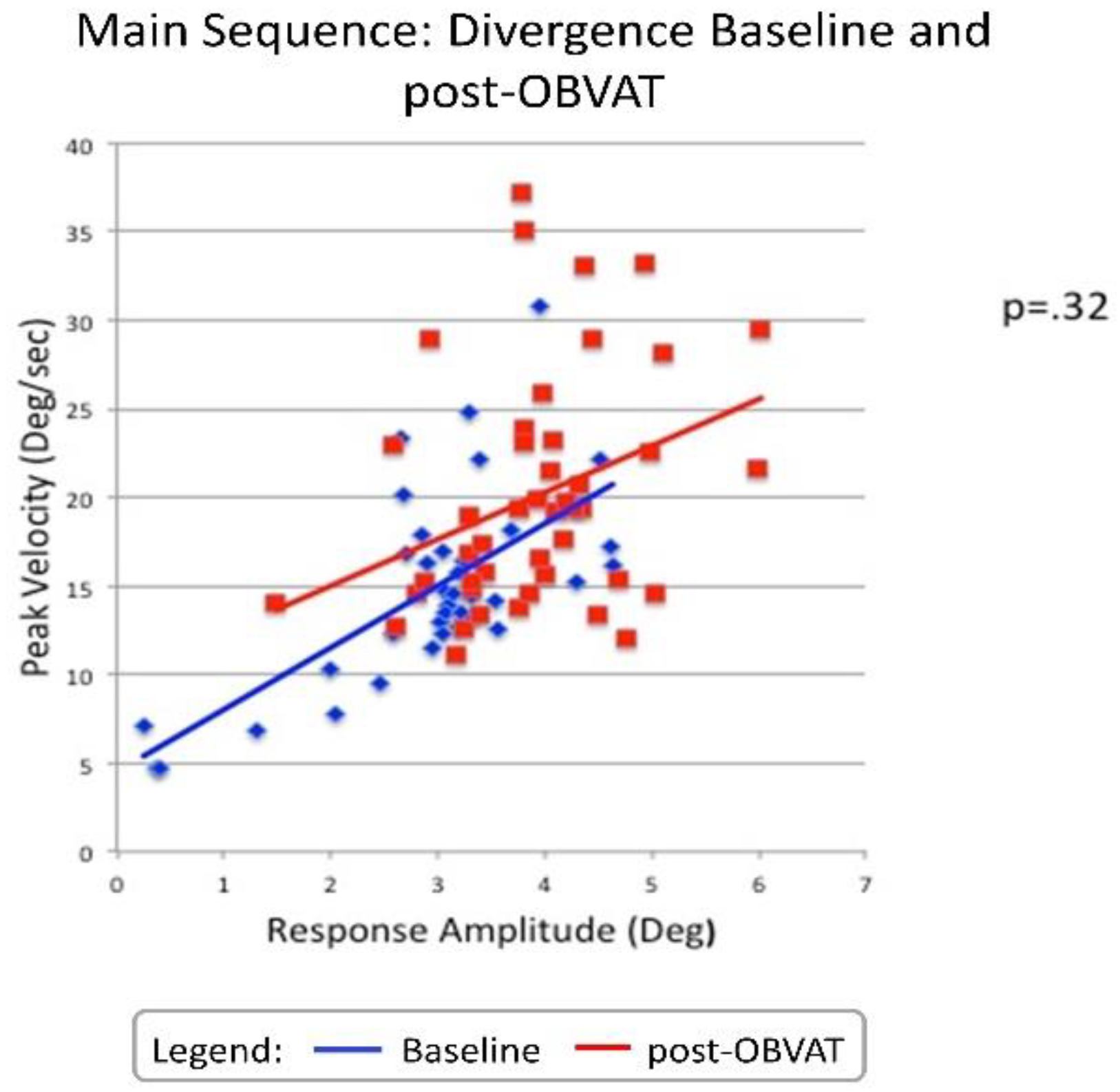
| Peak Velocity (°/sec) | 13.5 | 19.0 | 5.5 | P=,01 | 0.62 | 1.4 to 9.6 |
| Time to Peak Velocity(sec) | 0.43 | 0.40 | 0.03 | P=.25 | 0.27 | -0.03 to 0.09 |
| Response Amplitude (°) | 2.7 | 3.6 | 0.9 | P=.006 | 0.69 | -0.3 to 1.5 |
| Latency (sec) | 0.21 | 0.23 | 0.02 | P=0.46 | 0.16 | -0.8 to 0.03 |
| 6° Symmetrical Divergence | ||||||
| Peak Velocity (°/sec) | 15.8 | 20.3 | 4.5 | P=0.02 | 0.60 | 0.8 to 8.2 |
| Time to Peak Velocity(sec) | 0.53 | 0.56 | 0.03 | P=0.28 | 0.26 | 0.05 to 0.15 |
| Response Amplitude (°) | 3.1 | 4.3 | 1.2 | P=0.001 | 0.98 | 0.6 to 1.9 |
| Latency (sec) | 0.20 | 0.21 | 0.01 | P=0.17 | 0.33 | -0.03 to 0.01 |
| 5° Saccades | ||||||
| Function | Mean Pre OBVAT | Mean Post OBVAT | Mean Change | Sig | Cohen’s d Effect Size | Confidence Interval |
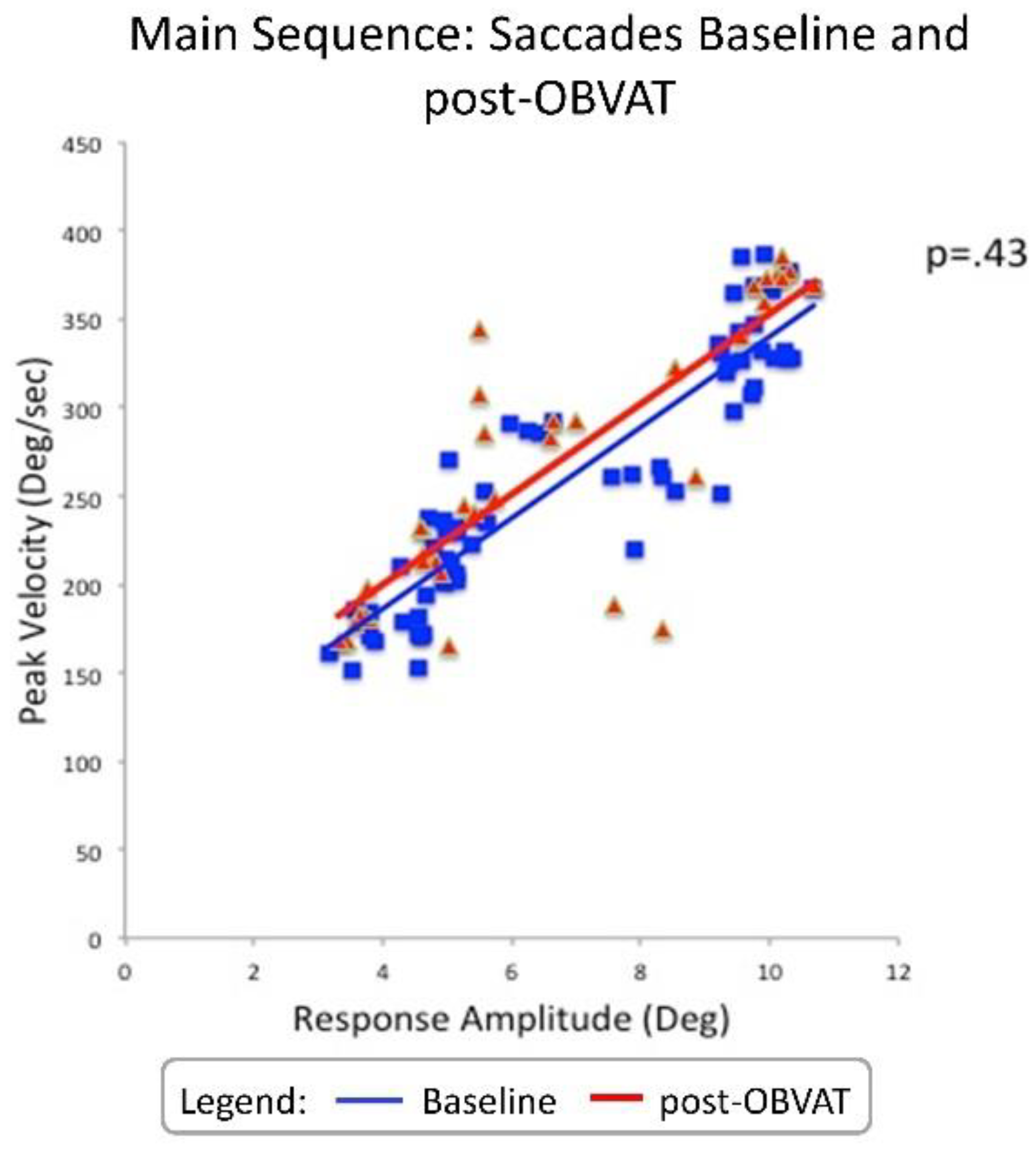
| Peak Velocity (°/sec) | 202.5 | 212.8 | 10.3 | P=.12 | 0.49 | -23.7 to 3.1 |
| Time to Peak Velocity(sec) | 0.24 | 0.27 | 0.03 | P=.35 | 0.28 | -0.09 to 0.03 |
| Response Amplitude (°) | 4.6 | 4.7 | 0.1 | P=.46 | 0.22 | -0.4 to 0.2 |
| Latency (sec) | 0.23 | 0.22 | 0.01 | P=.38 | 0.26 | -0.01 to 0.03 |
| 10° Saccades | ||||||
| Peak Velocity (°/sec) | 312.1 | 312.0 | 0.1 | P=0.99 | 0.003 | -24.9 to 25.1 |
| Time to Peak Velocity(sec) | 0.23 | 0.23 | 0.01 | P=0.38 | 0.27 | 0.01 to 0.03 |
| Response Amplitude (°) | 9.0 | 8.8 | 0.2 | P=0.68 | 0.14 | -0.4 to 0.6 |
| Latency (sec) | 0.21 | 0.21 | 0.01 | P=0.55 | 0.18 | -0.01 to 0.02 |
Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Scheiman, M.; Yaramothu, C.; Alvarez, T.L. Changes in the Disparity Vergence Main Sequence After Treatment of Symptomatic Convergence Insufficiency in Children. J. Eye Mov. Res. 2019, 12, 1-10. https://doi.org/10.16910/jemr.12.4.6
Scheiman M, Yaramothu C, Alvarez TL. Changes in the Disparity Vergence Main Sequence After Treatment of Symptomatic Convergence Insufficiency in Children. Journal of Eye Movement Research. 2019; 12(4):1-10. https://doi.org/10.16910/jemr.12.4.6
Chicago/Turabian StyleScheiman, Mitchell, Chang Yaramothu, and Tara L. Alvarez. 2019. "Changes in the Disparity Vergence Main Sequence After Treatment of Symptomatic Convergence Insufficiency in Children" Journal of Eye Movement Research 12, no. 4: 1-10. https://doi.org/10.16910/jemr.12.4.6
APA StyleScheiman, M., Yaramothu, C., & Alvarez, T. L. (2019). Changes in the Disparity Vergence Main Sequence After Treatment of Symptomatic Convergence Insufficiency in Children. Journal of Eye Movement Research, 12(4), 1-10. https://doi.org/10.16910/jemr.12.4.6



