Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Fennel Seeds
2.2. Sprouting of F. vulgare and Preparation of Aqueous and Ethanolic Extracts
2.3. Determination of Total Phenolic Content (TPC), Total Flavonoids (TF), and Total Flavonols (TFL) in FS and FSS
2.4. Antioxidant Capacity Determination
2.5. Quantification of Phenolic Compounds in F. vulgare and Its Sprouts by HPLC-DAD
2.6. Quantification of Volatile Components by GC-MS
2.7. Animals and Experimental Design
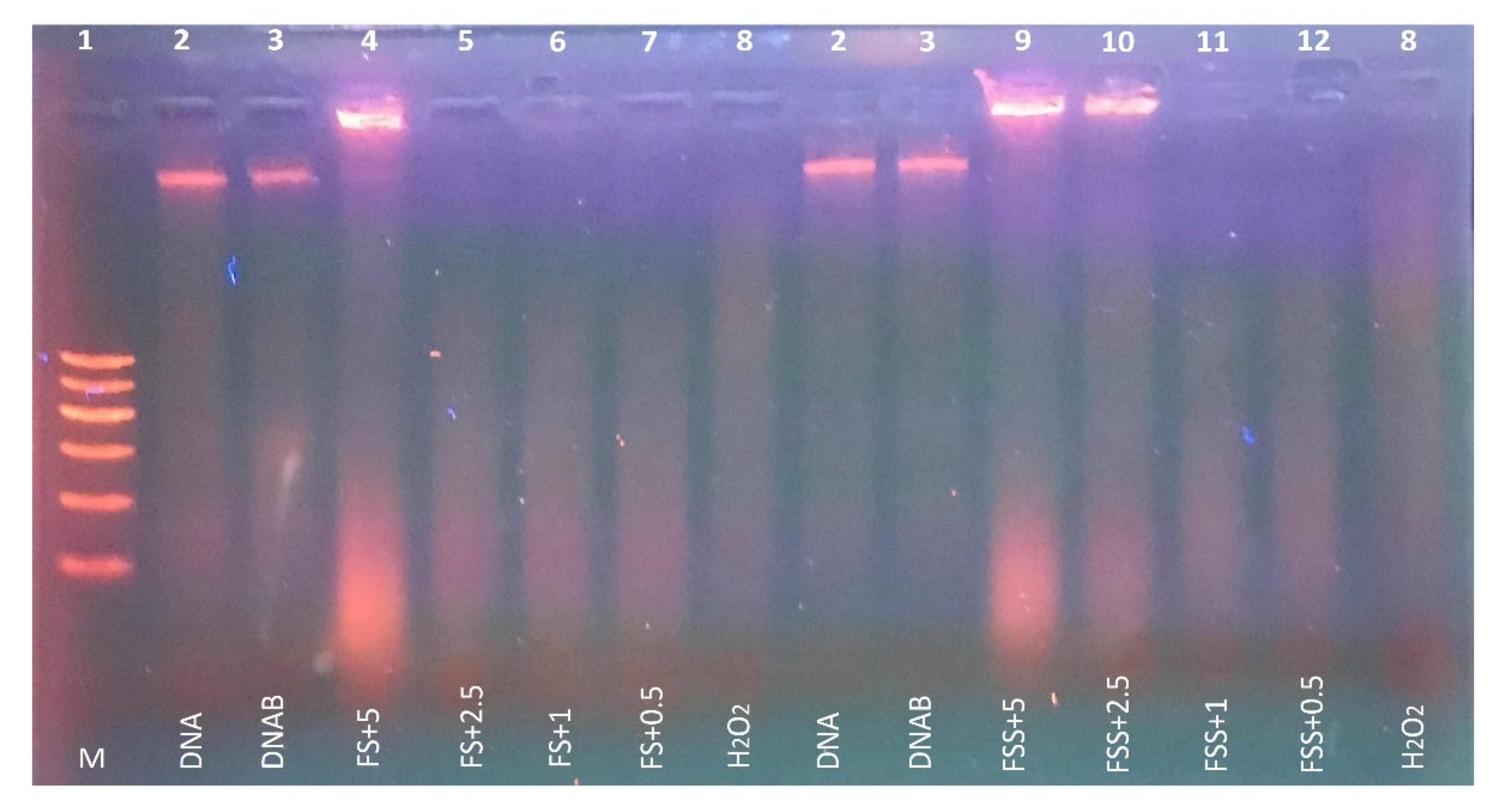
2.7.1. Protective Effect of FS and FSS Extracts against H2O2-Induced DNA Damage
2.7.2. Determination of Liver’s Functions
2.7.3. Oxidative Stress Biomarkers
2.8. Statistical Analysis
3. Results
3.1. Phytochemicals and Antioxidant Activity of F. vulgare Sprouts
3.2. Quantification of Phenolic Compounds in FS and FSS
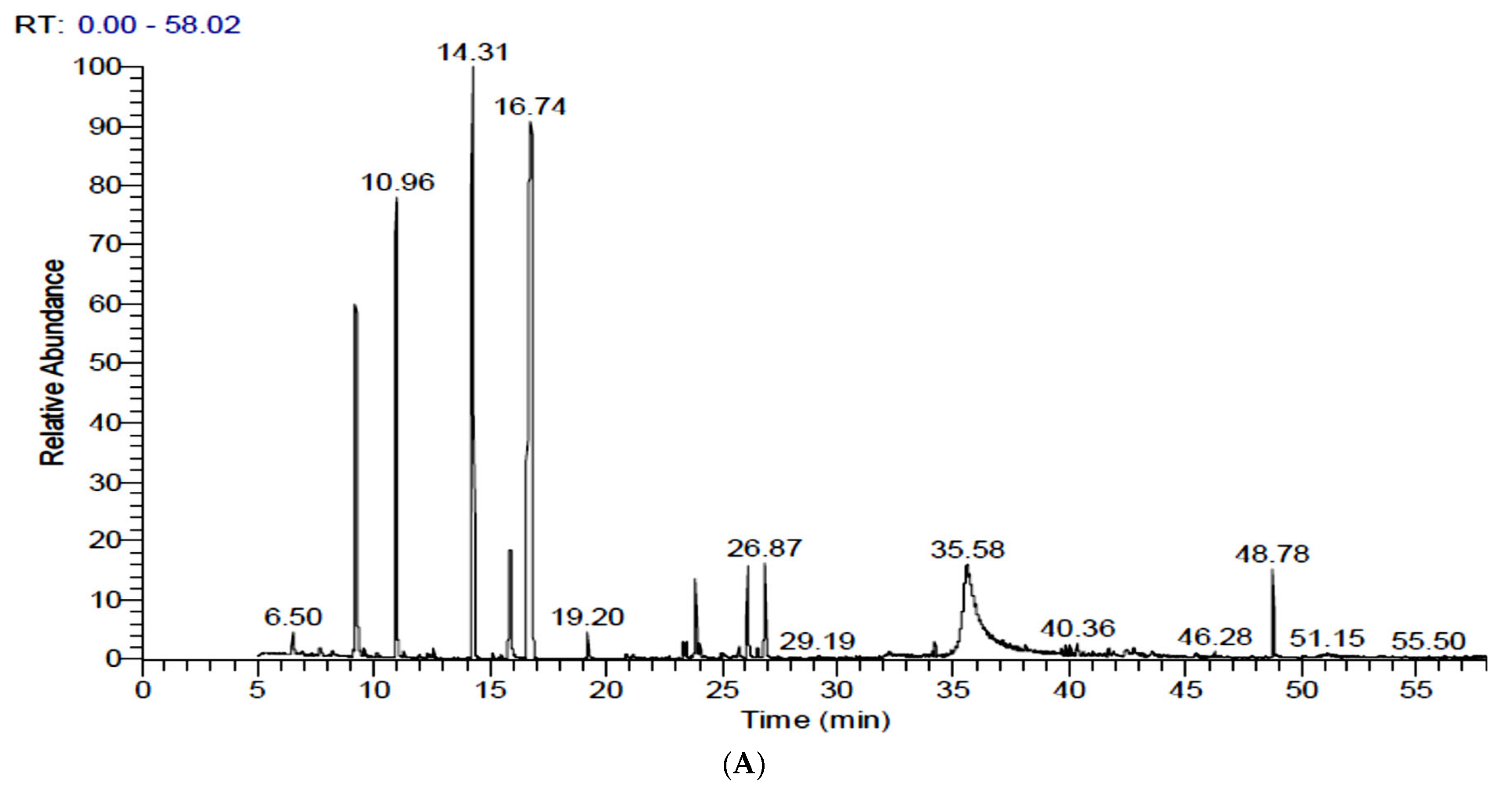
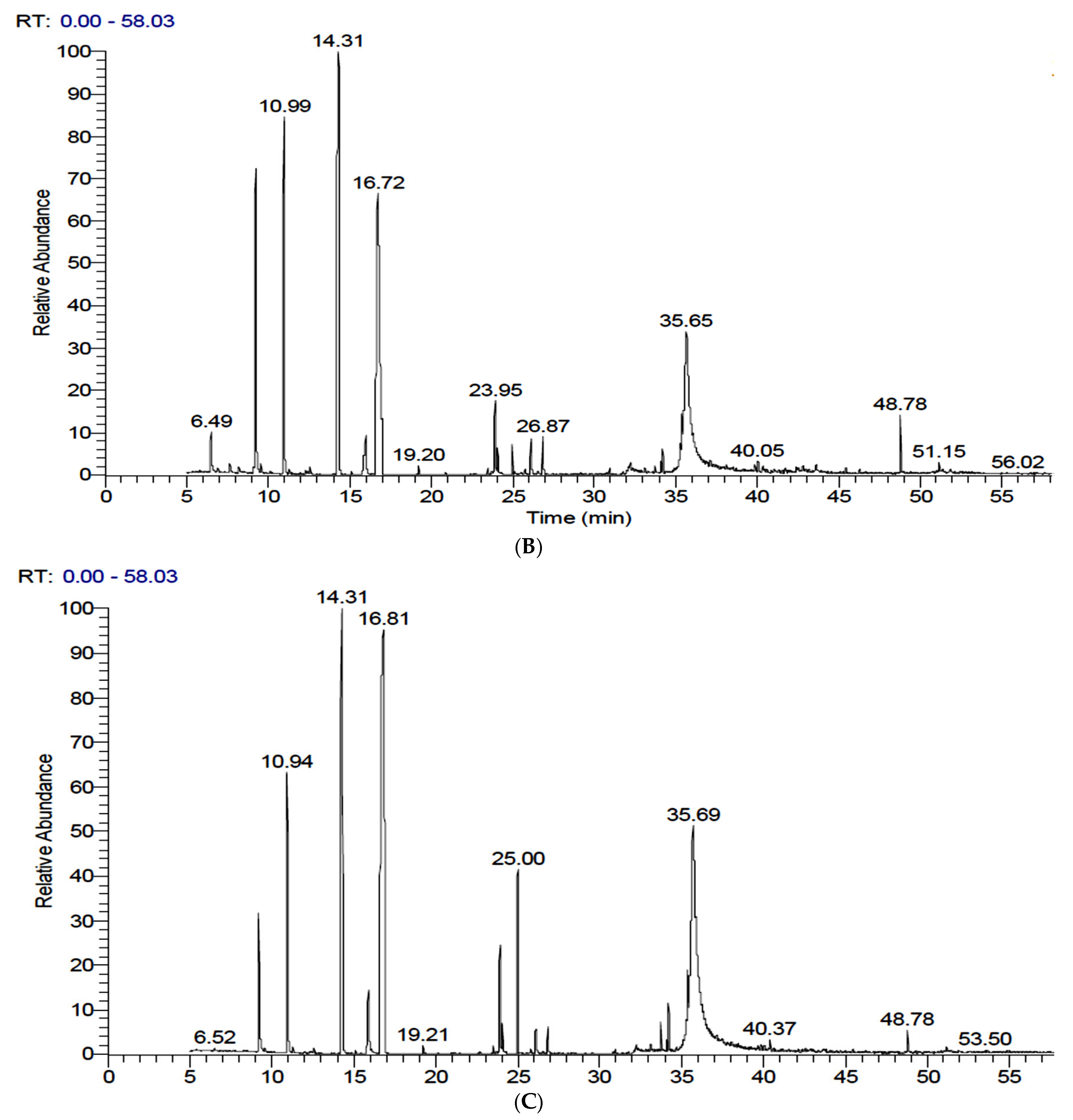
3.3. Identification and Quantification of Volatiles in FS and FSS by GC-MS
3.4. Protective Effect of FS and FSS Extracts on DNA Damage
3.5. The Liver’s Functions
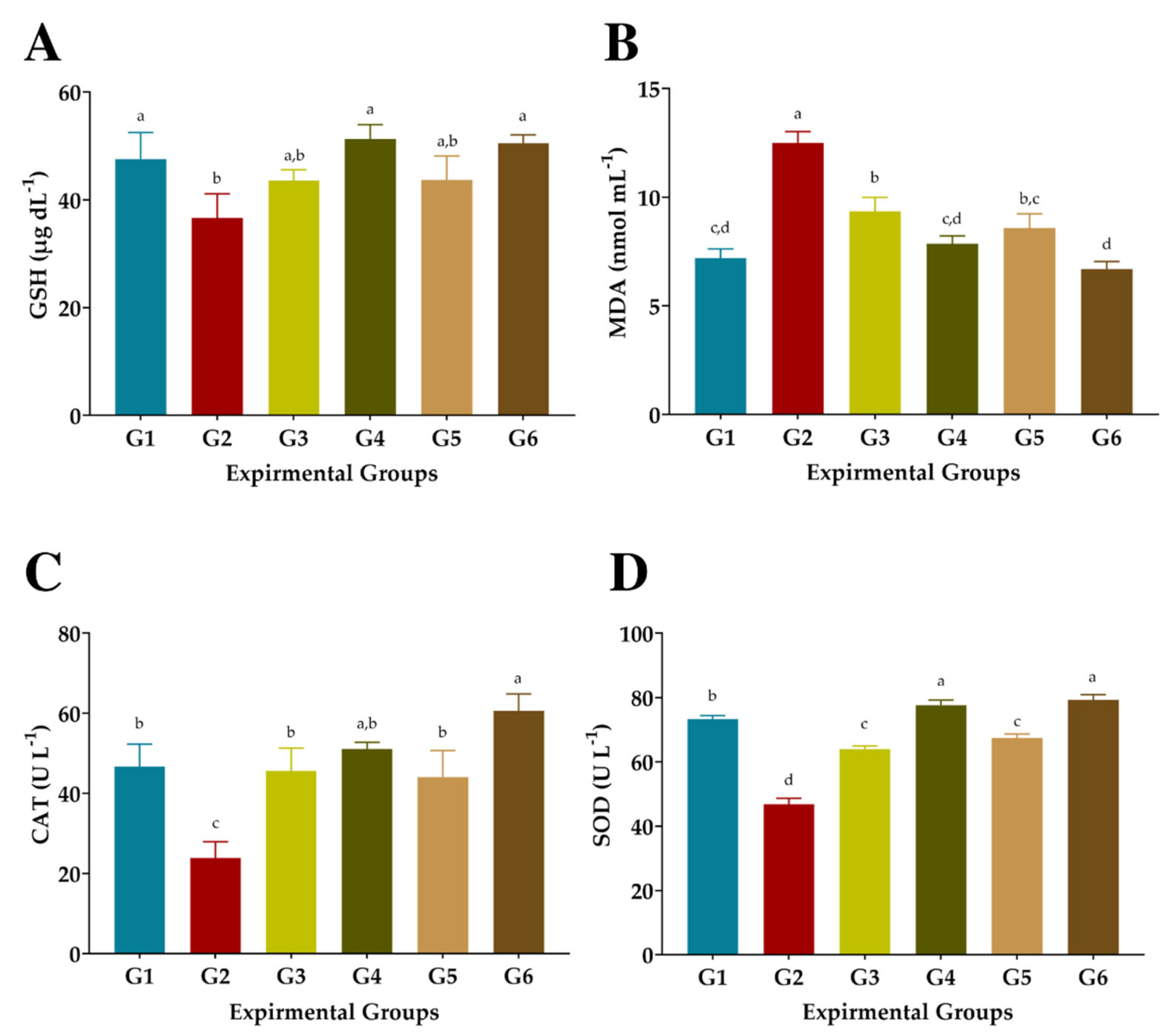
3.6. Antioxidant Biomarkers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Romá-Mateo, C.; Aguado, C.; García-Giménez, J.L.; Ibáñez-Cabellos, J.S.; Seco-Cervera, M.; Pallardó, F.V.; Knecht, E.; Sanz, P. Increased Oxidative Stress and Impaired Antioxidant Response in Lafora disease. Mol. Neurobiol. 2015, 51, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Reactive Oxygen Species and Cellular Defense System. In Free Radicals in Human Health and Disease, 1st ed.; Rani, V., Yadav, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and Oxidative Stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar]
- Jiménez-Fernández, S.; Gurpegui, M.; Díaz-Atienza, F.; Pérez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients with Major Depressive Disorder Compared to Healthy Controls Before and after Antidepressant Treatment: Results from A Meta-Analysis. J. Clin. Psychiat. 2015, 76, 1658–1667. [Google Scholar] [CrossRef]
- Moreno, D.A.; Pérez-Balibrea, S.; García-Viguera, C. Phytochemical Quality and Bioactivity of Edible Sprouts. Nat. Prod. Commun. 2006, 1, 1934578X0600101120. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the Nutritional, Functional and Technological Effects of the Sourdough Fermentation of Sprouted Flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Peñas, E.; Martínez-Villaluenga, C. Advances in Production, Properties and Applications of Sprouted Seeds. Foods 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, R.F.; Lopes, J.d.O.; Antunes, A.E.C. The Use of Sprouts to Improve the Nutritional Value of Food Products: A Brief Review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Gawlik-Dziki, U. Effects of Sprouting and Postharvest Storage under Cool. Temperature Conditions on Starch Content and Antioxidant Capacity of Green Pea, Lentil and Young Mung Bean Sprouts. Food Chem. 2015, 185, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Gawlik-Dziki, U.; Kowalczyk, D.; Złotek, U. Impact of Germination Time and Type of Illumination on the Antioxidant Compounds and Antioxidant Capacity of Lens culinaris Sprouts. Sci. Hortic. 2012, 140, 87–95. [Google Scholar] [CrossRef]
- Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. [Google Scholar] [CrossRef]
- Shojaiefar, S.; Sabzalian, M.R.; Mirlohi, A.; Mirjalili, M.H. Seed Yield Stability with Modified Essential Oil Content and Composition in Self-Compatible Progenies of Bitter Fennel (Foeniculum vulgare Mill.). Ind. Crops Prod. 2022, 182, 114821. [Google Scholar] [CrossRef]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef]
- Yang, I.J.; Lee, D.U.; Shin, H.M. Anti-Inflammatory and Antioxidant effects of Coumarins Isolated From Foeniculum vulgare in Lipopolysaccharide-Stimulated Macrophages and 12-O-Tetradecanoylphorbol-13-Acetate-Stimulated Mice. Immunopharmacol. Immunotoxicol. 2015, 37, 308–317. [Google Scholar] [CrossRef]
- Gerhke, S.A.; Shibli, J.A.; Salles, M.B. Potential of the Use of An Antioxidant Compound to Promote Peripheral Nerve Regeneration After Injury. Neural. Regen. Res. 2015, 10, 1673–5374. [Google Scholar]
- Bhatti, S.; Ali Shah, S.A.; Ahmed, T.; Zahid, S. Neuroprotective Effects of Foeniculum vulgare Seeds Extract on Lead-Induced Neurotoxicity in Mice Brain. Drug Chem. Toxicol. 2018, 41, 399–407. [Google Scholar] [CrossRef]
- Joshi, H.; Parle, M. Cholinergic Basis of Memory-Strengthening Effect of Foeniculum vulgare Linn. J. Med. Food 2006, 9, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Koppula, S.; Kumar, H. Foeniculum vulgare Mill (Umbelliferae) Attenuates Stress and Improves Memory in Wister Rats. Trop. J. Pharm. Res. 2013, 12, 553–558. [Google Scholar] [CrossRef]
- Imran, A.; Xiao, L.; Ahmad, W.; Anwar, H.; Rasul, A.; Imran, M.; Aziz, N.; Razzaq, A.; Arshad, M.U.; Shabbir, A.; et al. Foeniculum vulgare (Fennel) Promotes Functional Recovery and Ameliorates Oxidative Stress Following A Lesion to the Sciatic Nerve in Mouse Model. J. Food Biochem. 2019, 43, e12983. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.; Tamta, G.; Nand, V.; Singh, J.P. Nutritional Profiling, Antibacterial Potential, and Cluster Analysis in Foeniculum vulgare Seeds Against Human Pathogenic Bacteria. J. Food Process. Preserv. 2022, 46, e16763. [Google Scholar] [CrossRef]
- Kalleli, F.; Bettaieb Rebey, I.; Wannes, W.A.; Boughalleb, F.; Hammami, M.; Saidani Tounsi, M.; M’Hamdi, M. Chemical Composition and Antioxidant Potential of Essential Oil and Methanol. Extract from Tunisian and French Fennel (Foeniculum vulgare Mill.) seeds. J. Food Biochem. 2019, 43, e12935. [Google Scholar] [CrossRef]
- Burkhardt, A.; Sintim, H.Y.; Gawde, A.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Schlegel, V. Method for Attaining Fennel (Foeniculum vulgare Mill.) Seed Oil Fractions with Different Composition and Antioxidant Capacity. J. Appl. Res. Med. Aromat. Plants 2015, 2, 87–91. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Bouhlali, E.d.T.; Derouich, M.; El-Rhaffari, L. Essential Oil and Chemical Composition of Wild and Cultivated Fennel (Foeniculum vulgare Mill.): A Comparative Study. S. Afr. J. Bot 2020, 135, 93–100. [Google Scholar] [CrossRef]
- Smoum, R.; Haj, C.; Hirsch, S.; Nemirovski, A.; Yekhtin, Z.; Bogoslavsky, B.; Bakshi, G.K.; Chourasia, M.; Gallily, R.; Tam, J.; et al. Fenchone Derivatives as a Novel Class of CB2 Selective Ligands: Design, Synthesis, X-ray Structure and Therapeutic Potential. Molecules 2022, 27, 1382. [Google Scholar] [CrossRef]
- Dobrikov, G.M.; Valcheva, V.; Nikolova, Y.; Ugrinova, I.; Pasheva, E.; Dimitrov, V. Enantiopure antituberculosis candidates synthesized from (-)-fenchone. Eur. J. Med. Chem. 2014, 77, 243–247. [Google Scholar] [CrossRef]
- Keskin, I.; Gunal, Y.; Ayla, S.; Kolbasi, B.; Sakul, A.; Kilic, U.; Gok, O.; Koroglu, K.; Ozbek, H. Effects of Foeniculum vulgare Essential Oil Compounds, Fenchone and Limonene, on Experimental Wound Healing. Biotech. Histochem. 2017, 92, 274–282. [Google Scholar] [CrossRef]
- Chang, W.; An, J.; Seol, G.H.; Han, S.H.; Yee, J.; Min, S.S. Trans-Anethole Alleviates Trimethyltin Chloride-Induced Impairments in Long-Term Potentiation. Pharmaceutics 2022, 14, 1422. [Google Scholar] [CrossRef] [PubMed]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef] [PubMed]
- Odeh, A.; Allaf, A.W. Determination of Polyphenol. Component Fractions and Integral Antioxidant Capacity of Syrian Aniseed and Fennel Seed Extracts Using Gc–Ms, Hplc Analysis, and Photochemiluminescence Assay. Chem. Pap. 2017, 71, 1731–1737. [Google Scholar] [CrossRef]
- Alam, P.; Abdel-Kader, M.S.; Alqarni, M.H.; Zaatout, H.H.; Ahamad, S.R.; Shakeel, F. Chemical Composition of Fennel Seed Extract and Determination of Fenchone in Commercial Formulations by GC–MS Method. J. Food Sci.Technol. 2019, 56, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Spielvogel, A.; Hassan, M.; El-Desouky, A.; El-Mansy, H.; Rath, F.; Meyer, V.; Stahl, U. The Antifungal Protein AFP from Aspergillus giganteus Prevents Secondary Growth of Different Fusarium Species on Barley. Appl. Microbiol. Biotechnol. 2010, 87, 617–624. [Google Scholar] [CrossRef]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Saavedra, D.; Pérez-Ramírez, I.F.; Ramos-Gómez, M.; Mendoza-Díaz, S.; Loarca-Piña, G.; Reynoso-Camacho, R. Phytochemical Characterization and Effect of Calendula officinalis, Hypericum perforatum, and Salvia officinalis Infusions on Obesity-Associated Cardiovascular Risk. Med. Chem. Res. 2016, 25, 163–172. [Google Scholar] [CrossRef]
- Yawadio Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant Activity of Various Extracts and Fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Mohdaly, A.A.A.; Hassanien, M.F.R.; Mahmoud, A.; Sarhan, M.A.; Smetanska, I. Phenolics Extracted from Potato, Sugar Beet, and Sesame Processing By-Products. Int. J. Food Prop. 2012, 16, 1148–1168. [Google Scholar] [CrossRef]
- Barakat, H.; Rohn, S. Effect of Different Cooking Methods on Bioactive Compounds in Vegetarian, Broccoli-based Bars. J. Funct. Foods 2014, 11, 407–416. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid Profiles and Antioxidant Activities of Wheat Bran Extracts and the Effect of Hydrolysis Conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition ad HOC Writing Committee on the Reformulation of the AIN-76a Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Barakat, H.; Almundarij, T.I. Phenolic Compounds and Hepatoprotective Potential of Anastatica hierochuntica Ethanolic and Aqueous Extracts Against CCl4-Induced Hepatotoxicity in rats. J. Tradit. Chin. Med. 2020, 40, 947–955. [Google Scholar]
- Moradabadi, L.; Montasser Kouhsari, S.; Fehresti Sani, M. Hypoglycemic Effects of Three Medicinal Plants in Experimental Diabetes: Inhibition of Rat Intestinal α-glucosidase and Enhanced Pancreatic Insulin and Cardiac Glut-4 mRNAs Expression. Iran. J. Pharm. Sci. 2013, 12, 387–397. [Google Scholar]
- Arseneau, J.R.; Steeves, R.; Laflamme, M. Modified Low-Salt CTAB Extraction of High-Quality DNA from Contaminant-Rich Tissues. Mol. Ecol. Resour. 2016, 17, 686–693. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, E.K.; Hwang, J.W.; Oh, H.J.; Cheong, S.H.; Moon, S.H.; Jeon, B.T.; Lee, S.M.; Park, P.J. Purification and Characterisation of an Antioxidative Peptide from Enzymatic Hydrolysates of Duck Processing By-Products. Food Chem. 2010, 123, 216–220. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Mirmiran, P.; Bahadoran, Z.; Azizi, F. Functional Foods-Based Diet as a Novel Dietary Approach for Management of Type 2 Diabetes and its Complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef]
- Eva, Y.; Annisa, A.; Andrafikar. Effectiveness of Jicama Probiotic Yoghurt (Pachyrhizus erosus) on Blood Glucose in Diabetic Mice. KnE. Life Sci. 2019, 4, 250–261. [Google Scholar] [CrossRef]
- Hasanein, P.; Felehgari, Z.; Emamjomeh, A. Preventive effects of Salvia officinalis L. Against Learning and Memory Deficit Induced by Diabetes in Rats: Possible Hypoglycaemic and Antioxidant Mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and Free Radical Scavenging Activity of H. officinalis L. var. Angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar]
- Xu, B.; Chang, S.K.C. Phenolic Substance Characterization and Chemical and Cell-Based Antioxidant Activities of 11 Lentils Grown in the Northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Free Radical-scavenging Capacity, Antioxidant Activity, and Phenolic Composition of Green Lentil (Lens culinaris). Food Chem. 2010, 121, 705–711. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; De Pascale, S.; Narváez, A.; Rodriguez-Carrasco, Y.; Ritieni, A. Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Polyphenolic Compounds from Nutraceutical Fennel Waste Extract. Molecules 2021, 26, 1968. [Google Scholar] [CrossRef]
- Vadivel, V.; Biesalski, H.K. Effect of Certain Indigenous Processing Methods on The Bioactive Compounds of Ten Different Wild Type Legume Grains. J. Food Sci. Technol. 2012, 49, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts During their Growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of Caffeic Acid in Peanut Sprouts and Evaluation of Its In Vitro Effectiveness Against Oxidative Stress-Induced Erythrocyte Hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea Fennel (Crithmum Maritimum L.): Phytochemical Profile, Antioxidative, Cholinesterase Inhibitory and Vasodilatory Activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Zaki, M.F. Antioxidant Activities of Phenolics, Flavonoids and Vitamin C in two Cultivars of Fennel (Foeniculum vulgare Mill.) in Responses to Organic and Bio-Organic Fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare Mill.) Seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Faudale, M.; Viladomat, F.; Bastida, J.; Poli, F.; Codina, C. Antioxidant Activity and Phenolic Composition of Wild, Edible, and Medicinal Fennel from Different Mediterranean Countries. J. Agric. Food Chem. 2008, 56, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Giambanelli, E.; D’Antuono, L.F. Fennel (Foeniculum vulgare Mill. Subsp. piperitum) Florets, A Traditional Culinary Spice in Italy: Evaluation of Phenolics and Volatiles in Local Populations, and Comparison with the Composition of Other Plant Parts. J. Sci. Food Agric. 2017, 97, 5369–5380. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Galván-D’Alessandro, L.; Vandendriessche, P.; Chollet, S. Effect of Germination and Fermentation Process on the Antioxidant Compounds of Quinoa Seeds. Plant Foods Hum. Nutr. 2016, 71, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, T.; Sun, Y.; Zhang, M.; Tian, J.; Chen, H.; Shen, Z.; Khan Abro, H.; Su, N.; Cui, J. Protective Effect of Selenium-Enriched Red Radish Sprouts on Carbon Tetrachloride-Induced Liver Injury in Mice. J. Food Sci. 2019, 84, 3027–3036. [Google Scholar] [CrossRef]
- López-Amorós, M.L.; Hernández, T.; Estrella, I. Effect of Germination on Legume Phenolic Compounds and their Antioxidant Activity. J. Food Compos. Anal. 2006, 19, 277–283. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare L.) and Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Singh, A.k.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.H.; El-Bastawesy, A.M.; Abdel-Monem, M.G.; Noor, A.M.; Al-Mehdar, H.A.R.; Sharawy, S.M.; El-Merzabani, M.M. Antioxidant and Anticarcinogenic Effects of Methanolic Extract and Volatile Oil of Fennel Seeds (Foeniculum vulgare). J. Med. Food 2011, 14, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Allaithy, S.A. Chemical Compound of Cumin and Fennel Seed Extracts Against Some Types of Pathogenic Bacteria. Iraq Med. J. 2017, 1, 1–6. [Google Scholar]
- Suleiman, W.B.; Helal, E.E.-H. Chemical Constituents and Potential Pleiotropic Activities of Foeniculum vulgare (Fennel) Ethanolic Extract; In Vitro Approach. Egypt. J. Chem. 2022, 65, 5–6. [Google Scholar] [CrossRef]
- Ilardi, V.; Badalamenti, N.; Bruno, M. Chemical Composition of the Essential Oil From Different Vegetative Parts of Foeniculum vulgare Subsp. Piperitum (Ucria) Coutinho (Umbelliferae) Growing Wild In Sicily. Nat. Prod. Res. 2022, 36, 3587–3597. [Google Scholar] [CrossRef]
- Hong, S.J.; Yoon, S.; Jo, S.M.; Jeong, H.; Youn, M.Y.; Kim, Y.J.; Kim, J.K.; Shin, E.-C. Olfactory Stimulation by Fennel (Foeniculum vulgare Mill.) Essential Oil Improves Lipid Metabolism and Metabolic Disorders in High Fat-Induced Obese Rats. Nutrients 2022, 14, 741. [Google Scholar] [CrossRef]
- Goswami, N.; Chatterjee, S. Assessment of Free Radical Scavenging Potential and Oxidative DNA Damage Preventive Activity of Trachyspermum ammi L. (Carom) and Foeniculum vulgare Mill. (Fennel) Seed Extracts. BioMed Res. Int. 2014, 2014, 582767. [Google Scholar] [CrossRef]
- El-Garawani, I.M.; El-Nabi, S.H.; Dawoud, G.T.; Esmail, S.M.; Abdel Moneim, A.E. Triggering of Apoptosis and Cell Cycle Arrest by Fennel and Clove Oils in Caco-2 Cells: The role of combination. Toxicol. Mech. Methods 2019, 29, 710–722. [Google Scholar] [CrossRef]
- El-Garawani, I.; Hassab, S.; Nabi, E.; El-Ghandour, E. The Protective Effect of (Foeniculum vulgare) Oil on Etoposide-Induced Genotoxicity on Male Albino Rats. Eur. J. Pharm. Med. Res. 2017, 4, 180–194. [Google Scholar]
- Hanan, M.S.; Sohair, S.A.; Hanan, A.A.; Areej, A.Y. Hepatoprotective Effect of Fennel and Tiger Nut on Biochemical Parameters and DNA Damage in Rats. Egypt. J. Chem. Environ. Health 2015, 1, 18–37. [Google Scholar]
- De Bleserc, P.J.; Jannes, P.; Van Buul-Offers, S.C.; Hoogerbrugge, C.M.; Van Schravendijk, C.F.H.; Niki, T.; Rogiers, V.; Van Den Brande, J.L.; Wisse, E.; Geerts, A. Insulinlike Growth Factor—Ii/Mannose 6-Phosphate Receptor is Expressed on CCl4-Exposed Rat Fat-Storing Cells and Facilitates Activation of Latent Transforming Growth Factor-Β in Cocultures with Sinusoidal Endothelial Cells. Hepatology 1995, 21, 1429–1437. [Google Scholar] [CrossRef]
- Ismail, R.S.; El-Megeid, A.A.; Abdel-Moemin, A.R. Carbon Tetrachloride-Induced Liver Disease in Rats: The Potential Effect of Supplement Oils with Vitamins E and C on the Nutritional Status. Ger. Med. Sci. GMS E-J. 2009, 7, Doc05. [Google Scholar] [CrossRef]
- Saxena, S.; Shahani, L.; Bhatnagar, P. Hepatoprotective effect of Chenopodium quinoa seed against CCL4-induced liver toxicity in Swiss albino male mice. Asian J. Pharm. Clin. Res. 2017, 10, 273–276. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Lee, S.-H.; Chun, S.Y.; Kim, D.H.; Jang, B.I.; Han, M.-H.; Lee, S.-O. In Vitro and In Vivo Protective Effects of Lentil (Lens culinaris) Extract against Oxidative Stress-Induced Hepatotoxicity. Molecules 2022, 27, 59. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.-C.; Anderson, A.; Coker, J.; Ondrus, M. Characterization of Lipid Oxidation Products in quinoa (Chenopodium quinoa). Food Chem. 2007, 101, 185–192. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem-Biol. Interact 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Dringen, R.; Pawlowski, P.G.; Hirrlinger, J. Peroxide Detoxification by Brain Cells. J. Neurosci. Res. 2005, 79, 157–165. [Google Scholar] [CrossRef]
- Dai, N.; Zou, Y.; Zhu, L.; Wang, H.-F.; Dai, M.-G. Antioxidant Properties of Proanthocyanidins Attenuate Carbon Tetrachloride (CCl4)–Induced Steatosis and Liver Injury in Rats via CYP2E1 Regulation. J. Med. Food 2014, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-Induced Liver Injury. Biol. Pharm. Bull. 2010, 33, 983–987. [Google Scholar] [CrossRef]
- Ingole, A.; Kadam, M.P.; Dalu, A.P.; Kute, S.M.; Mange, P.R.; Theng, V.D.; Lahane, O.R.; Nikas, A.P.; Kawal, Y.V.; Nagrik, S.U. A Review of the Pharmacological Characteristics of Vanillic Acid. J. Drug Deliv. Ther. 2021, 11, 200–204. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Jiang, Z.; Xie, W.; Zhang, X. Hepatoprotective Effect of Kaempferol. Against Alcoholic Liver Injury in Mice. Am. J. Chin. Med. 2015, 43, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.A.; Ke, B.J.; Cheng, C.S.; Wang, J.J.; Wei, B.L.; Lee, C.L. Red Quinoa Bran Extracts Protects Against Carbon Tetrachloride-Induced Liver Injury and Fibrosis in Mice via Activation of Antioxidative Enzyme Systems and Blocking TGF-β1 Pathway. Nutrients 2019, 11, 395. [Google Scholar] [CrossRef]
- Samadi-Noshahr, Z.; Hadjzadeh, M.-A.-R.; Moradi-Marjaneh, R.; Khajavi-Rad, A. The Hepatoprotective Effects of Fennel Seeds Extract and trans-Anethole in Streptozotocin-induced Liver Injury in Rats. Food Sci. Nutr. 2021, 9, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, H.F.; Algonaiman, R.; Barakat, H. Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats. Antioxidants 2022, 11, 1122. [Google Scholar] [CrossRef]
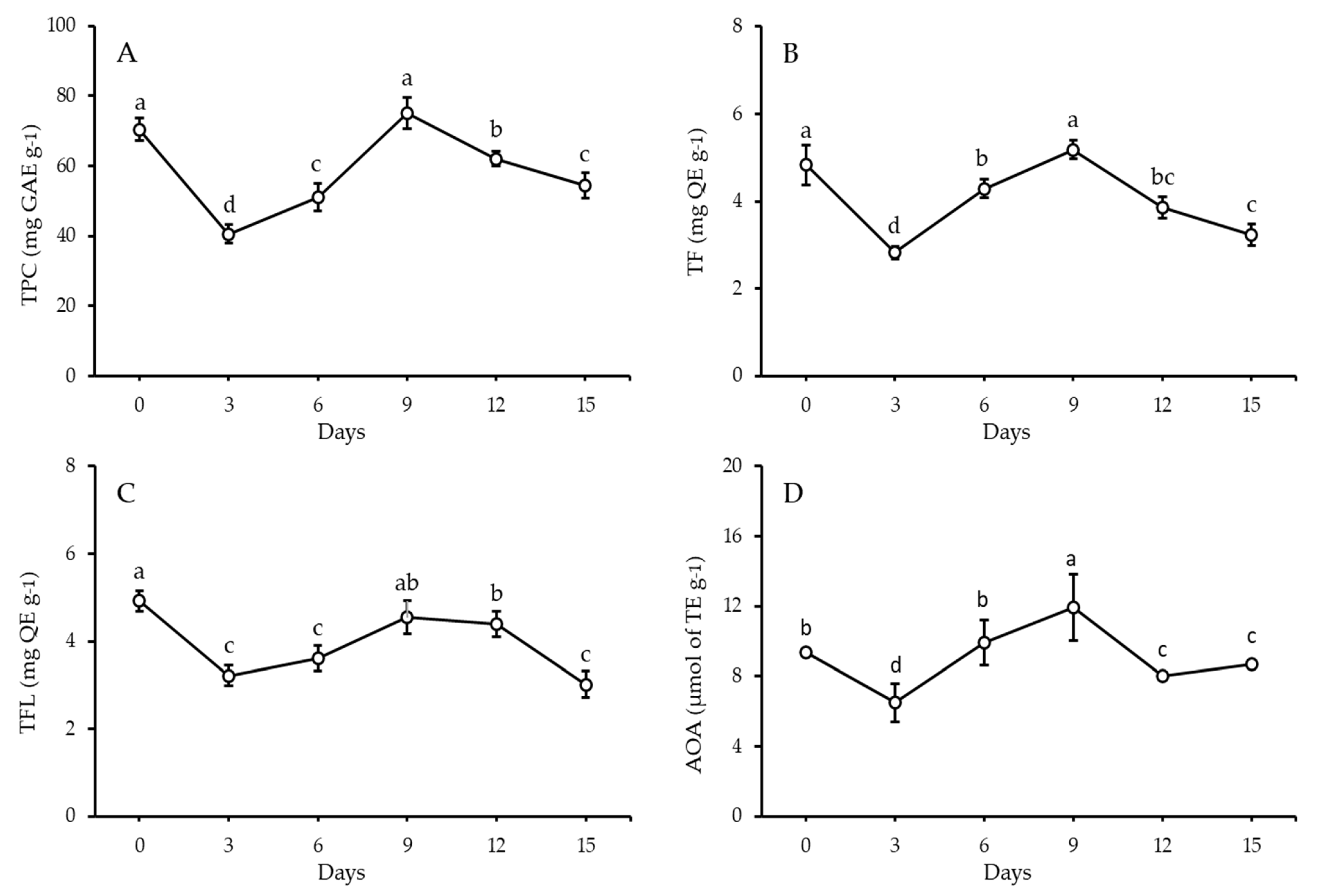

| Item | No. | Compound | Phenolics (µg g−1) * | ||
|---|---|---|---|---|---|
| Sprouting Period (day) | |||||
| Raw Fennel Seed | 6-Days Sprouts | 9-Days Sprouts | |||
| Phenolic acids | 1 | Pyrogallol | - | - | - |
| 2 | Quinol | - | - | - | |
| 3 | 3-Hydroxytyrosol | - | - | - | |
| 4 | Catechol | 9.26 | 40.98 | 72.26 | |
| 5 | p-Hydroxy benzoic acid | 32.25 | 83.45 | 32.60 | |
| 6 | Caffeic acid | 26.72 | 2.40 | 48.22 | |
| 7 | Chlorogenic acid | 6.79 | 17.88 | 71.25 | |
| 8 | Cinnamic acid | 9.38 | 14.18 | 18.83 | |
| 9 | Ellagic acid | 25.35 | 46.20 | 52.30 | |
| 10 | Vanillic acid | 587.40 | 105.31 | 129.08 | |
| 11 | Ferulic acid | 20.01 | 20.39 | 48.51 | |
| 12 | Gallic acid | - | - | - | |
| 13 | O-coumaric acid | 112.77 | 9.76 | 8.44 | |
| 14 | p-coumaric acid | 18.46 | 11.05 | 19.89 | |
| 15 | Benzoic acid | 30.38 | 90.74 | 110.35 | |
| 16 | Rosmarinic acid | 64.41 | 53.25 | 124.71 | |
| 17 | Syringic acid | 9.72 | 10.13 | 66.08 | |
| Flavonoids | 1 | Catechin | 123.46 | 151.42 | 151.46 |
| 2 | Kaempferol | 5913.55 | 8.24 | 2357.57 | |
| 3 | Myricetin | 236.93 | 42.17 | 166.94 | |
| 4 | Quercetin | 28.71 | 187.88 | 192.35 | |
| 5 | Rutin | 423.28 | 817.03 | 985.29 | |
| 6 | Resveratrol | 472.19 | 159.37 | 402.24 | |
| 7 | Naringenin | - | - | - | |
| No. | Rt | Compound | MW | Chemical Formula | Peak Area % | ||
|---|---|---|---|---|---|---|---|
| Raw Fennel Seed | 6-Days Sprouts | 9-Days Sprouts | |||||
| 1 | 6.50 | α-Pinene | 136 | C10H16 | 0.44 | 1.14 | - |
| 2 | 7.63 | (E)-3-Propylidenecyclopentene | 108 | C8H12 | 0.27 | - | - |
| 3 | 8.21 | α-Myrcene | 136 | C10H16 | 0.14 | - | - |
| 4 | 9.21 | 1,7-Octadiene, 2-methyl-6-methylene-Cyclohexene | 136 | C10H16 | 7.17 | - | 3.32 |
| 5 | 9.55 | α-Pinene | 136 | C10H16 | 0.17 | 0.19 | 0.1 |
| 6 | 10.13 | ç-Terpinene | 136 | C10H16 | 0.1 | - | - |
| 7 | 10.95 | Fenchone | 152 | C10H16O | 11.18 | 14.19 | 7.42 |
| 8 | 11.24 | 2,6,10-trimethyl-tridecane | 226 | C16H34 | 0.14 | 0.13 | - |
| 9 | 11.96 | cis-Verbenol | 152 | C10H16O | 0.07 | - | - |
| 10 | 12.28 | cis-Limonene oxide | 152 | C10H16O | 0.08 | 0.1 | - |
| 11 | 12.41 | trans-Limonene oxide | 152 | C10H16O | 0.09 | 0.1 | 0.06 |
| 12 | 12.54 | Naphthalene, decahydro-2-methyl | 152 | C11H20 | 0.19 | 0.2 | 0.16 |
| 13 | 14.30 | trans-Anethole (Benzene, 1-methoxy-4-(2-propenyl)) | 148 | C10H12O | 23.65 | 33.47 | 22.89 |
| 14 | 15.10 | Exobornyl acetate | 168 | C12H20O2 | 0.1 | - | 0.09 |
| 15 | 15.45 | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl) | 150 | C10H14O | 0.13 | - | - |
| 16 | 15.82 | 4-Methoxybenzaldehyde (4-Anisaldehyde) | 136 | C8H8O2 | 1.45 | 2.38 | 2.32 |
| 17 | 15.87 | Hydrazine, phenyl, monohydrochloride | 144 | C6H9ClN2 | 1.81 | ||
| 18 | 16.77 | Benzene, [1-(2-propenyloxy)-3-butenyl] (trans-Anethole) | 188 | C13H16O | 38.41 | 19.01 | 42.32 |
| 19 | 19.20 | (4R,5S)-1-Ethoxy4methoxy-5-[(4-methoxybenzyl)oxy]hept-1-yn-6-ene | 304 | C18H24O4 | 0.43 | - | 0.18 |
| 20 | 20.89 | (3R,3aR)-1,2,3,4,5,6-hexahydro-3-methyl-3aH-indene-3a-carbaldehyde | 196 | C11H16O | 0.11 | - | 0.05 |
| 21 | 21.17 | m-Anisic acid, 3,4-dichlorophenyl ester | 296 | C14H10Cl2O3 | 0.1 | - | - |
| 22 | 23.35 | 1,2-Dimethyl-3-nitro-4-nitrosobenzene | 180 | C8H8N2O3 | 0.3 | - | - |
| 23 | 23.47 | Trans-2-Tridecenal | 196 | C13H24O | 0.24 | - | 0.14 |
| 24 | 23.87 | Benzeneacetic acid, α-hydroxy-4-methoxy | 182 | C9H10O4 | 1.65 | 3.18 | 3.34 |
| 25 | 24.03 | z-isomer, 2-(2-hydroxyethylidene)-3-methoxynorbornane | 168 | C10H16O2 | 0.21 | 0.52 | 0.46 |
| 26 | 25.08 | 1-(4-Methoxyphenyl) propan-1-ol | 166 | C10H14O2 | 0.12 | - | - |
| 27 | 25.76 | Ethanone, 1-(1-hydroxy-2,6,6-trimethyl-2,4-cyclohexadien-1-yl) | 180 | C11H16O2 | 0.26 | - | - |
| 28 | 26.13 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl) | 180 | C10H12O3 | 1.8 | 0.98 | 0.51 |
| 29 | 26.55 | (4-Methoxy-phenyl)-(2-nitrocyclohexyl)-methanol | 265 | C14H19NO4 | 0.19 | 0.11 | 0.52 |
| 30 | 26.87 | 4-Methoxyphenylethyleneglycol | 168 | C9H12O3 | 1.86 | - | - |
| 31 | 32.21 | Pentamethyl Pentaphenyl Cyclopentasiloxane | 680 | C35H40O5Si5 | 0.13 | - | - |
| 32 | 34.08 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 294 | C19H34O2 | 0.11 | 0.23 | 0.18 |
| 33 | 34.2 | 6-Octadecenoic acid, methyl ester | 296 | C19H36O2 | 0.23 | 0.59 | 0.89 |
| 34 | 35.35 | 9-Octadecenoic acid (Z), ethyl ester | 310 | C20H38O2 | 0.09 | 0.09 | 0.75 |
| 35 | 35.57 | 4,4′-Di(3-butenyl)-2,2′-bipyridine | 264 | C18H20N2 | 3.12 | - | - |
| 36 | 37.12 | Benzaldehyde N,N-dimethylhydrazone | 148 | C9H12N2 | 0.09 | 0.09 | - |
| 37 | 37.47 | Z-7-Pentadecenol | 226 | C15H30O | 0.08 | 0.12 | - |
| 38 | 39.63 | Cyclohexane,1,1′-dodecylidenebis[4-methyl] | 362 | C26H50 | 0.11 | - | - |
| 39 | 39.83 | 1-Ethyl-2-formyl-9-methy-l-4-oxo1,2,3,4-tetrahydr-o-α-carboline | 256 | C15H16N2O2 | 0.2 | 0.2 | 0.13 |
| 40 | 40.04 | 6-Methyl-6-(3′-isopropeny-l-2′-methyl-cycloprop-1′-en1′-yl)-2-heptanol | 222 | C15H26O | 0.15 | - | - |
| 41 | 40.36 | 9-Octadecenoic acid (Z) | 282 | C18H34O2 | 0.29 | 0.22 | 0.24 |
| 42 | 40.61 | Ethanol, 2-ethoxy, acetate (6-Tridecene) | 139 | C9H15D2N | 0.08 | - | - |
| 43 | 41.7 | Tert-Butyl ester of 3,4-Dimethyl-5-(2-nitroethyl)-pyrrol-2-carboxylic acid | 268 | C13H20N2O4 | 0.14 | - | - |
| 45 | 41.9 | 1-(p-hydroxy tolyl) propan-1-ol (impure) | 166 | C10H14O2 | 0.08 | 0.17 | - |
| 46 | 42.46 | Ethanone, 2-hydroxy-1,2-bis(4-methoxyphenyl) | 272 | C16H16O4 | 0.19 | - | - |
| 47 | 42.8 | Ethanone, 2-hydroxy-1,2-bis(4-methoxyphenyl) | 272 | C16H16O4 | 0.17 | - | - |
| 48 | 43.54 | Nonacosane | 408 | C29H60 | 0.08 | 0.15 | 0.08 |
| 49 | 46.28 | Docosane | 310 | C22H46 | 0.1 | 0.11 | - |
| 50 | 48.79 | 2-Diisobutylcarbamoyl-cyclohexane carboxylic acid, decyl ester | 423 | C26H49NO3 | 1.57 | - | - |
| 51 | 51.15 | {[3E)-2-[(Dimethylcarbamoyl)methyl]-3-ethylidene-13,17-bis[2′(methoxycarbonyl)ethyl]2,7,12,18-tetramethyl-2,3dihydroporphytinato]}vzinc (II) | 713 | C38H43N5O5Zn | 0.08 | - | - |
| 52 | - | unknown | - | - | 0.05 | 0.17 | 0.01 |
| Total | 100 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barakat, H.; Alkabeer, I.A.; Aljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Mohamed, A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants 2022, 11, 2318. https://doi.org/10.3390/antiox11122318
Barakat H, Alkabeer IA, Aljutaily T, Almujaydil MS, Algheshairy RM, Alhomaid RM, Almutairi AS, Mohamed A. Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants. 2022; 11(12):2318. https://doi.org/10.3390/antiox11122318
Chicago/Turabian StyleBarakat, Hassan, Ibrahim Ali Alkabeer, Thamer Aljutaily, Mona S. Almujaydil, Reham M. Algheshairy, Raghad M. Alhomaid, Abdulkarim S. Almutairi, and Ahmed Mohamed. 2022. "Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats" Antioxidants 11, no. 12: 2318. https://doi.org/10.3390/antiox11122318
APA StyleBarakat, H., Alkabeer, I. A., Aljutaily, T., Almujaydil, M. S., Algheshairy, R. M., Alhomaid, R. M., Almutairi, A. S., & Mohamed, A. (2022). Phenolics and Volatile Compounds of Fennel (Foeniculum vulgare) Seeds and Their Sprouts Prevent Oxidative DNA Damage and Ameliorates CCl4-Induced Hepatotoxicity and Oxidative Stress in Rats. Antioxidants, 11(12), 2318. https://doi.org/10.3390/antiox11122318







