Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
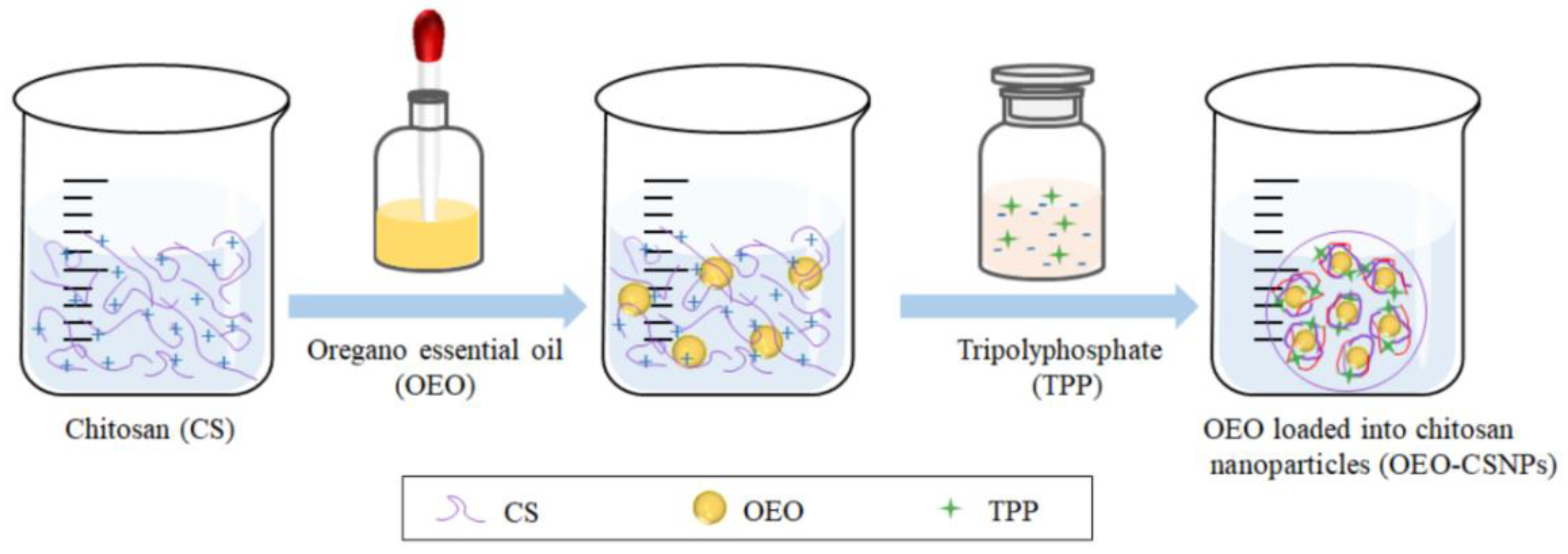
2.2. Preparation of OEO-CSNPs
2.3. Optimization of the Preparation of OEO-CSNPs Using BBD
2.4. Maximum Absorption Wavelength and Standard Curve of OEO
2.5. Determination of Encapsulation Efficiency and Loading Capacity
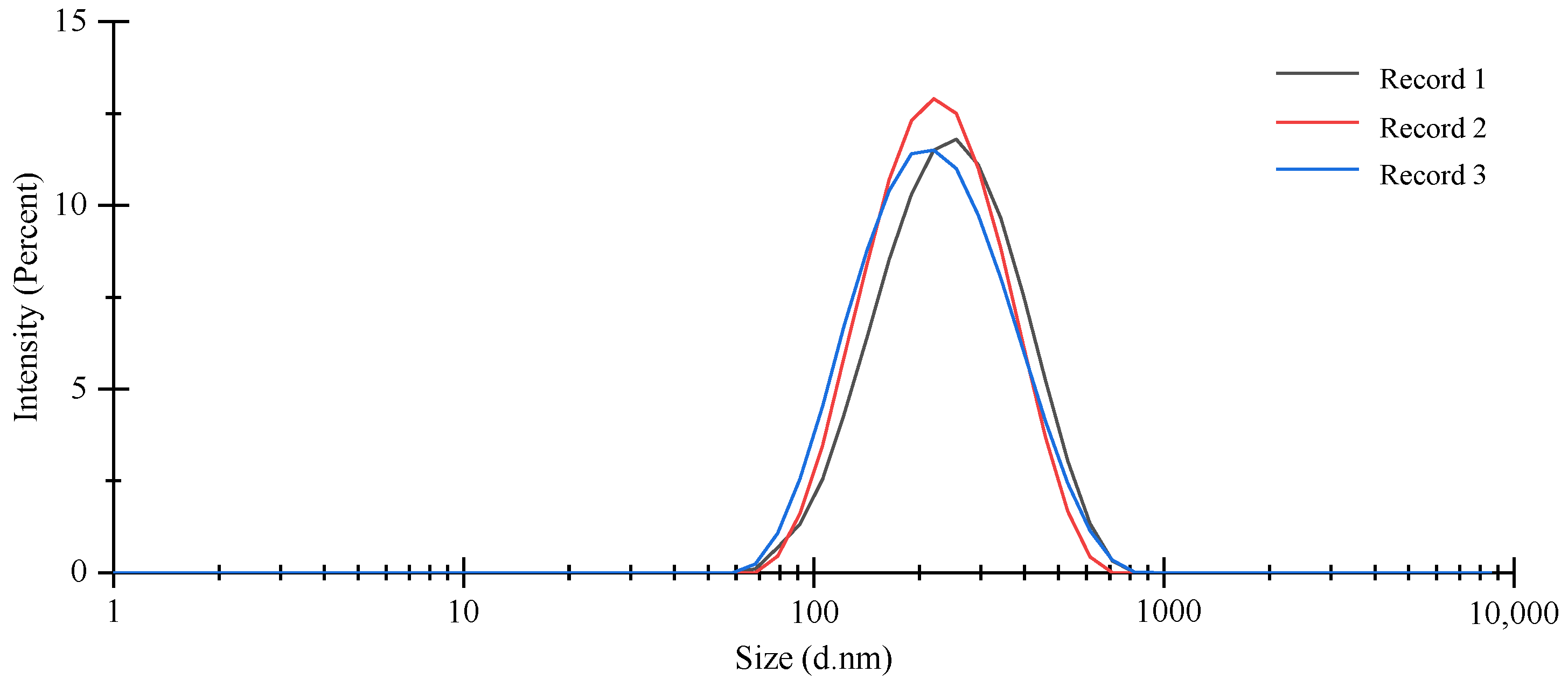
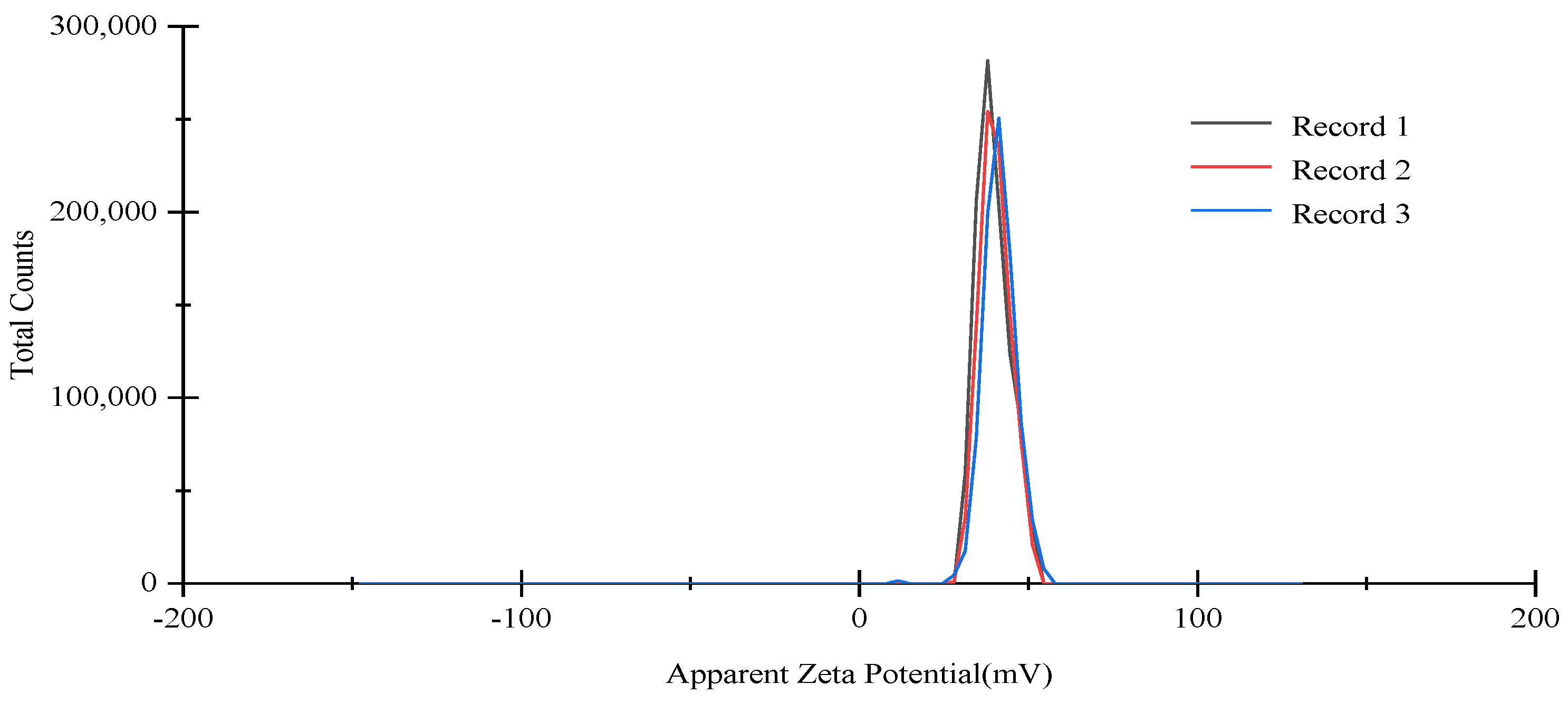
2.6. Particle Size, PDI, and Zeta Potential
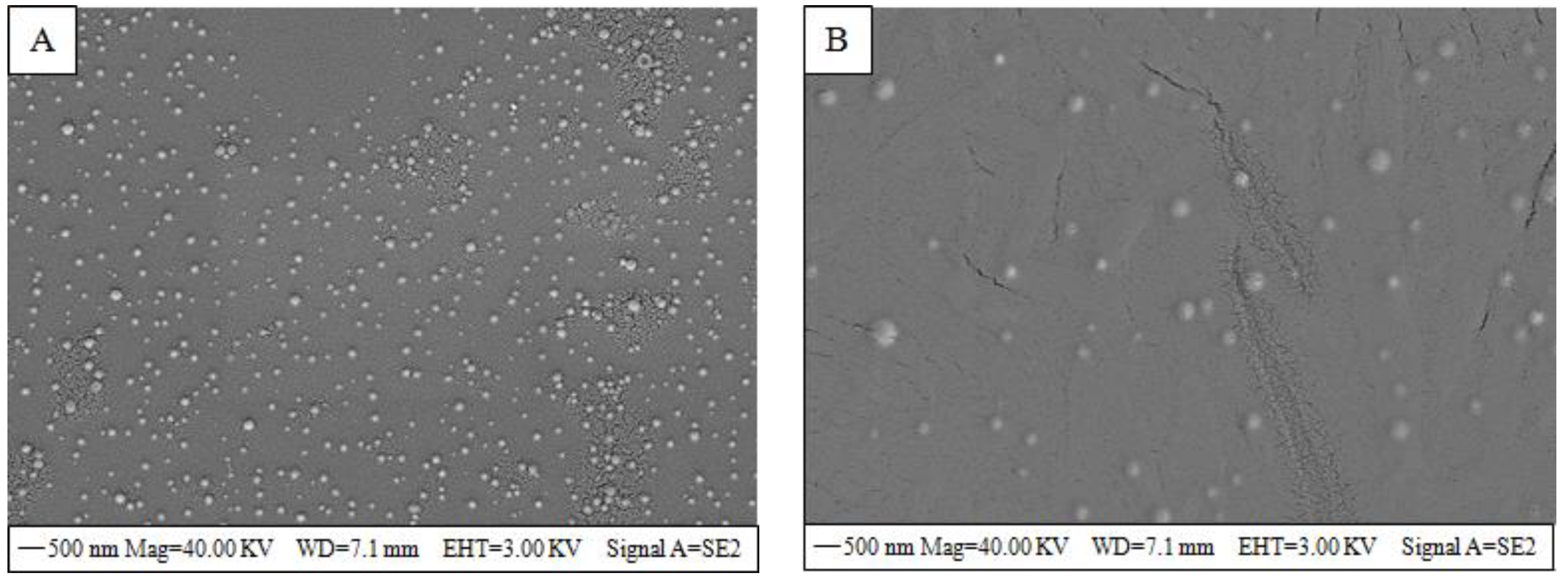
2.7. Scanning Electron Microscopy (SEM)
2.8. Fourier Transformed Infrared Spectroscopy (FT-IR)
2.9. Thermal Characterization by Thermogravimetric Analysis (TGA)
2.10. In Vitro Release Studies
2.11. Determination of Antimicrobial Activity
2.12. Statistical Analysis
3. Results
3.1. BBD Experimental Results and Analysis
3.2. Characterization of OEO-CSNPs
3.3. SEM Analysis
3.4. FT-IR Analysis
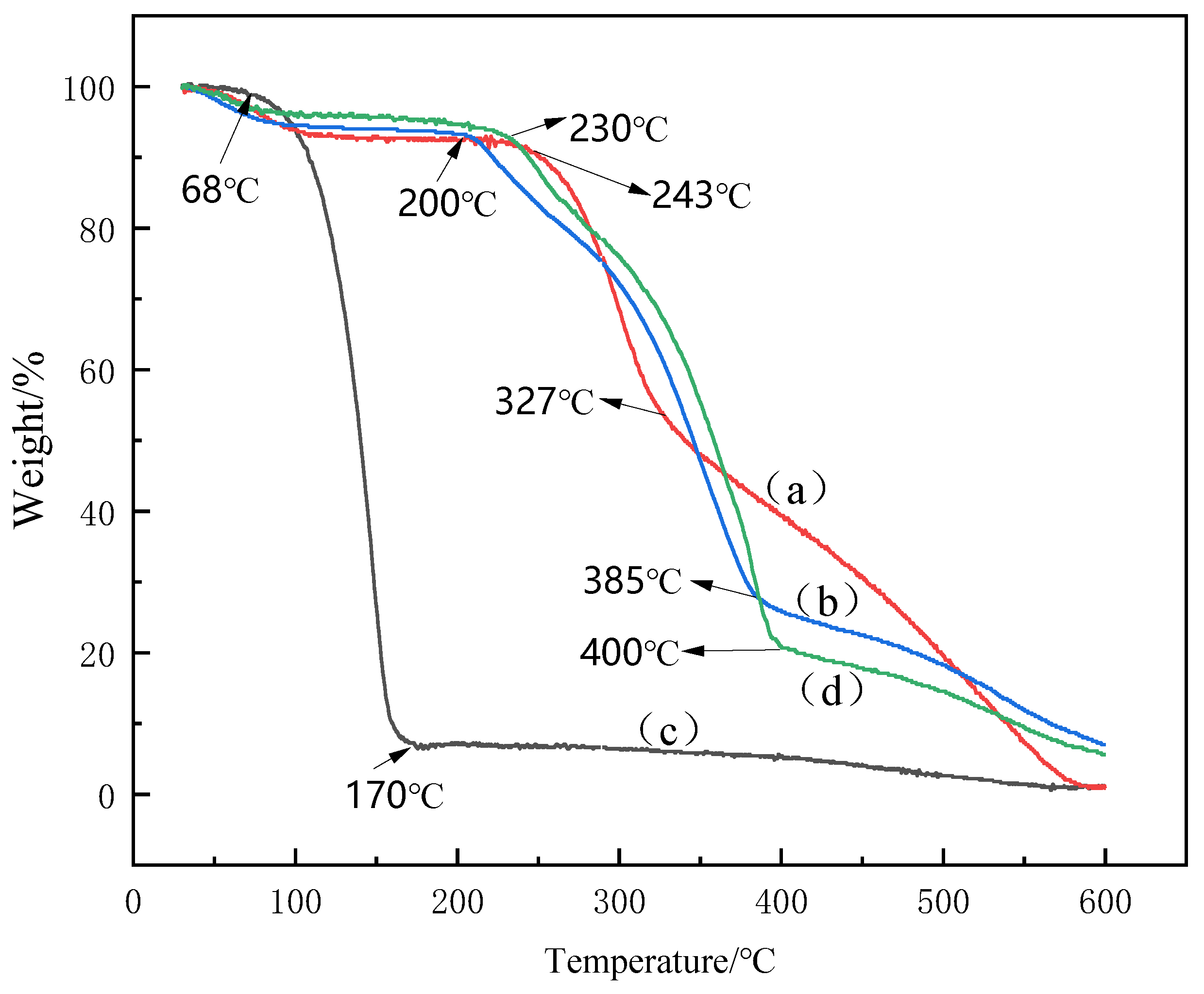
3.5. TGA Analysis
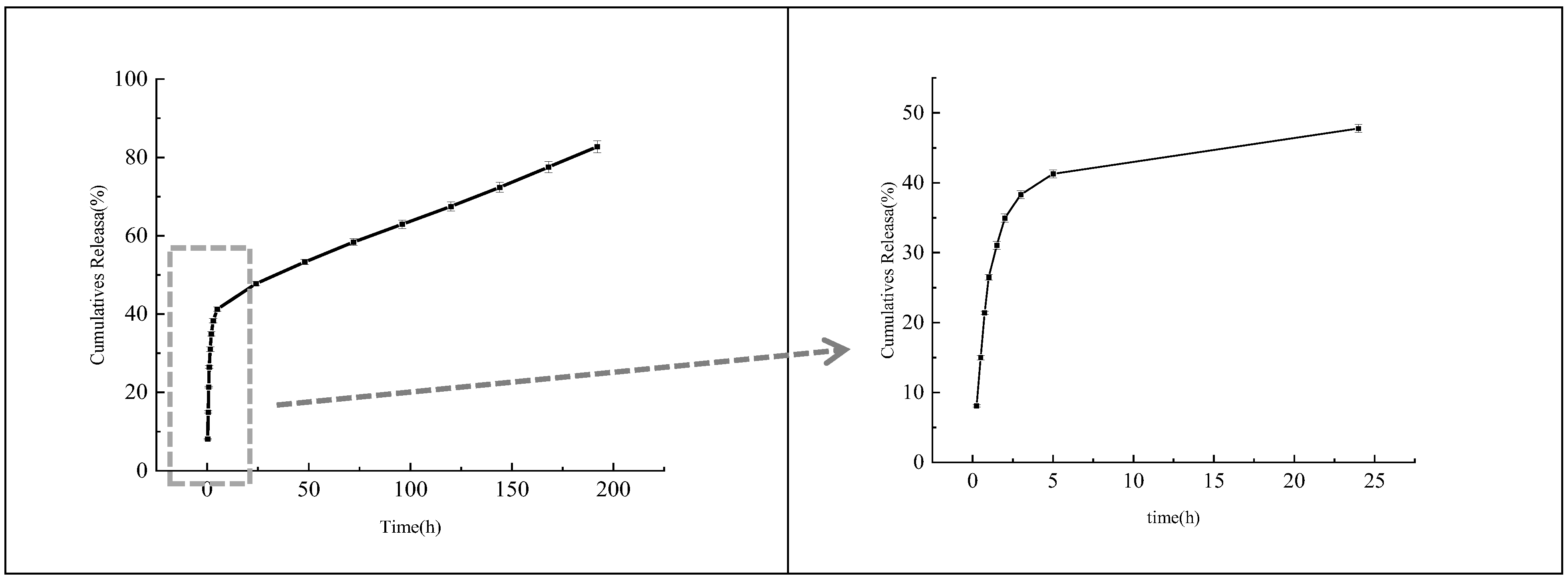
3.6. In Vitro Release Analysis
3.7. Antibacterial Activity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT—Food Sci. Technol. 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2019, 148, 1280–1289. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Jaspal, M.H.; Ijaz, M.; Haq, H.A.U.; Yar, M.K.; Asghar, B.; Manzoor, A.; Badar, I.H.; Ullah, S.; Islam, M.S.; Hussain, J. Effect of oregano essential oil or lactic acid treatments combined with air and modified atmosphere packaging on the quality and storage properties of chicken breast meat. LWT—Food Sci. Technol. 2021, 146, 111459. [Google Scholar] [CrossRef]
- Stefanakisa, M.K.; Touloupakisa, E.; Anastasopoulosb, E.; Ghanotakisa, D.; Makridisc, P. Antibacterial activity of essential oils from plants of the genus origanum. Food Control. 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crop. Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Chang, Y.; Han, J. Oregano essential oil-based natural antimicrobial packaging film to inactivate Salmonella enterica and yeasts/molds in the atmosphere surrounding cherry tomatoes. Food Microbiol. 2017, 65, 114–121. [Google Scholar] [CrossRef]
- Plati, F.; Paraskevopoulou, A. Micro- and Nano-encapsulation as Tools for Essential Oils Advantages’ Exploitation in Food Applications: The Case of Oregano Essential Oil. Food Bioprocess Technol. 2022, 15, 949–977. [Google Scholar] [CrossRef]
- Abdollah, H.S.; Amir, M. Feeding broilers with thyme essential oil loaded in chitosan nanoparticles: An efficient strategy for successful delivery. British Poultry Sci. 2018, 59, 669–678. [Google Scholar]
- Bazana, M.T.; Codevilla, C.F.; de Menezes, C.R. Nanoencapsulation of bioactive compounds: Challenges and perspectives. Curr. Opin. Food Sci. 2019, 26, 47–56. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hosseini, S.M.; Hashemi, M. Emerging chitosan nanoparticles loading-system boosted the antibacterial activity of Cinnamomum zeylanicum essential oil. Ind. Crop. Prod. 2020, 155, 112824. [Google Scholar] [CrossRef]
- Ferreira, L.M.; dos Santos, A.M.; Boni, F.I.; dos Santos, K.C.; Robusti, L.M.G.; de Souza, M.P.; Ferreira, N.N.; Carvalho, S.G.; Cardoso, V.M.; Chorilli, M.; et al. Design of chitosan-based particle systems: A review of the physicochemical foundations for tailored properties. Carbohydr. Polym. 2020, 250, 116968. [Google Scholar] [CrossRef] [PubMed]
- Weißpflog, J.; Vehlow, D.; Müller, M.; Kohn, B.; Scheler, U.; Boye, S.; Schwarz, S. Characterization of chitosan with different degree of deacetylation and equal viscosity in dissolved and solid state—Insights by various complimentary methods. Int. J. Biol. Macromol. 2021, 171, 242–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2018, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef]
- My, A.; Nk, A.; Amm, B.; Kd, C. Preparation optimization and characterization of chitosan-tripolyphosphate microcapsules for the encapsulation of herbal galactagogue extract. Int. J. Biol. Macromolecules. 2019, 140, 920–928. [Google Scholar]
- Nouri, A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- At, A.; Mb, A.; Sd, B.; Rk, A. Shelf life extension of bell pepper by application of chitosan nanoparticles containing heracleum persicum fruit essential oil. Postharvest Biol. Technol. 2020, 170, 111313. [Google Scholar]
- Caldera, L.; Franzetti, L.; Van Coillie, E.; De Vos, P.; Stragier, P.; De Block, J.; Heyndrickx, M. Identification, enzymatic spoilage characterization and proteolytic activity quantification of Pseudomonas spp. isolated from different foods. Food Microbiol. 2016, 54, 142–153. [Google Scholar] [CrossRef]
- Zeinab, A.; Moosa, S.; Ali, A.; Reza, M.G. Encapsulation of Satureja Hortensis L. (lamiaceae) in chitosan/tpp nanoparticles with enhanced acaricide activity against tetranychus urticae koch (acari: Tetranychidae). Ecotoxicol. Environ. Saf. 2018, 161, 111–119. [Google Scholar]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Application of Box-Behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route*. Int. J. Biol. Macromol. 2016, 89, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Medeiros-Neves, B.; Prá, M.D.; Schuh, R.S.; Veras, K.S.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Braganhol, E.; Teixeira, H.F. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery—In vitro studies. Carbohydr. Polym. 2018, 199, 572–582. [Google Scholar] [CrossRef]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.; Gavlighi, H.A.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A.; Khan, A.A.; Khan, S.; Almalik, A.; Alshamsan, A. Optimizing indomethacin-loaded chitosan nanoparticle size, encapsulation, and release using Box–Behnken experimental design. Int. J. Biol. Macromol. 2016, 87, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Chang, R.; Yang, J.; Ge, S.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Preparation and characterization of essential oil-loaded starch nanoparticles formed by short glucan chains. Food Chem. 2017, 221 Pt 2, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crop. Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Asgari, A. In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int. J. Biol. Macromol. 2015, 81, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles pre-pared by liquid–liquid dispersion method. LWT—Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through en-capsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Alibadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef]
- Shadab, M.D.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Preparation, Characterization and Evaluation of Bromocriptine Loaded Chitosan Nanoparticles for Intranasal Delivery. Sci. Adv. Mater. 2012, 4, 949–960. [Google Scholar] [CrossRef]
- Almeida, K.B.; Ramos, A.S.; Nunes, J.B.; Silva, B.O.; Ferraz, E.R.; Fernandes, A.S.; Felzenszwalb, I.; Amaral, A.C.F.; Roullin, V.G.; Falcao, D.Q. PLGA nanoparticles optimized by box-behnken for efficient encapsulation of therapeutic Cymbopogon citratus essential oil. Colloids Surf. B Biointerfaces 2019, 181, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Liew, S.N.; Utra, U.; Alias, A.K.; Tan, T.B.; Tan, C.P.; Yussof, N.S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT—Food Sci. Technol. 2020, 128, 109388. [Google Scholar] [CrossRef]
- López-Meneses, A.K.; Plascencia-Jatomea, M.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Rodríguez-Félix, F.; Mouriño-Pérez, R.R.; Cortez-Rocha, M.O. Schinus molle L. essential oil-loaded chitosan nanoparticles: Preparation, characterization, antifungal and anti-aflatoxigenic properties. LWT—Food Sci. Technol. 2018, 96, 597–603. [Google Scholar] [CrossRef]
- Hesami, G.; Darvishi, S.; Zarei, M.; Hadidi, M. Fabrication of chitosan nanoparticles incorporated with Pistacia atlantica subsp. kurdica hulls’ essential oil as a potential antifungal preservative against strawberry grey mould. Int. J. Food Sci. Technol. 2021, 56, 4215–4223. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.E.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Corona-Rangel, M.L. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT—Food Sci. Technol. 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Seyed, F.H.; Mojgan, Z.; Masoud, R.; Farhid, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar]
- Da Costa, R.C.; Daitx, T.S.; Mauler, R.S.; da Silva, N.M.; Miotto, M.; Crespo, J.S.; Carli, L.N. Poly(hydroxybutyrate-co-hydroxyvalerate)-based nanocomposites for antimicrobial active food packaging containing oregano essential oil. Food Packag. Shelf Life 2020, 26, 100602. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Masson, M.M.; Silva, N.C.; Esposto, B.S.; Barros, T.T.; Assis, O.B.; Tapia-Blácido, D. Soybean straw nanocellulose produced by enzymatic or acid treatment as a reinforcing filler in soy protein isolate films. Carbohydr. Polym. 2018, 198, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, V.; Cerbu, C.; Witkowski, L.; Potarniche, A.V.; Timar, M.C.; Żychska, M.; Sabliov, C.M. Nanodelivery of essential oils as efficient tools against antimicrobial resistance: A review of the type and physical-chemical properties of the delivery systems and applications. Drug Deliv. 2022, 29, 1007–1024. [Google Scholar] [CrossRef]
- Pereira, M.C.; Hill, L.E.; Rui, C.Z.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phyto-chemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa o. berg) study. LWT—Food Sci. Technol. 2015, 63, 100–107. [Google Scholar] [CrossRef]
- Barbosa, R.F.D.S.; Yudice, E.D.C.; Mitra, S.K.; Rosa, D.D.S. Characterization of Rosewood and Cinnamon Cassia essential oil polymeric capsules: Stability, loading efficiency, release rate and antimicrobial properties. Food Control 2020, 121, 107605. [Google Scholar] [CrossRef]
- Yin, H.; Wang, C.; Yue, J.; Deng, Y.; Jiao, S.; Zhao, Y.; Zhou, J.; Cao, T. Optimization and characterization of 1,8-cineole/hydroxypropyl-β-cyclodextrin inclusion complex and study of its release kinetics. Food Hydrocoll. 2021, 110, 106159. [Google Scholar] [CrossRef]
- Van Long, N.N.; Joly, C.; Dantigny, P. Active packaging with antifungal activities. Int. J. Food Microbiol. 2016, 220, 73–90. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; Yilmaz, A.; Akman, P.K.; Bozkurt, F.; Dertli, E.; Basahel, A.; Al-Sasi, B.; Taylan, O.; Sagdic, O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innov. Food Sci. Emerg. Technol. 2018, 52, 166–178. [Google Scholar] [CrossRef]


| Independent Variables | Code | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CS concentration (mg/mL) | A | 1 | 1.5 | 2 |
| TPP concentration (mg/mL) | B | 1 | 1.5 | 2 |
| OEO (%) | C | 0.125 | 0.25 | 0.5 |
| Dependent variables | Applied constrains | |||
| Y1 = %EE | Maximize | |||
| Y2 = Size (nm) | Minimize | |||
| Run | A Chitosan | B TPP | C OEO | Y1 EE | Y2 Size |
|---|---|---|---|---|---|
| /mg/mL | /mg/mL | /% | /% | /nm | |
| 1 | −1 | 0 | −1 | 81.65 | 586.23 |
| 2 | 0 | 1 | −1 | 77.54 | 286.37 |
| 3 | 0 | 0 | 0 | 91.58 | 192.33 |
| 4 | 0 | 0 | 0 | 91.94 | 226.4 |
| 5 | −1 | 0 | 1 | 85.01 | 684.37 |
| 6 | 0 | 1 | 1 | 83.41 | 583.17 |
| 7 | 0 | 0 | 0 | 91.13 | 188.13 |
| 8 | −1 | −1 | 0 | 87.49 | 515.37 |
| 9 | 1 | 0 | −1 | 81.91 | 450.73 |
| 10 | −1 | 1 | 0 | 81.02 | 506.67 |
| 11 | 1 | 1 | 0 | 79.99 | 417.4 |
| 12 | 0 | −1 | −1 | 87.47 | 486.5 |
| 13 | 0 | 0 | 0 | 91.11 | 186.63 |
| 14 | 0 | 0 | 0 | 92.18 | 217.17 |
| 15 | 1 | −1 | 0 | 88.46 | 283.87 |
| 16 | 0 | −1 | 1 | 88.56 | 328.1 |
| 17 | 1 | 0 | 1 | 87.96 | 375.4 |
| Source | Y1-EE% | Y2-Size | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | F- Value | p-Value Prob > F | Sum of Squares | df | Mean Square | F- Value | p-Value Prob > F | |
| Model | 348.28 | 9 | 38.7 | 90.23 | <0.0001 ** | 4.03 × 105 | 9 | 44,789.02 | 56.58 | <0.0001 ** |
| A | 1.24 | 1 | 1.24 | 2.89 | 0.1328 | 73,199.03 | 1 | 73,199.03 | 92.47 | <0.0001 ** |
| B | 112.65 | 1 | 112.65 | 262.67 | <0.0001 ** | 4039.66 | 1 | 4039.66 | 5.1 | 0.0584 |
| C | 33.5 | 1 | 33.5 | 78.11 | <0.0001 ** | 3248.58 | 1 | 3248.58 | 4.1 | 0.0824 |
| AB | 1 | 1 | 1 | 2.33 | 0.1706 | 5057.34 | 1 | 5057.34 | 6.39 | 0.0394 * |
| AC | 1.81 | 1 | 1.81 | 4.22 | 0.0791 | 7522.96 | 1 | 7522.96 | 9.5 | 0.0177 ** |
| BC | 5.71 | 1 | 5.71 | 13.32 | 0.0082 ** | 51,801.76 | 1 | 51,801.76 | 65.44 | <0.0001 ** |
| A2 | 58.59 | 1 | 58.59 | 136.61 | <0.0001 ** | 1.16 × 105 | 1 | 1.16 × 105 | 146.44 | <0.0001 ** |
| B2 | 55.11 | 1 | 55.11 | 128.5 | <0.0001 ** | 16,591.9 | 1 | 16,591.9 | 20.96 | 0.0025 ** |
| C2 | 58.43 | 1 | 58.43 | 136.25 | <0.0001 ** | 1.03 × 105 | 1 | 1.03 × 105 | 129.66 | <0.0001 ** |
| Residual | 3 | 7 | 0.43 | 5540.94 | 7 | 791.56 | ||||
| Lack of Fit | 2.09 | 3 | 0.7 | 3.05 | 0.1546 | 4193.42 | 3 | 1397.81 | 4.15 | 0.1014 |
| Pure Error | 0.91 | 4 | 0.23 | 1347.52 | 4 | 336.88 | ||||
| Cor Total | 351.28 | 16 | 4.09 × 105 | 16 | ||||||
| R2 | 0.9915 | 0.9864 | ||||||||
| Adjusted R2 | 0.9805 | 0.9690 | ||||||||
| Responses | Predicted Value | Experimental Value | Percentage Prediction Error (%) |
|---|---|---|---|
| EE (%) | 92.65 | 92.90 ± 0.35 | 0.58 |
| Size (nm) | 184.83 | 182.77 ± 4.83 | 0.78 |
| CSNPs | OEO | OEO-CSNPs | Anhydrous Ethanol | Sterile Water | |
|---|---|---|---|---|---|
| P. fluorescens | 6.09 ± 0.03 Bb | 6.69 ± 0.11 Ab | 6.86 ± 0.05 Ac | 0.00 | 0.00 |
| P. gessardii | 6.63 ± 0.20 Ba | 6.07 ± 0.01 Cc | 8.30 ± 0.05 Ab | 0.00 | 0.00 |
| P. jessenii | 6.12 ± 0.02 Cb | 8.85 ± 0.10 Ba | 9.35 ± 0.04 Aa | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, P.; Ye, K.; He, Y.; Chen, S.; Yuan, A.; Chen, F.; Yang, W. Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles. Foods 2022, 11, 3756. https://doi.org/10.3390/foods11233756
Ma Y, Liu P, Ye K, He Y, Chen S, Yuan A, Chen F, Yang W. Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles. Foods. 2022; 11(23):3756. https://doi.org/10.3390/foods11233756
Chicago/Turabian StyleMa, Yuan, Ping Liu, Kunyue Ye, Yezheng He, Siqi Chen, Anqi Yuan, Fang Chen, and Wanli Yang. 2022. "Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles" Foods 11, no. 23: 3756. https://doi.org/10.3390/foods11233756
APA StyleMa, Y., Liu, P., Ye, K., He, Y., Chen, S., Yuan, A., Chen, F., & Yang, W. (2022). Preparation, Characterization, In Vitro Release, and Antibacterial Activity of Oregano Essential Oil Chitosan Nanoparticles. Foods, 11(23), 3756. https://doi.org/10.3390/foods11233756




