The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of the Animal Model
2.3. Pathological Section and Mast Cell Staining Analysis
2.4. Glucose and Insulin Tolerance Test
2.5. ELISA
2.6. Real-Time PCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
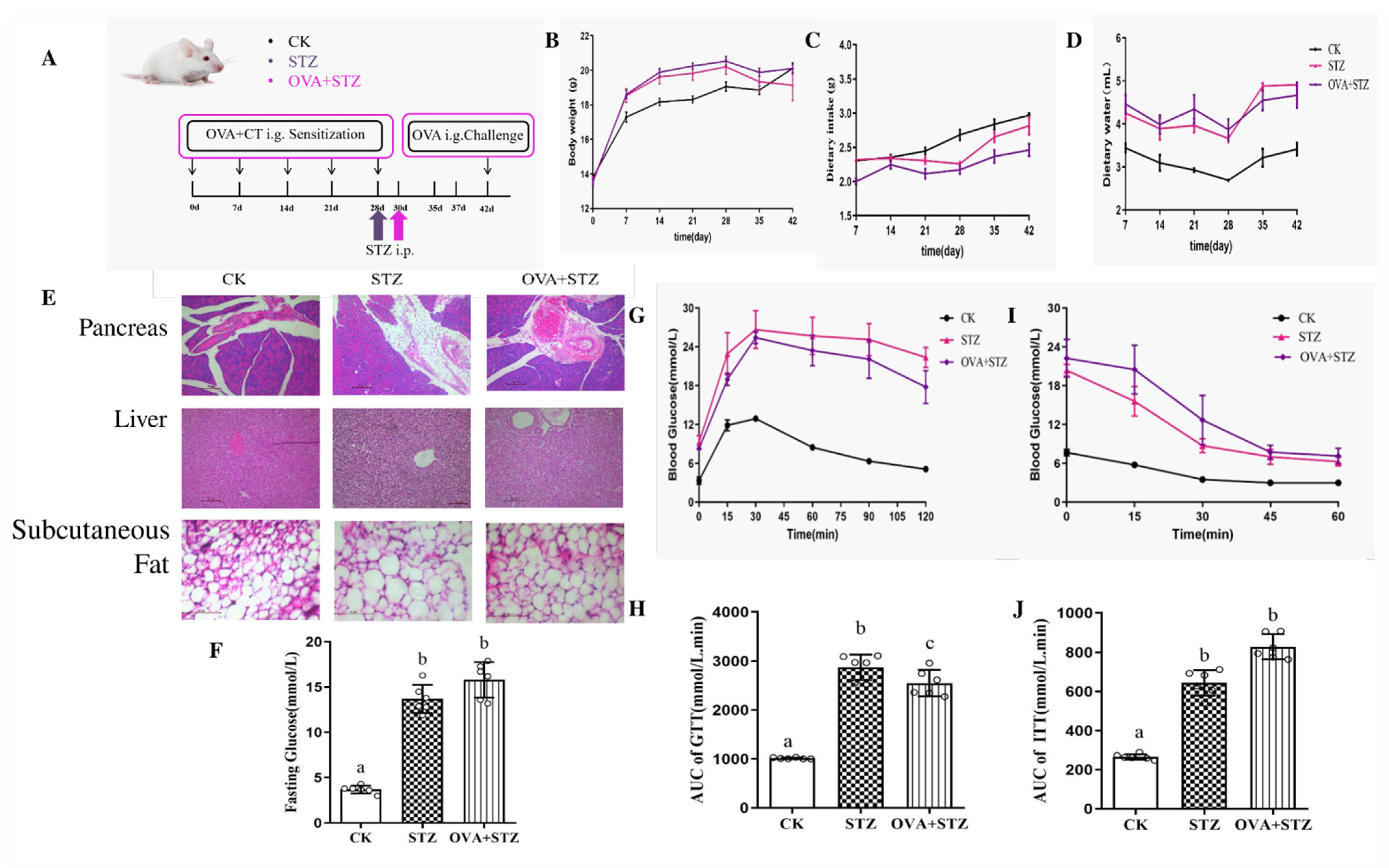
3.1. Establishment of Diabetic Allergic (STZ + OVA) Mice Model
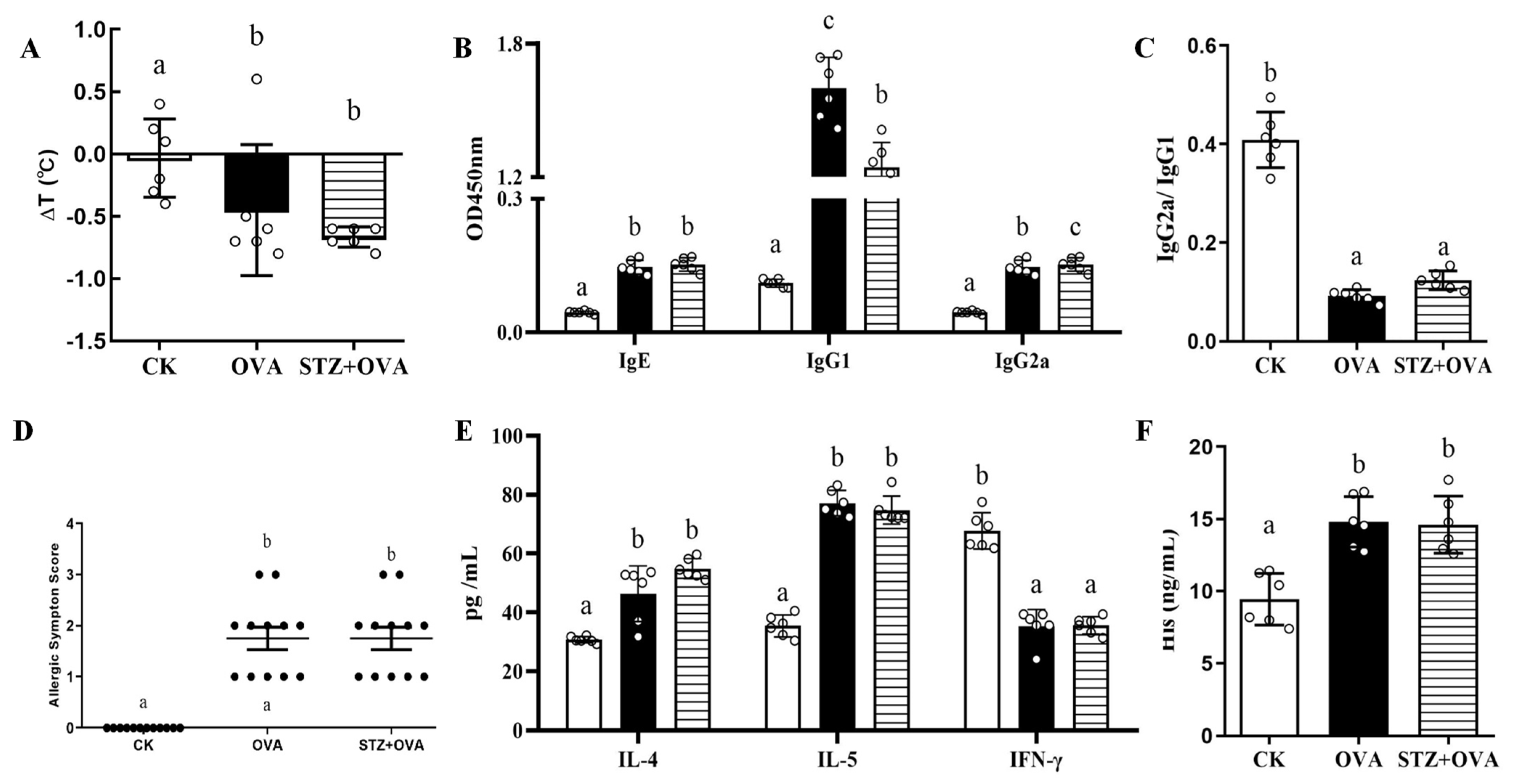
3.2. Effects of Diabetes on Food Allergy
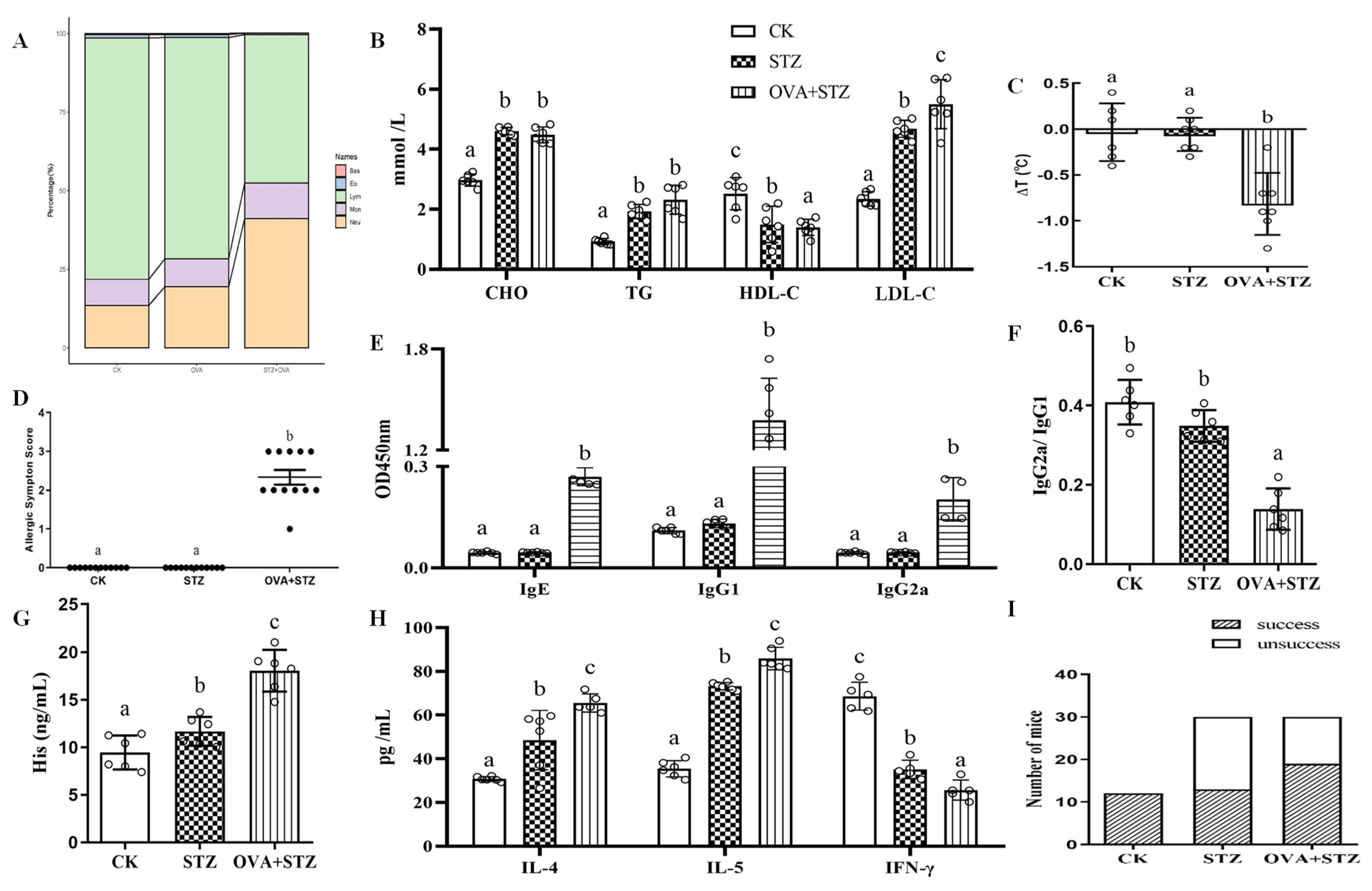
3.3. Establishment of Allergic Diabetic (OVA + STZ) Mice Model
3.4. Effects of Food Allergy on Diabetes
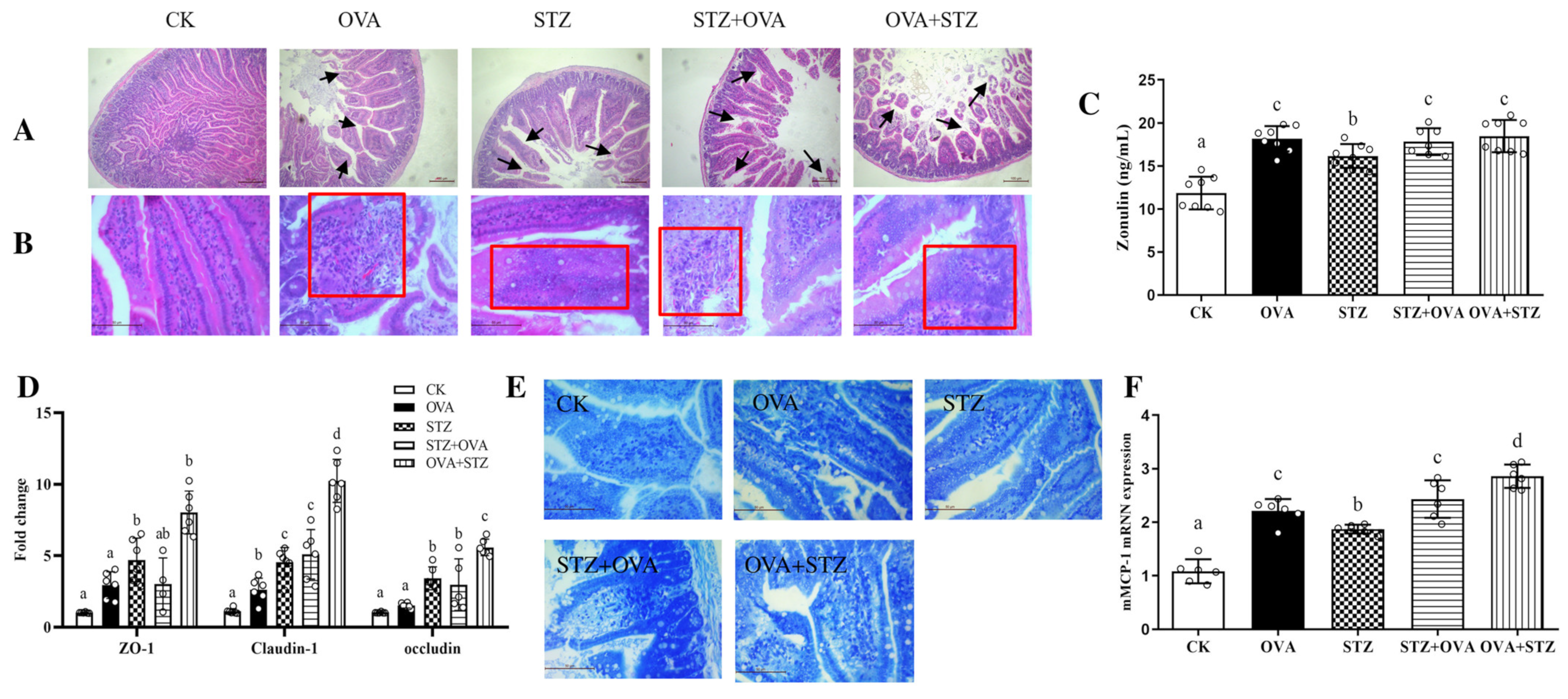
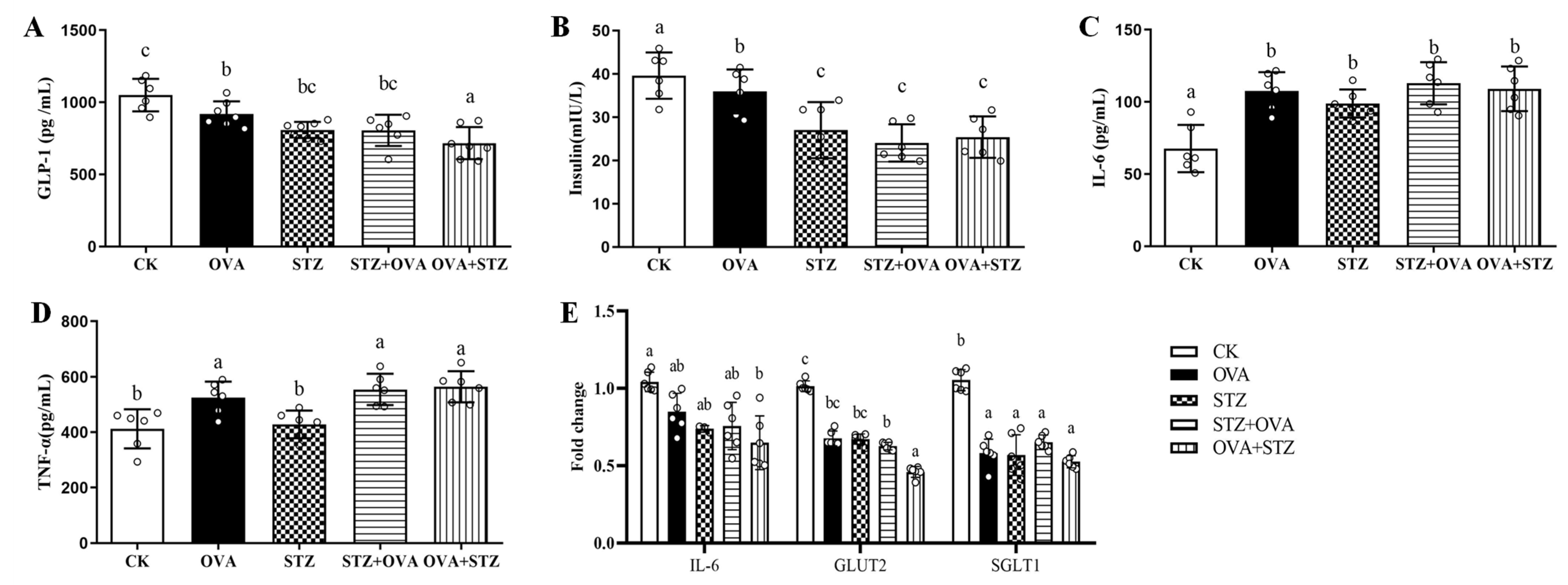
3.5. Food Allergy Affects Diabetes by Demoting the Secretion of GLP-1
3.6. Food Allergy Affects Diabetes by Upregulating the Expression of PI3K/Akt/mTOR/NF-κB p65
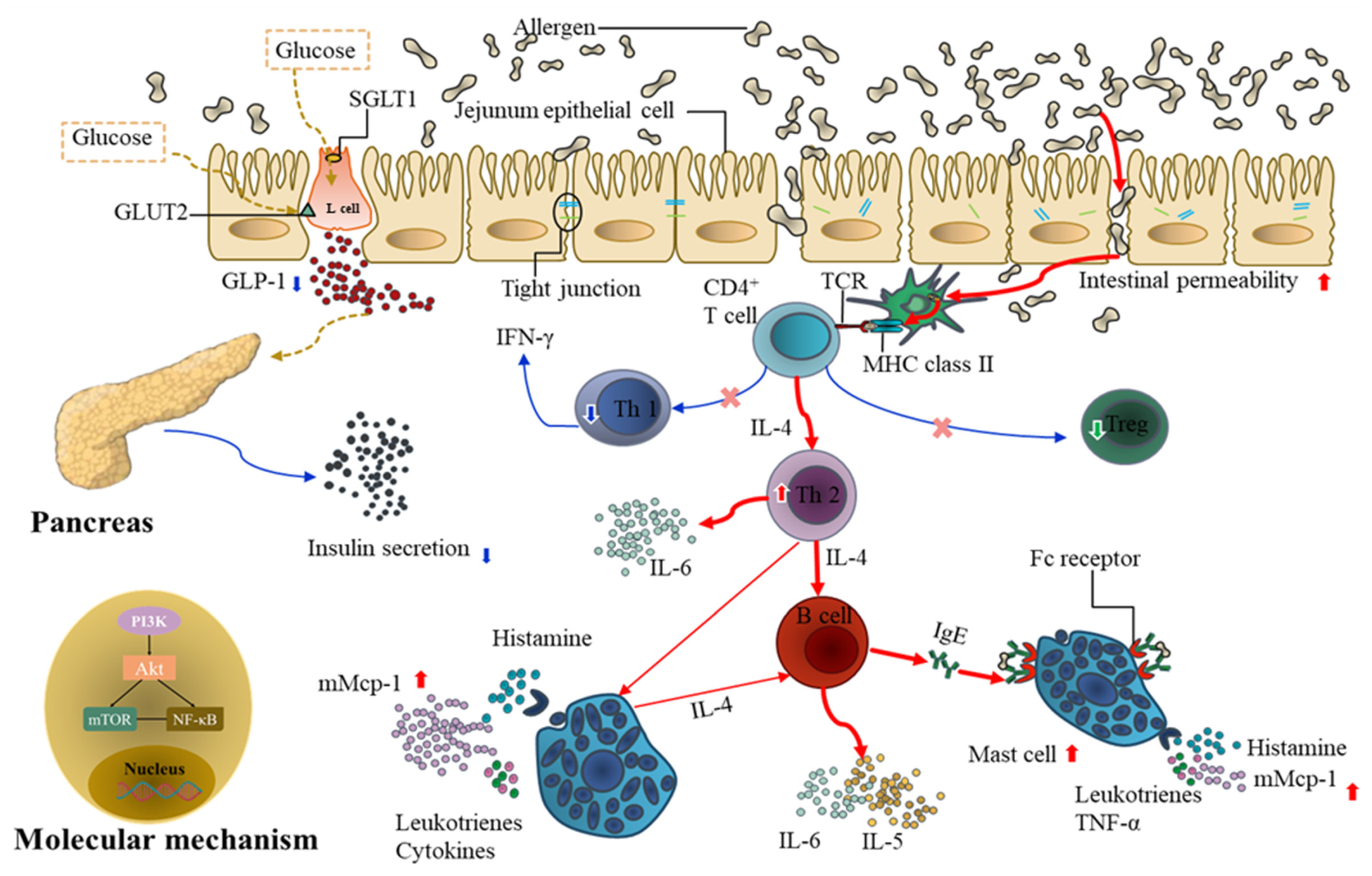
4. Discussion
5. Conclusions
- (1)
- The diabetic allergic (STZ + OVA) model was successfully developed by basic growth status and glucose sensitivity. Compared to the STZ group, STZ treatment could not further disrupt the secretion of immunoglobulin and cytokines induced by OVA administration;
- (2)
- Allergic diabetic (OVA + STZ) model was successfully developed by basic growth status and glucose sensitivity. Compared to the OVA group, when mice were in a state of sensitization, the pathological morphology of the viscera, the composition of peripheral blood leukocytes, and the metabolic level were altered, the secretion of immunoglobulin and cytokines could further disrupt and would have a higher susceptibility to diabetes;
- (3)
- Food allergy affects diabetes by promoting jejunal barrier destruction through the damage of tight junction proteins and the proliferation and activation of mast cells;
- (4)
- Food allergy affects diabetes by demoting the secretion of GLP-1. When mice were in the OVA-sensitized state, secretion of GLP-1 secretion is inhibited, in accompany by decreased insulin, IL-6, TNF-α, SGLT1, and GLUT2 levels;
- (5)
- Food allergy affects diabetes by upregulating the expression of PI3K/Akt/mTOR/NF-κB p65, especially mTOR, which plays an important role in the mechanism by which food allergy affects diabetes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wioletta, A.Z.; Paula, W.; Piotr, A.; Przemysław, K. Causes, symptoms and prevention of food allergy. Postepy Dermatol. Alergol. 2013, 30, 113–116. [Google Scholar]
- Novembre, E.; de Martino, M.; Vierucci, A. Foods and respiratory allergy. J. Allergy Clin. Immunol. 1988, 81, 1059–1065. [Google Scholar] [CrossRef]
- Sampath, V.; Sindher, S.B.; Zhang, W.; Nari, C.N. New treatment directions in food allergy. Ann. Allergy Asthma Immunol. 2018, 120, 254–262. [Google Scholar] [CrossRef]
- Suzuki, S.; Bandoh, N.; Goto, T.; Uemura, A.; Sasaki, M.; Harabuchi, Y. Severe laryngeal edema caused by Pseudoterranova species: A case report. Medicine 2021, 100, e24456. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, X.; Sun, S.; Che, H. High-Fat Diet-Induced Obesity Aggravates Food Allergy by Intestinal Barrier Destruction and Inflammation. Int. Arch. Allergy Immunol. 2022, 183, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Yang, Z.Y.; Dai, W.K.; Huang, J.Q.; Li, Y.H.; Zhang, J.; Qiu, C.Z.; Wei, C.; Zhou, Q.; Sun, X.; et al. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and β-lactoglobulin-induced intestinal food allergy mouse models. World J. Gastroenterol. 2017, 23, 2149–2158. [Google Scholar] [CrossRef]
- Kashiwakura, J.I.; Ando, T.; Karasuyama, H.; Kubo, M.; Matsumoto, K.; Matsuda, T.; Kawakami, T. The basophil-IL-4-mast cell axis is required for food allergy. Allergy 2019, 74, 1992–1994. [Google Scholar] [CrossRef]
- Oguntibeju, O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- De Fronzo, R.A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. North Am. 2004, 88, 787–835. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- American, D.A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- Nickerson, H.D.; Dutta, S. Diabetic complications: Current challenges and opportunities. J. Cardiovasc Transl. Res. 2012, 5, 375–379. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Kumar, R.; Hong, X.; Story, R.; Yu, Y.; Pongracic, J.; Wang, X. Gestational Diabetes is Associated with Food Allergen Sensitization in an Inner-City Population. J. Allergy Clin. Immunol. 2009, 123, S268. [Google Scholar] [CrossRef]
- Azad, M.B.; Becker, A.B.; Kozyrskyj, A.L. Association of maternal diabetes and child asthma. Pediatr. Pulmonol. 2013, 48, 545–552. [Google Scholar] [CrossRef]
- Haataja, P.; Korhonen, P.; Ojala, R.; Hirvonen, M.; Paassilta, M.; Gissler, M.; Luukkaala, T.; Tammela, O. Asthma and atopic dermatitis in children born moderately and late preterm. Eur. J. Pediatr. 2016, 175, 799–808. [Google Scholar] [CrossRef]
- Husemoen, L.; Glümer, C.; Lau, C.; Pisinger, C.; Mørch, L.; Linneberg, A. Association of obesity and insulin resistance with asthma and aeroallergen sensitization. Allergy 2008, 63, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Lamminsalo, A.; Lundqvist, A.; Virta, L.J.; Gissler, M.; Kaila, M.; Metsälä, J.; Virtanen, S.M. Cow’s milk allergy in infancy and later development of type 1 diabetes–nationwide case-cohort study. Pediatr. Diabetes 2021, 22, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Klamt, S.; Vogel, M.; Kapellen, T.M.; Hiemisch, A.; Prenzel, F.; Zachariae, S.; Ceglarek, U.; Thiery, J.; Kiess, W. Association between IgE-mediated allergies and diabetes mellitus type 1 in children and adolescents. Pediatr. Diabetes 2015, 16, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Cavagni, G.; Pierdomenico, R.; Chiari, G.; Spattini, A.; Vanelli, M. Coexistence of IgE-mediated allergy and type 1 diabetes in childhood. Int. Arch. Allergy Immunol. 2004, 134, 288–294. [Google Scholar] [CrossRef]
- Mirghani, H.O.; Alhazmi, K.; Alghamdi, S.; Alraddadi, M. The Cross-Talk between Atopic Dermatitis and Diabetes Mellitus: A Meta-Analysis. Cureus 2021, 13, e13750. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.-E.; Vaillant, J.-C.; Oppert, J.-M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 3222, 604054. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Lau, J.J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, J.; Gao, L.; Bi, Y.; Li, P.; Wang, H.; Zhu, D.; Feng, W. Liraglutide attenuates nonalcoholic fatty liver disease by modulating gut microbiota in rats administered a high-fat diet. BioMed. Res. Int. 2020, 2020, 2947549. [Google Scholar] [CrossRef] [PubMed]
- Malm-Erjefalt, M.; Ekblom, M.; Vouis, J.; Zdravkovic, M.; Lennernas, H. Effect on the gastrointestinal absorption of drugs from different classes in the biopharmaceutics classification system, when treating with liraglutide. Mol. Biopharm. 2015, 12, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wu, X.; Zhang, W.; Xiao, M. Glucagon like peptide-1 (GLP-1) modulates OVA-induced airway inflammation and mucus secretion involving a protein kinase A (PKA)-dependent nuclear factor-κB (NF-κB) signaling pathway in mice. Int. J. Mol. Sci. 2015, 16, 20195–20211. [Google Scholar] [CrossRef] [PubMed]
- McCravy, M.; Ingram, J.L.; Que, L.G. Dysregulated Metabolism in the Pathophysiology of Non-Allergic Obese Asthma. J. Asthma Allergy 2021, 14, 179. [Google Scholar] [CrossRef]
- Khan, F.; Mat, A.; Hogan, A.; Kent, B.D.; Eigenheer, S.; Corrigan, M.A.; O’Shea, D.; Butler, M. Preliminary asthma-related outcomes following glucagon-like peptide 1 agonist therapy. QJM Int. J. Med. 2017, 110, 853–854. [Google Scholar] [CrossRef]
- Xu, G.; Li, Z.; Ding, L.; Tang, H.; Guo, S.; Liang, H.; Wang, H.; Zhang, W. Intestinal mTOR regulates GLP-1 production in mouse L cells. Diabetologia 2015, 58, 1887–1897. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jin, G.Y.; Li, L.C.; Yan, G.H. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS ONE 2013, 8, e81773. [Google Scholar] [CrossRef]
- Mohseni, A.H.; Casolaro, V.; Bermúdez-Humarán, L.G.; Keyvani, H.; Taghinezhad-S, S. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes. 2021, 13, 1886844. [Google Scholar] [CrossRef]
- Tuo, Y.; Xiang, M. mTOR: A double-edged sword for diabetes. J. Leukocyte Biol. 2019, 106, 385–395. [Google Scholar] [CrossRef]
- Diesner, S.; Knittelfelder, R.; Krishnamurthy, D.; Pali-Schöll, I.; Gajdzik, L.; Jensen-Jarolim, E.; Untersmayr, E. Dose-dependent food allergy induction against ovalbumin under acid-suppression: A murine food allergy model. Immunol. Lett. 2008, 121, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Azman, S.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Wahidin, S.; Lum, P.T.; Dhadde, S.B. Traditional medicinal plants conferring protection against ovalbumin-induced asthma in experimental animals: A review. J. Asthma Allergy 2021, 14, 641. [Google Scholar] [CrossRef] [PubMed]
- Mestecky, J.; Lamm, M.E.; Ogra, P.L.; Strober, W.; Bienenstock, J.; McGhee, J.R.; Mayer, L. Mucosal Immunology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lin, H.; Huang, H.; Chuang, Y. In vivo rodent models of type 2 diabetes and their usefulness for evaluating flavonoid bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Kennard, M.; Daniels, L.; Roberts, A.; White, E.; Nandi, M.; King, A. The use of mice in diabetes research: The impact of experimental protocols. Diabetic Med. 2021, 38, e14705. [Google Scholar] [CrossRef]
- Li, Y.; Hou, J.; Liu, Z.; Gong, X.; Hu, J.; Wang, Y.; Li, W. Alleviative effects of 20 (R)-Rg3 on HFD/STZ-induced diabetic nephropathy via MAPK/NF-κB signaling pathways in C57BL/6 mice. J. Ethnopharmacol. 2021, 267, 113500. [Google Scholar] [CrossRef]
- Zhiping, W.; Junbiao, W.; Qun, Z.; Yifei, W.; Tongsheng, C. Berberine nanosuspension enhances hypoglycemic efficacy on streptozotocin induced diabetic C57BL/6 mice. J. Evid.-Based Complement. Altern. Med. 2015, 2015, 239749. [Google Scholar]
- Rubinstein, M.R.; Genaro, A.M.; Wald, M.R. Differential effect of hyperglycaemia on the immune response in an experimental model of diabetes in BALB/cByJ and C57Bl/6J mice: Participation of oxidative stress. Clin. Exp. Immunol. 2013, 171, 319–329. [Google Scholar] [CrossRef]
- Wang, J.; Lian, D.W.; Yang, X.F.; Xu, Y.F.; Chen, F.J.; Lin, W.J.; Wang, R.; Tang, L.Y.; Ren, W.K.; Fu, L.J.; et al. Suo quan wan protects mouse from early diabetic bladder dysfunction by mediating motor protein myosin va and transporter protein SLC17A9. Front. Pharmacol. 2019, 10, 552. [Google Scholar] [CrossRef]
- Perrier, C.; Thierry, A.C.; Mercenier, A.; Corthesy, B. Allergen-specific antibody and cytokine responses, mast cell reactivity and intestinal permeability upon oral challenge of sensitized and tolerized mice. J. Allergy Clin. Immunol. 2010, 4, 153–162. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Zheng, Y.; Yue, F.; Russell, R.D.; Zeng, Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res. Clin. Pract. 2014, 106, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Albert-Bayo, M.; Paracuellos, I.; González-Castro, A.M.; Rodríguez-Urrutia, A.; Rodríguez-Lagunas, M.J.; Alonso-Cotoner, C.; Santos, J.; Vicario, M. Intestinal mucosal mast cells: Key modulators of barrier function and homeostasis. Cells 2019, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.; DeYoung, M.; Lowe, C.; Parkes, D. GLP-1 receptor activated insulin secretion from pancreatic β-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Kahles, F.; Meyer, C.; Möllmann, J.; Diebold, S.; Findeisen, H.M.; Lebherz, C.; Trautwein, C.; Koch, A.; Tacke, F.; Marx, N.; et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6–dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes 2014, 63, 3221–3229. [Google Scholar] [CrossRef]
- Lehrskov-Schmidt, L.; Lehrskov-Schmidt, L.; Nielsen, S.T.; Holst, J.J.; Møller, K.; Solomon, T.P. The effects of TNF-α on GLP-1-stimulated plasma glucose kinetics. J. Clin. Endocrinol. Metab. 2015, 100, E616–E622. [Google Scholar] [CrossRef]
- Rowlands, J.; Heng, J.; Newsholme, P.; Carlessi, R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front. Endocrinol. 2018, 9, 672. [Google Scholar] [CrossRef]
- Dales, R.; Chen, Y.; Lin, M.; Karsh, J. The association between allergy and diabetes in the Canadian population: Implications for the Th1-Th2 hypothesis. Eur. J. Epidemiol. 2005, 20, 713–717. [Google Scholar] [CrossRef]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The streptozotocin-induced diabetic nude mouse model: Differences between animals from different sources. Comp. Med. 2011, 61, 356–360. [Google Scholar]
- Villa, M.P.; Cacciari, E.; Bernardi, F.; Cicognani, A.; Salardi, S.; Zapulla, F. Bronchial reactivity in diabetic patients: Relationship to duration of diabetes and degree of glycemic control. Am. J. Dis. Child. 1988, 142, 726–729. [Google Scholar] [CrossRef]
- Thomsen, S.F.; Duffy, D.; Kyvik, K.; Skytthe, A.; Backer, V. Risk of asthma in adult twins with type 2 diabetes and increased body mass index. Allergy 2011, 66, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Smeeth, L.; Hubbard, R. There is no evidence of an inverse relationship between TH2-mediated atopy and TH1-mediated autoimmune disorders: Lack of support for the hygiene hypothesis. J. Allergy Clin. Immunol. 2003, 11, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Alam, C.; Bittoun, E.; Bhagwat, D.; Valkonen, S.; Saari, A.; Jaakkola, U.; Hänninen, A. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia 2011, 5, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Dong, G.; Guo, L.; Graves, D.T. The function of dendritic cells in modulating the host response. Mol. Oral Microbiol. 2018, 3, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Maisey, A. A practical approach to gastrointestinal complications of diabetes. Diabetes Ther. 2016, 7, 379–386. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, D.; Zhao, J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World J. Diabetes 2017, 8, 249. [Google Scholar] [CrossRef]
- Borgström, B.; Dahlqvist, A.; Lundh, G.; Sjövall, J. Studies of intestinal digestion and absorption in the human. J. Clin. Investig. 1957, 36, 1521–1536. [Google Scholar] [CrossRef]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Zanzi, D.; Rapacciuolo, L.; Franzese, A.; Sblattero, D.; et al. Majority of children with type 1 diabetes produce and deposit anti-tissue transglutaminase antibodies in the small intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef]
- Cox, A.J.; Zhang, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef]
- El Bahrawey, M.; Imbaby, S.; El Fazary, H.; Badrah, M.; El Deen, H.T. Diabetes, Endocrinology. Study of serum zonulin level as an early predictor for gestational diabetes in Egyptian females. Egypt. J. Obes. Diabetes Endocrinol. 2020, 6, 54. [Google Scholar]
- Salinari, S.; le Roux, C.W.; Bertuzzi, A.; Rubino, F.; Mingrone, G. Duodenal-jejunal bypass and jejunectomy improve insulin sensitivity in Goto-Kakizaki diabetic rats without changes in incretins or insulin secretion. Egypt. J. Obes. Diabetes Endocrinol. Diabetes 2014, 63, 1069–1078. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samadi, N.; Klems, M.; Untersmayr, E. The role of gastrointestinal permeability in food allergy. Ann. Allergy Asthma Immunol. 2018, 121, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- MacGlashan, J.D.; Lavens-Phillips, S.; Katsushi, M. IgE-mediated desensitization in human basophils and mast cells. Front. Biosci.-Landmark 1998, 3, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hotamisligil, G.S. Inflammation, stress, and diabetes. J. Clin. Investig. 2005, 115, 1111–1119. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef]
- Filippidou, F.M.; Kirsch, A.H.; Thelen, M.; Kétszeri, M.; Artinger, K.; Aringer, I.; Schabhüttl, C.; Mooslechner, A.A.; Frauscher, B.; Pollheimer, M.; et al. Glucagon-like peptide-1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T-cell proliferation. Am. J. Pathol. 2020, 190, 400–411. [Google Scholar] [CrossRef]
- Morrow, N.M.; Hanson, A.A.; Mulvihill, E.E. Distinct Identity of GLP-1R, GLP-2R, and GIPR Expressing Cells and Signaling Circuits within the Gastrointestinal Tract. Front. Cell Dev. Biol. 2021, 9, 703966. [Google Scholar] [CrossRef]
- He, S.; Kahles, F.; Rattik, S.; Nairz, M.; McAlpine, C.S.; Anzai, A.; Selgrade, D.; Fenn, A.M.; Chan, C.T.; Mindur, J.E.; et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 2019, 566, 115–119. [Google Scholar] [CrossRef]
- Wu, A.Y.; Peebles, R.S. The GLP-1 receptor in airway inflammation in asthma: A promising novel target? Expert Rev. Clin. Immunol. 2021, 17, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose sensing in L cells: A primary cell study. Cell Metab. 2008, 8, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Ellingsgaard, H.; Seelig, E.; Timper, K.; Coslovsky, M.; Soederlund, L.; Lyngbaek, M.P.; Albrechtsen, N.J.W.; Schmidt-Trucksäss, A.; Hanssen, H.; Frey, W.O.; et al. GLP-1 secretion is regulated by IL-6 signalling: A randomised, placebo-controlled study. Diabetologia 2020, 63, 362–373. [Google Scholar] [CrossRef]
- Kappe, C.; Tracy, L.M.; Patrone, C.; Iverfeldt, K.; Sjöholm, Å.J. GLP-1 secretion by microglial cells and decreased CNS expression in obesity. J. Neuroinflamm. 2012, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Wueest, S.; Laesser, C.I.; Böni-Schnetzler, M.; Item, F.; Lucchini, F.C.; Borsigova, M.; Müller, W.; Donath, M.Y.; Konrad, D. IL-6–type cytokine signaling in adipocytes induces intestinal GLP-1 secretion. Diabetes 2018, 67, 36–45. [Google Scholar] [CrossRef]
- Deo, S.S.; Mistry, K.J.; Kakade, A.M.; Niphadkar, P.V. Role played by Th2 type cytokines in IgE mediated allergy and asthma. Lung India 2010, 27, 66. [Google Scholar] [CrossRef]
- Sun, E.W.; de Fontgalland, D.; Rabbitt, P.; Hollington, P.; Sposato, L.; Due, S.L.; Wattchow, D.A.; Rayner, C.K.; Deane, A.M.; Young, R.L.; et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes 2017, 66, 2144–2149. [Google Scholar] [CrossRef]
- Xu, G.; Hong, X.; Tang, H.; Jiang, S.; Liu, F.; Shen, Z.; Li, Z.; Zhang, W. Ghrelin regulates GLP-1 production through mTOR signaling in L cells. Mol. Cell Endocrinol. 2015, 416, 9–18. [Google Scholar] [CrossRef]
- Kimura, R.; Okouchi, M.; Fujioka, H.; Ichiyanagi, A.; Ryuge, F.; Mizuno, T.; Imaeda, K.; Okayama, N.; Kamiya, Y.; Asai, K.; et al. Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. BMC Neurosci. 2009, 162, 1212–1219. [Google Scholar] [CrossRef]
- Bader, A.G.; Kang, S.; Zhao, L.; Vogt, P.K. Oncogenic PI3K deregulates transcription and translation. Nat. Rev. Cancer 2005, 5, 921–929. [Google Scholar] [CrossRef]
- Weichhart, T.; Säemann, M. The PI3K/Akt/mTOR pathway in innate immune cells: Emerging therapeutic applications. Ann. Rheum. Dis. 2008, 67, iii70–iii74. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wuniqiemu, T.; Tang, W.; Teng, F.; Bian, Q.; Yi, L.; Qin, J.; Zhu, X.; Wei, Y.; Dong, J. Luteolin inhibits autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling and inhibiting Beclin-1-PI3KC3 complex. Int. Immunopharmacol. 2021, 94, 107460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Xu, Z.; Hao, L.; Zhang, Y.; Liu, Z. PI3K-AKT-mTOR signaling pathway: The intersection of allergic asthma and cataract. Die Pharm.-Int. J. Pharm. Sci. 2019, 74, 598–600. [Google Scholar]
- Bathina, S.; Das, U.N. Dysregulation of PI3K-Akt-mTOR pathway in brain of streptozotocin-induced type 2 diabetes mellitus in Wistar rats. Lipids Health Dis. 2018, 17, 168. [Google Scholar] [CrossRef]
- Wang, B.; Zhong, Y.; Li, Q.; Cui, L.; Huang, G. Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy. Aging 2018, 10, 2772. [Google Scholar] [CrossRef]
- Huang, C.; Lin, M.Z.; Cheng, D.; Braet, F.; Pollock, C.A.; Chen, X.M. 1 mediates dysfunction of tubular autophagy in diabetic kidneys via PI3k/Akt/mTOR signaling pathways. Sci. Rep. 2016, 6, 23884. [Google Scholar] [CrossRef]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Role of the PI3K/AKT (mTOR and GSK3β) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019, 50, 52–59. [Google Scholar] [CrossRef]
- Jacot, J.L.; Sherris, D.J. Potential therapeutic roles for inhibition of the PI3K/Akt/mTOR pathway in the pathophysiology of diabetic retinopathy. J. Ophthalmol. 2011, 2011, 589813. [Google Scholar] [CrossRef]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]


| Gene Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| β-actin | GATTACTGCTCTGGCTCCTAGC | GACTCATCGTACTCCTGCTTGC |
| ZO-1 | TTTTTGACAGGGGGAGTGG | TGCTGCAGAGGTCAAAGTTCAAG |
| Claudin-1 | CGGGTTGCCTGCAAAGT | ATGTCCGGCCGATGCTCTC |
| occludin | CTTTGGCTGCTGTTGGGTCTG | AGCCAGGAGCCTCGCCCCGCAGCTGCA |
| mMCP-1 | CAGATGTGGTGGGTTTCTCA | GCTCACATCATGAGCTCCAA |
| IL-6 | CGTGGAAATGAGAAAAGAGTTGTGC | ATGCTTAGGCATAACGCACTAGGT |
| SGLT1 | CGGAAGAAGGCATCTGAGAA | AATCAGCACGAGGATGAACA |
| GLUT2 | TCTTCACGGCTGTCTCGTG | AATCATCCCGGTTAGGAACA |
| mTOR | ACCGGCACACATTTGAAGAAG | CTCGTTGAGGATCAGCAAGG |
| PI3K | CACTCAGCCCATCTATTTCCAG | TCTTGGATCTTCACCTTCAGC |
| AKT | GACTGACACCAGGTATTTCGATGA | CTCCGCTCACTGTCCACACA |
| NF-κB p65 | GCATTCTGACCTTGCCTAT | ACCGCCACTACCGAACAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Yao, L.; Jiang, T.; Che, H. The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum. Foods 2022, 11, 3758. https://doi.org/10.3390/foods11233758
Gu Y, Yao L, Jiang T, Che H. The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum. Foods. 2022; 11(23):3758. https://doi.org/10.3390/foods11233758
Chicago/Turabian StyleGu, Yanjun, Lu Yao, Tianyi Jiang, and Huilian Che. 2022. "The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum" Foods 11, no. 23: 3758. https://doi.org/10.3390/foods11233758
APA StyleGu, Y., Yao, L., Jiang, T., & Che, H. (2022). The Interaction of Food Allergy and Diabetes: Food Allergy Effects on Diabetic Mice by Intestinal Barrier Destruction and Glucagon-like Peptide 1 Reduction in Jejunum. Foods, 11(23), 3758. https://doi.org/10.3390/foods11233758





