Abstract
Bone substitutes are being increasingly used in craniofacial surgery and cranio- maxillofacial trauma. We will review the history of the biomaterials and describe the ideal characteristics of bone substitutes, with a specific emphasis on craniofacial reconstruction. Some of the most commonly used bone substitutes are discussed in more depth, such as calcium phosphate and hydroxyapatite ceramics and cements, bioactive glass, and polymer products. Areas of active research and future directions include tissue engineering, with an increasing emphasis on bioactivity of the implant.
Bone substitutes are being increasingly used in craniofacial surgery. This is due to their ease of use and handling, improved safety profiles, intraoperative cost and time advantages, and adaptability to a variety of clinical challenges. A wide variety of bone substitutes have been employed over the past 50 years, as shown in Table 1. Biomaterials used in the osseous reconstruction of the craniofacial skeleton can be broadly categorized into calcium phosphate-based ceramics and cements, synthetic polymers, and, most recently, tissue-engi- neered bone substitutes. We will review some of the most important biomaterials in each of these categories. The art of repairing a bone defect has been accomplished and refined by man for thousands of years. Excavations of a Neolithic era Peruvian tribal chief revealed a frontal bone defect repaired with a hammer- applied gold plate [1]. Examination of a skull from 2000 BC uncovered evidence of healing around a 7-mm defect repaired with a piece of animal bone [2]. The Egyptians practiced some extremely advanced orthopedic proce- dures for the time. Anthropologists have studied the mummy of a priest who had a 23-cm prosthesis inserted at the left knee. Examination of the device revealed that it had been made of pure iron and cemented with resin, and it was inserted while the priest was still alive [3].

Table 1.
Commonly Used Calcium-Based and Polymer Bone Substitutes.
Surgeon Job van Meekeren heralded the modern era of bone replacement in 1668 when he successfully performed the first heterologous graft by inserting the fragment of a dog skull into the skull of an injured soldier [4]. Because of the blasphemous nature of the operation, the soldier was excommunicated from the church. When he went to von Meekeren to have the graft removed, it had already become fully incorporated into his skull. In 1820, a Germany surgeon by the name of Philips von Walter performed the first autologous graft, replacing a cranium fragment after trepanation [5].
Although autologous bone grafting is still con- sidered the ‘‘gold standard’’ in bony defect repair, the past century has seen significant advances in alternatives to natural bone. A bone substitute can be defined as ‘‘a synthetic, inorganic or biologically organic combination which can be inserted for the treatment of a bone defect instead of autogenous or allogenous bone.’’[6] The first reported modern use of a bone substitute took place in 1892 when Dressmann used calcium sulfate, or plaster of Paris, to fill bony defects [7].
Methylmethacrylate, an acrylic resin, was first introduced in the 1940s and remains a popular choice as a bone substitute. It has more recently been combined with various metallic meshes to facilitate fixation and provide additional strength. The latter half of the 20th century has seen the evolution of the hydroxyapatite and calcium phosphate-based cements and ceramics. Current advances are being made with the development of tissue- engineered products, incorporating growth factors and stem cells.
The ideal bone substitute must have several im- portant properties. It must be biocompatible with the host and not evoke an adverse inflammatory response. It should be able to be easily molded to a bony defect with a practical time to set. Durability of the implant is im- portant so that it maintains its shape and volume over time. Radiolucency is ideal to allow for optimal radio- graphic assessment. Finally, the ideal bone substitute should also be thermally nonconductive, bioactive, able to be sterilized, and readily available, at a reasonable cost to purchase and use.
Hydroxyapatite [Ca10(PO4)6(OH)2] (HA) is a calcium phosphate compound that is the primary min- eral component of teeth and bone. For the past 30 years, it has been popular in craniofacial and orthognathic surgery, filling bony defects and smoothing contour irregularities. HA ceramics come in both naturally oc- curring and synthetic forms. Clinically available, natu- rally occurring forms of HA include the coral-based products Interpore and Pro-osteon (Interpore Interna- tional, Inc., Irvine, CA) as well as bovine derived products such as Bio-Oss (Geistlich Biomaterials, Geist- lich, Switzerland), Osteograf-N (CeraMed Co., Denver, CO), and Endobon (Merck Co., Darmstadt, Germany). The synthetic HA product is Calcitite (Sulzer Calcitek, Carlsbad, CA). HA ceramics are manufactured in a variety of forms including granules and porous blocks (Figure 1). HA is also frequently used as a coating on ring HA in the human body exists in a substituted form. Carbonate and silicates, among other ions, may replace hydroxyl or phosphate groups of the apatite structure. Investigators have attempted to produce carbonate- and silicon-substituted synthetic HA in an effort to produce HA that more closely resembles the mineral content of native bone, enhancing bioactivity and osteoconduc- tion [9]. Although there are few of products in clinical use at this time, HA substitution will likely remain an active area of research.
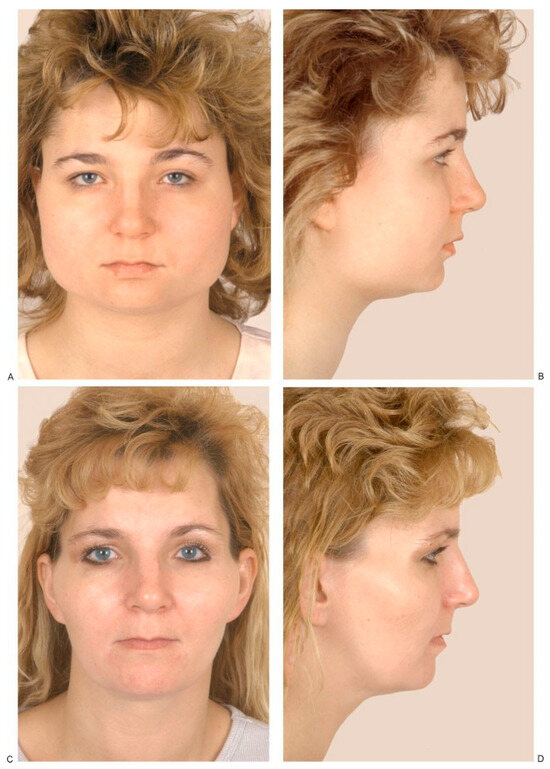
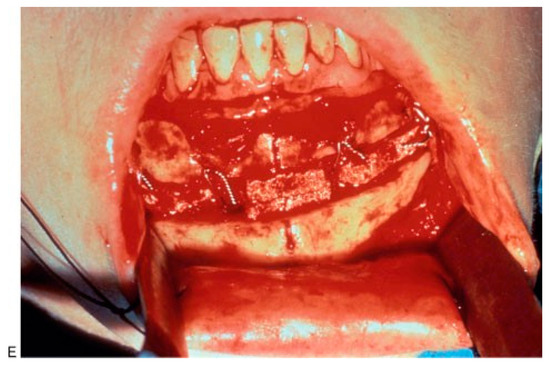
Figure 1.
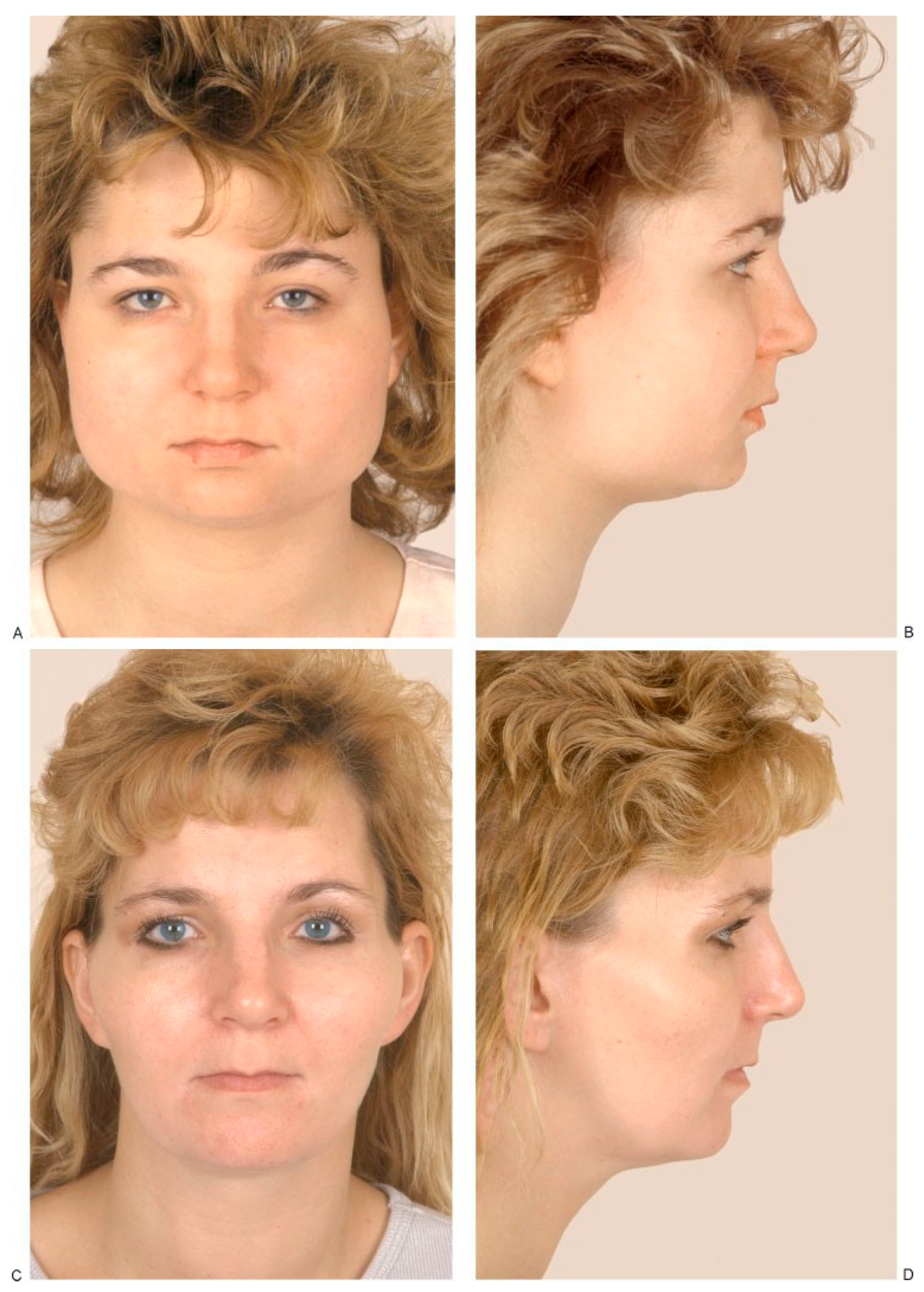
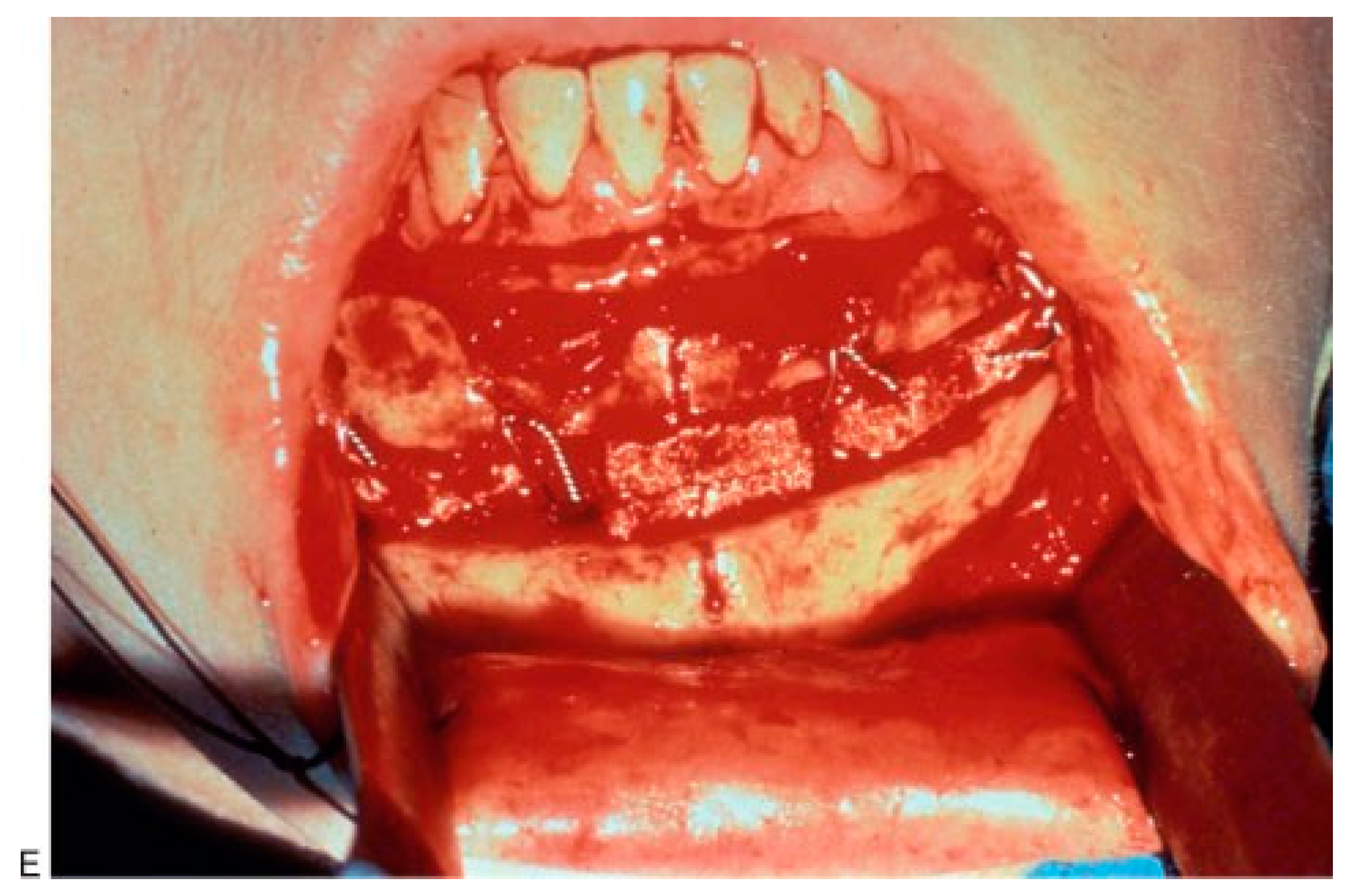
(A,B) Masseteric hypertrophy and vertical microgenia. Correction was performed by gonial angle resection bilaterally and vertical lengthening genioplasty using block hydroxyapatite. Front and profile views after surgery. (C,D) Frontal and profile views 8 years later. (E) Intraoperative view of vertical lengthening genioplasty using block hydroxyapatite. (Reprinted with permission from Zins JE, et al. Contour alteration of the facial skeleton. In: Achauer BM, Guyuron B, eds. Plastic Surgery: Indications, Operatons, Outcomes. Philadelphia: Elsevier; 2000:2824. Copyright Elsevier 2000.).
Tricalcium phosphate [Ca3(PO4)2] (TCP) is a synthetic compound created by sintering precipitated calcium-deficient apatite with calcium phosphate in a ratio of 1:5. TCP is more soluble than HA due to its small granule size and porosity. A pure TCP product is commercially available as Vitoss (Orthovita, Inc., Phil- adelphia, PA). This product is engineered to resemble cancellous bone and is used to fill traumatic cancellous bone defects. This product has more limited applications in craniofacial reconstruction (Figure 2), but many other calcium phosphate and HA cement products are more widely used.
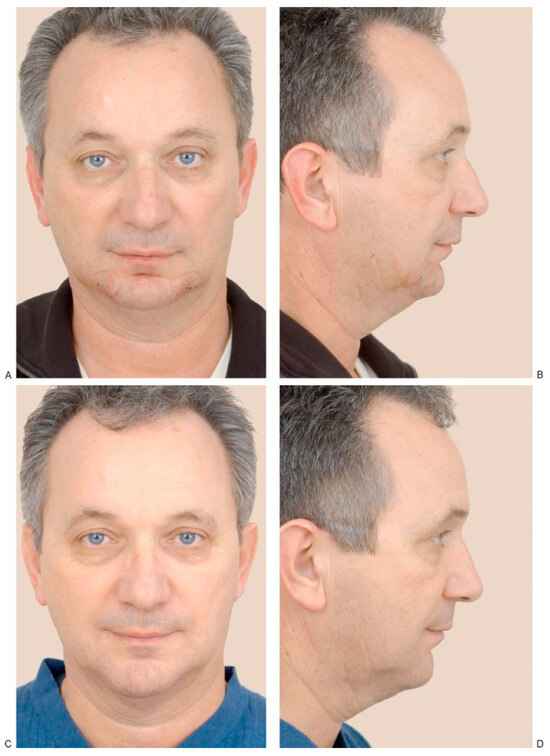
Figure 2.
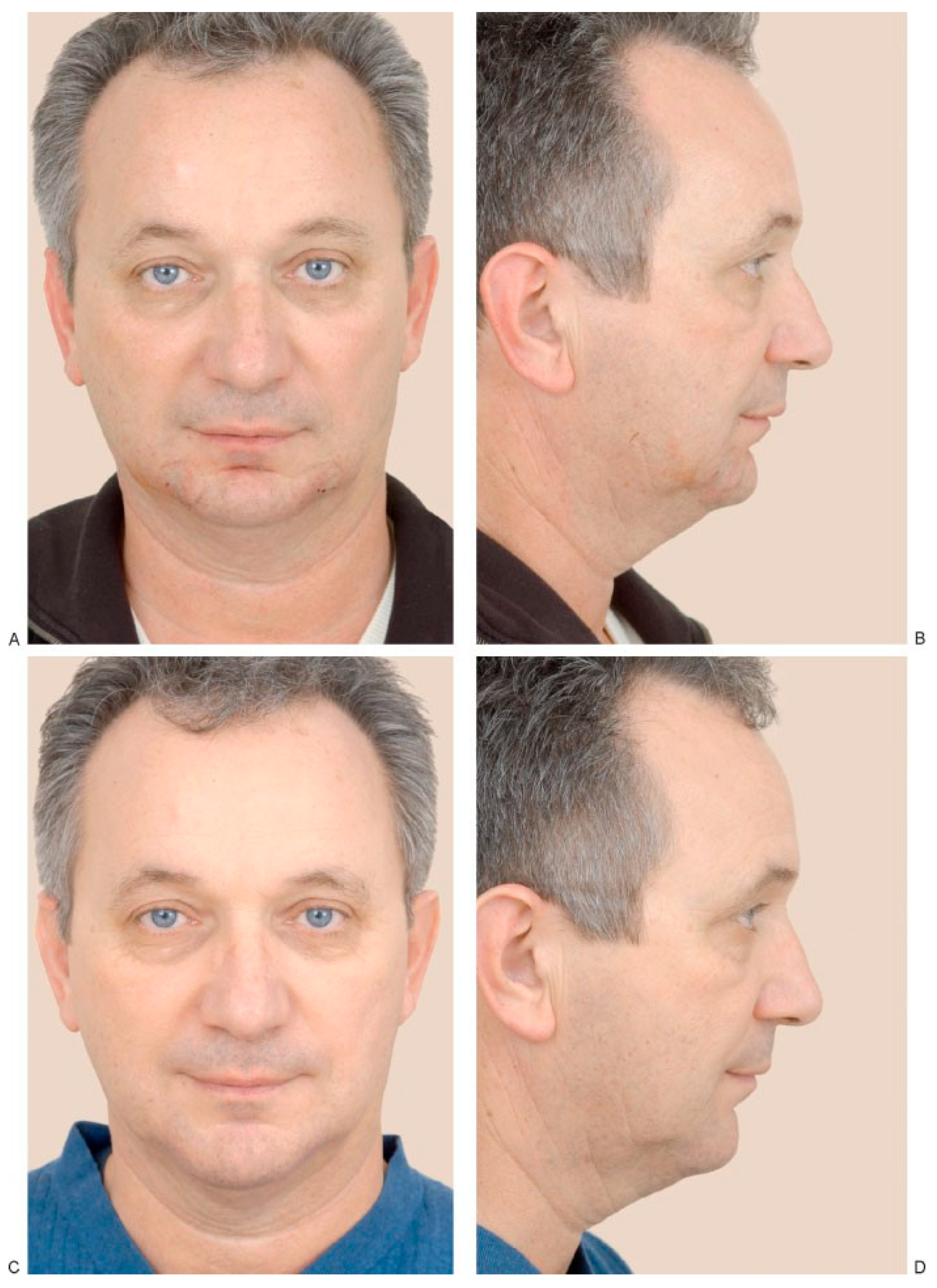
(A,B) A 55-year-old man who presented with a decrease in his lower vertical facial height due to vertical microgenia. Postoperative (C) frontal and (D) profile views following vertical lengthening genioplasty and interposition implant of tricalcium phosphate. (Reprinted with permission from Zins JE. Aesthetic surgery of the aging face and neck. In: Siemionow M, Eisenmann-Klein M, eds. Plastic and Reconstructive Surgery Series. Berlin: Springer; 2009:Figure 26.4).
Since the early 1990s, calcium phosphate cements have become one of the most versatile bone substitutes, filling calvarial defects and smoothing contour abnor- malities of the facial skeleton. These calcium phos- phate-based cements do not have sufficient tensile and compressive strength to be used in load-bearing appli- cations, but their ability to be precisely sculpted has made them extremely useful. A comparison of the three most commonly used HA cements is summarized in Table 2.

Table 2.
Comparison of the Properties of Calcium Phosphate Cements.
Norian CRS Bone Cement (Synthes, Paoli, PA) is a pliable calcium phosphate cement that is mixed in vivo to form dahllite, a carbonated apatite, once set. Originally approved by the Food and Drug Adminis- tration (FDA) for use in distal radius fractures [10], it has found broad application in craniofacial surgery. Norian CRS bone cement is prepared by mixing sodium phos- phate solution with calcium powder to form a bone putty. This putty begins to harden in 2 min during an exothermic reaction that may reach as high as 428C and is set in 10 min. Maximum strength is not achieved until 24 h [10]. In a recent study of Norian by Zins et al., reconstructed skull bone defects in sheep showed excellent osteointegration but almost no osteo- conductivity over the course of 12 months [11]. Drop-tests resulted in fractures of in the center of the construct rather than at the bone-cement interface. Norian is slowly absorbed over time and is not intended for use in load-bearing areas or in the presence of active infection.
Bone Source (Stryker-Leibinger, Kalamazoo, MI) is a self-setting calcium phosphate cement originally approved for use in filling burr holes and for facial skeleton augmentation [12] (Figure 3). It is prepared by mixing calcium phosphate salts in a sodium phosphate buffer, forming a bone putty that remains malleable for approx- imately 20 min. Bone Source hardens into HA, and like other HA cements, is very slowly absorbed over time. It is not intended to fill defects over 25 cm2 and lacks sufficient strength for load-bearing applications.
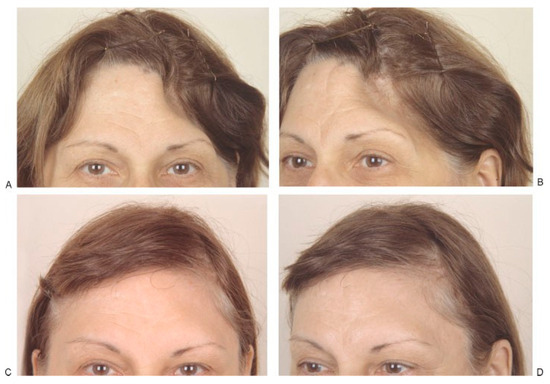
Figure 3.
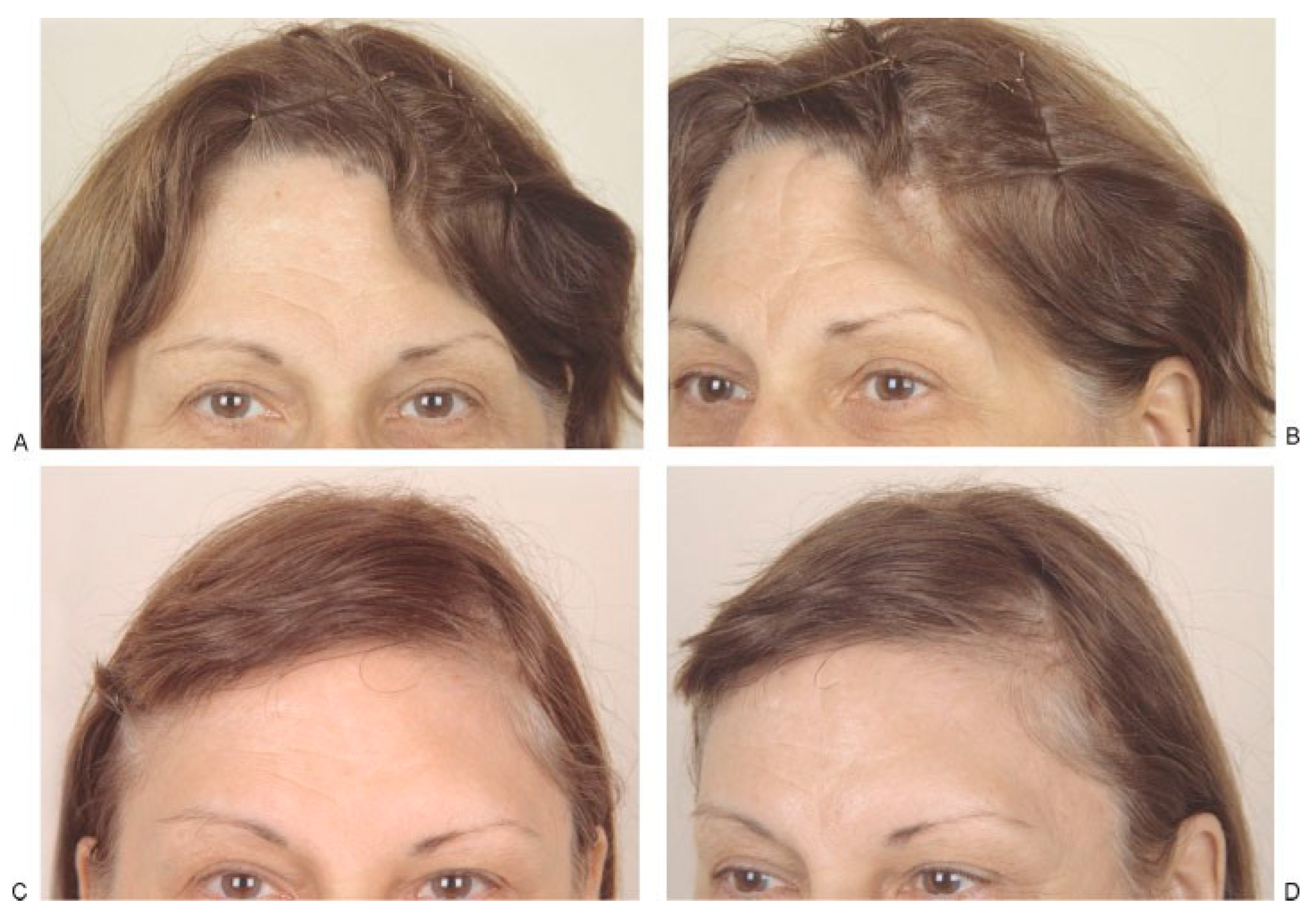
(A,B) Left frontotemporal defect in 48-year-old woman caused by removal of infected bone flap 2 years earlier. Frontal and three-quarter views shown before surgery. (C,D) Frontal and three-quarter views 6 months after reconstruction of full-thickness defect with hydroxyapatite bone paste (Bone Source). (Reprinted with permission from Zins JE, et al. Contour alteration of the facial skeleton. In: Achauer BM, Guyuron B, eds. Plastic Surgery: Indications, Operatons, Outcomes. Philadelphia: Elsevier; 2000:2829. Copyright Elsevier 2000).
Mimix Bone Void Filler (W. Lorenz Surgical, Jacksonville, FL), like Bone Source, achieved FDA ap- proval for use in filling burr hole and craniotomy defects and in smoothing facial skeletal contour abnormalities over a surface area of no larger than 25 cm2 [13]. This cement product is prepared by mixing dry components of calcium phosphate powder and sodium citrate dehydrate with an anhydrous citric acid solution. As it cures, Mimix hardens into HA and is mildly exothermic. Mimix Quickset is rapidly prepared, remains malleable for 3 to 4 min, and is completely set in 4 to 6 min, offering a potential advantage over other commercially available HA cement products that take longer to set and cure [14].
Since the introduction of HA cements, they have found broad use and application in craniofacial defects. The largest review of HA cements in craniofacial re- construction was published by Burstein and colleagues [15]. They reviewed 150 patients who underwent orbitocra- nial reconstruction using Bone Source and Mimix HA cements over 7 years. The majority of patients were reconstructed with an onlay technique, with or without adjunctive absorbable or titanium mesh. Excellent results were reported, with 92% of patients having a satisfactory result over a minimum of 1-year follow-up. The overall complication rate was 9% but no infections were reported.
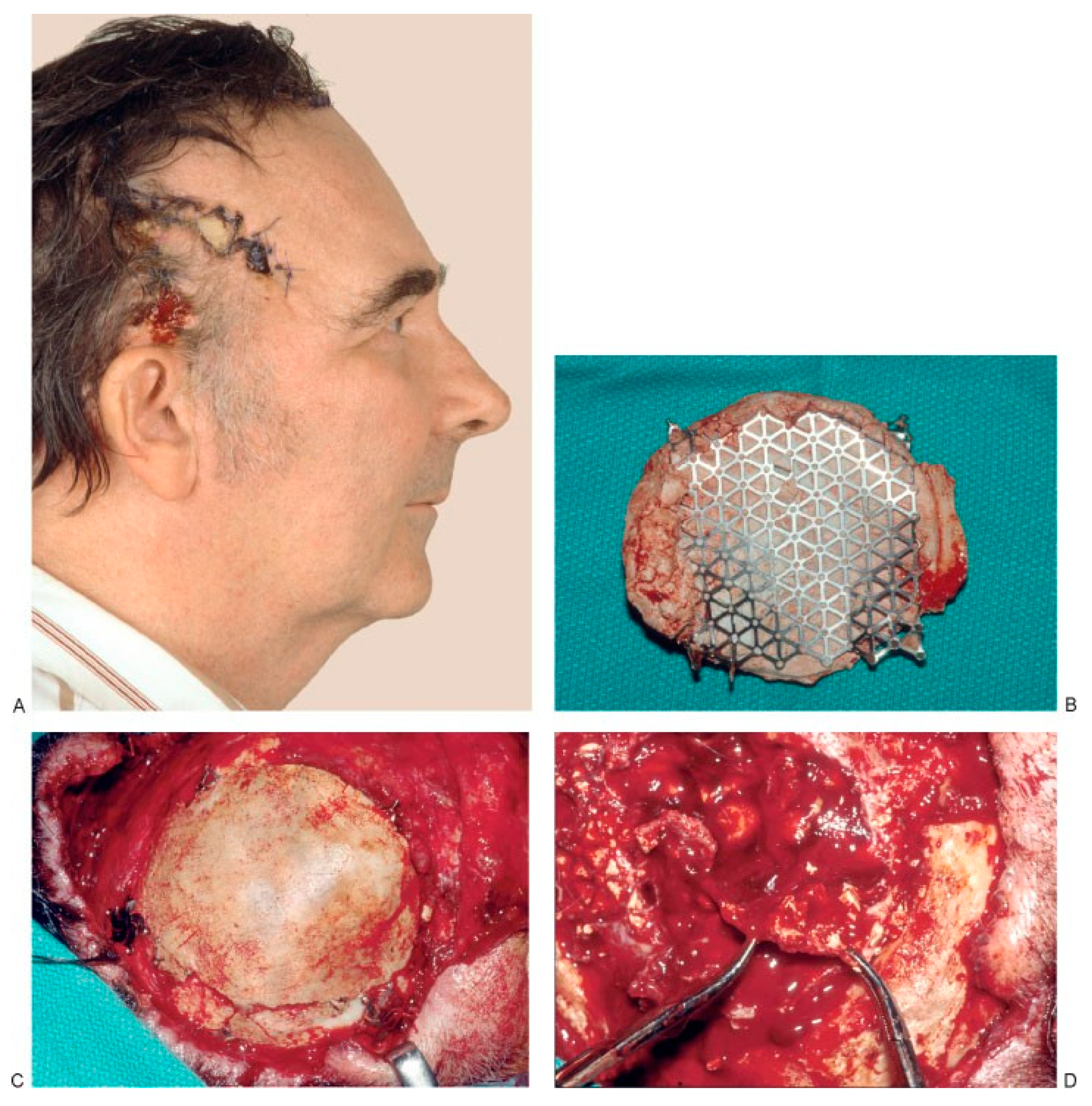
Other series have reported much higher rates of infection/exposure [16,17,18,19,20]. Moreira-Gonzalez et al. found that infection or extrusion occurred in 22.4%, with an increased risk for infection in the vicinity of the frontal sinus [19]. This finding has also been corroborated by Verret et al. [18]. In their review of 102 patients undergoing craniofacial reconstruction for traumatic and malig- nancy-related defects, they reported a 12% incidence of infection/foreign body reaction requiring implant re- moval. Tissue irradiation and frontal sinus involvement both increased the risk for these complications. Micro- fragmentation has also been reported as a complication of HA cement [15,16,20,21]. Losee et al. attribute microfrag- mentation to brain pulsations in cranial reconstruction and suggest that this risk can be mitigated in large defects by including mesh as an adjunct to cement [21]. Combined use of both titanium and absorbable mesh products with HA cements has been shown to be safe and effective by several authors [21,22,23]. Choice of HA cement may also affect the rate of microfragmentation, with Norian hav- ing recently demonstrated the highest mean fracture force required to fracture a standardized test piece [22]. Zins et al. reviewed 121 patients undergoing craniofacial recon- structions using Norian and Bone Source with and with- out mesh adjuncts and found an overall major complication rate of 15% [20]. However, in a subset of patients undergoing reconstructions for large (>25 cm2) defects, major complications occurred in 63% of patients (Figure 4). As a result of these findings, Zins recommends autogenous reconstruction for large cranial defects, even if mesh is utilized.
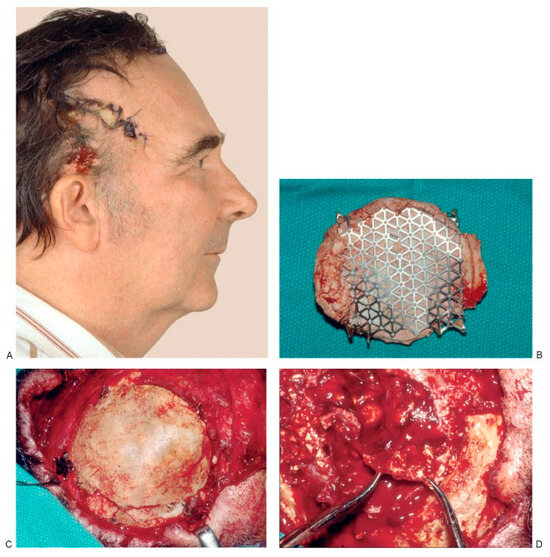

Figure 4.
(A) Preoperative view of a 54-year old man who underwent surgery for brain tumor removal and immediate reconstruction with calcium-based bone cement elsewhere. The cement became exposed, and two attempts to close the area using local flaps were performed. The patient was then referred to us for free flap coverage. (B–D) The patient underwent reoperation, and all calcium-based bone cement plus the titanium mesh used for reconstruction was removed. Part of the dura had to be excised because it was attached to the calcium-based bone cement. A dural patch was used for reconstruction. Calcium-based bone cement was used to reconstruct a 132-cm2 cranial defect 21 months later. (E) Postoperative view. Unfortu- nately, this patient underwent a fifth operation and the calcium-based bone cement was once again removed 2 years later because of infection and wound breakdown. (Reprinted with permission from Zins JE, Moreira-Gonzalez A, Papay FA. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg 2007;120(5):1332–1342.).
Bioactive glass is a synthetic, osteoconductive, silica-containing particulate bone filler that forms an osteoconductive apatite layer at the bone-implant in- terface. This enhances bone attachment and promotes new bone growth [24]. Collagen, mucopolysaccharides, and glycoproteins are recruited from the adjacent bone, facilitating early bonding of the bioactive glass with surrounding bone. Once mature, this bond has been shown to be stronger than the native bone itself. Fracturing is more likely to occur within the native bone or the bioglass substance rather than at the inter- face between them [24,25]. In addition to its osteoconduc- tive properties, bioactive glass has also been reported to be osteoinductive. The bioactive surface becomes coated with osteogenic stem cells in response to the controlled release of soluble silicon from the glass surface [26,27].
NovaBone (Porex Surgical, College Park, GA) is a commercially available bioactive glass intended for filling surgical or traumatic bone gaps [28]. Its composition is 45% silica dioxide, 45% sodium oxide, 5% calcium, and 5% phosphate [24]. NovaBoneAR, a second-generation NovaBone product, has two components: a slowly ab- sorbing, melt-derived calcium phosphosilicate bioglass and a more rapidly absorbed solution-gelation calcium phosphosilicate component. The latter is more rapidly absorbed, leaving more space for bone infiltration in the interstices between the more slowly absorbed melt- derived component. NovaBone thus acts as a scaffold for the ingrowth of new bone and is substantially resorbed within 6 months. NovaBone Putty is similar to NovaBoneAR except that the bioglass particulate material is mixed with a gelatin binding agent to form a malleable putty that can be packed into osseous defects [29]. The gelatin component will also reabsorb over time, leaving the osteoconductive bioglass matrix to promote bony ingrowth. NovaBone is not intended for heavy load-bearing applications prior to completion of bony ossification.
Cho and Gosain reviewed the role of bioactive glass in craniofacial surgery, describing its use in perio- dontal, alveolar, orbital floor, maxillofacial, and cranial applications [24,26]. Bioactive glass has been used in a mixture with autogenous bone particles and demineral- ized bone matrix, resulting in accelerated bone healing compared with bone grafting alone [26,27,30,31]. Complica- tion rates from the use of bioactive glass in craniofacial reconstruction have been described as high as 20% in one series [32].
Polymethylmethacrylate (PMMA) is an acrylic- based resin with broad applications. It may be prepared into a cement by mixing powdered methylmethacrylate polymer and liquid methylmethacrylate monomer, which polymerize during an exothermic reaction. PMMA is also available in block form. PMMA cement has been used for many years to secure orthopedic prosthetics and fill craniofacial defects. This polymer is rigid, biologically inert, and minimally reabsorbed. It is also relatively inexpensive and easy to obtain [24,33]. Dis- advantages of its use include lack of bioactivity, excessive heat produced with the polymerization reaction, lack of remodeling or replacement by new bone, and lifelong susceptibility to infection or extrusion.
Hard Tissue Replacement (HTR; Walter Lorenz Surgical, Inc., Jacksonville, FL) is a PMMA product that is prefabricated. HTR alloplastic implants are con- structed based on high-resolution computed tomo- graphic models. The custom-made implants come packaged for immediate use and can fit any number of complex craniofacial defects.
Advantages of the HTR include good strength, durability, surface osteoconductivity, biocompatibility, and some tissue ingrowth and revascularization. Addi- tionally, there is no need for intraoperative mixing of reagents or waiting, leading to decreased operating room time. Disadvantages include the need to plan procedures in advance to allow time for prefabrication and life- long risk of extrusion or infection. Infection rates in cranial reconstruction have been estimated at 5%, with an increased risk when the nose or frontal sinus is involved [24,33].
Eppley and colleagues written extensively regard- ing the clinical use of PMMA and HTR in craniofacial reconstruction [34,35,36,37]. In an animal study, they reported the HTR polymer to be biocompatible, with no evidence of infection, inflammatory reaction, or bone resorption around the implants. They also noted that the best osteoconductive effects were observed when the implants were exposed to bleeding cortical marrow as inlay grafts. Eppley also reviewed his experience in seven patients who had cranial reconstructions with prefabricated PMMA implants [36]. In cases where the frontal sinus was in proximity to the implant, it was either cranialized and obliterated with a pericranial flap or obliterated with HA cement. Eppley reported excellent cosmetic results and no complications with a minimum of 1 year of follow-up. More recently, Eppley looked at the rigidity of the various forms of the PMMA implants, concluding that all forms of PMMA compare favorably with native bone in terms of measured impact resistance [37].
Porous polyethylene or Medpor (Porex Surgical, College Park, GA) is a biocompatible, porous, high- density polyethylene that has been used extensively in orbital reconstruction and facial contouring for the past 20 years [38]. Available commercially in sheets, blocks, or preformed shapes, Medpor’s high degree of porosity, with an average pore size of 100 mm and pore volume of ~50%, promotes tissue ingrowth. The material is flexible enough to bend yet rigid enough to cut sharply. Medpor alloplastic implants may be placed subperiosteally and secured by closure of the periosteum and soft tissue over the implant. Alternatively, titanium or absorbable screw fixation may be used. Medpor products are also now available with titanium plates or meshes already incor- porated to allow for easy screw fixation or increased structural support.
Yaremchuk reviewed his experience with 370 Medpor implants in 162 consecutive patients over 11 years [38]. Implants were placed for a variety of acquired and congenital craniofacial deficits. All implants were placed in a subperiosteal pocket, and the majority se- cured using titanium screw fixation. Infections were reported in 3% of patients, and the overall reoperation rate was 10%, including operative removal for facial recontouring and infection. No implant extrusions were reported.
Cenzi and colleagues also reported a series of 285 Medpor implants placed in 187 patients over 7 years [39]. Implants were used almost exclusively for craniofacial reconstruction and were placed as both inlays and onlays. Over a mean follow-up of 5 years, Cenzi et al. reported an overall complication rate of 6.3%, with implant exposure and infection being the most common. Risk factors for implant extrusion and infection included placement in the maxilla or ear, as well as placement in areas where soft tissue coverage was thin and/or scarred from irradi- ation or previous surgery.
Menderes et al. reviewed their experience recon- structing craniofacial defects using 83 high density porous polyethylene implants in 71 patients over 7 years [40]. Subperiosteal placement was performed in the vast majority of patients, and fixation was accom- plished using titanium screws, absorbable screws and miniplates, or a stainless steel wire circulage. At a minimum of 1-year follow-up, the authors reported a nearly 10% reoperation rate for problems such as con- touring irregularities and extrusion/infection. They con- cluded that the use of porous polyethylene is safe, easy, and effective and associated with low morbidity.
The rapidly expanding field of tissue engineering seeks to combine the stimulatory effects of bone growth factors, such as bone morphogenetic protein-2 and osteogenic protein-1 with bone substitute carriers to provide structural support during healing while deliver- ing critical growth factors to the fracture site, promoting more rapid bone growth and healing. Bone mesenchy- mal cells have also been explored as a potential compo- nent in engineered bone substitutes for similar reasons. Potential delivery systems have included demineralized bone matrix, collagen composites, fibrin, calcium phos- phate, polylactide, polylactide-co-glycolide, polylactide- polyethylene glycol, HA, dental plaster, titanium, and bioglass [41,42,43,44,45,46,47,48,49,50,51,52,53].
Although the early history of bone substitution in craniofacial surgery gave emphasis to the physical prop- erties of the material itself, the science of biomaterials and bone substitution is now increasingly focused on the biologic effects of the implant on the surrounding tissue, such as its ability to promote new bone growth [54]. It seems certain that the biologic interface between the alloplastic implant and host will guide the future of biomaterials to bring ever improving products to our patients.
References
- Urist, M.R.; O’Conner, B.T.; Burwell, R.G. Bone Graft, Derivatives and Substitutes; Butterworth- Heinemann: Cambridge, UK, 1994. [Google Scholar]
- Flati, G.; Di Stanislao, C. Chirurgia nella preistoria. Parte I. Prov. Med. Aquil. 2004, 2, 8–11. [Google Scholar]
- Donati, D.; Zolezzi, C.; Tomba, P.; Vigano`, A. Bone grafting: historical and conceptual review, starting with an old manuscript by Vittorio Putti. Acta Orthop. 2007, 78, 19–25. [Google Scholar] [CrossRef]
- De Boer, H.H. The history of bone grafts. Clin. Orthop. Relat. Res. 1988, 226, 292–298. [Google Scholar] [CrossRef]
- von Walter, P. J. Chir. Und Augen-Heilkd. 1821, 2, 571.
- Schlickewie, W.; Schlickewie, C. The use of bone substitutes in the treatment of bone defects—the clinical view and history. Macromol. Symp. 2007, 253, 10–23. [Google Scholar] [CrossRef]
- Dressmann, H. Ueber Knochenplombierung bei Hohlenfor- migen Defekten des Knochens. Beitr. Klin. Chir. 1892, 9, 804–810. [Google Scholar]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- FDA. Services, H., Ed.; Pre-Market Approval for Norian SRS Cement (PMA P970010); FDA: Silver Spring, MD, USA, 1998. [Google Scholar]
- Zins, J.E.; Moreira-Gonzalez, A.; Parikh, A.; Arslan, E.; Bauer, T.; Siemionow, M. Biomechanical and histologic evaluation of the Norian craniofacial repair system and Norian Craniofacial Repair System Fast Set Putty in the long-term reconstruction of full-thickness skull defects in a sheep model. Plast. Reconstr. Surg. 2008, 121, 271e–282e. [Google Scholar] [CrossRef]
- FDA. Services, H., Ed.; Bone Source HAC Marketing Approval; FDA: Silver Spring, MD, USA, 2002. [Google Scholar]
- FDA. Services, H., Ed.; Mimix Bone Void Filler Marketing Approval; FDA: Silver Spring, MD, USA, 2002. [Google Scholar]
- Goebel, J.A.; Jacob, A. Use of Mimix hydroxyapatite bone cement for difficult ossicular reconstruction. Otolaryngol. Head Neck Surg. 2005, 132, 727–734. [Google Scholar] [CrossRef]
- Burstein, F.D.; Williams, J.K.; Hudgins, R.; et al. Hydroxyapatite cement in craniofacial reconstruction: experience in 150 patients. Plast. Reconstr. Surg. 2006, 118, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.B.; Weinzweig, J.; Kirschner, R.E.; Bartlett, S.P. Applications of a new carbonated calcium phosphate bone cement: early experience in pediatric and adult craniofacial reconstruction. Plast. Reconstr. Surg. 2002, 109, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Go’mez, E.; Mart’ın, M.; Arias, J.; Carceller, F. Clinical applications of Norian SRS (calcium phosphate cement) in craniofacial reconstruction in children: our experience at Hospital La Paz since 2001. J Oral Maxillofac Surg 2005, 63, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Verret, D.J.; Ducic, Y.; Oxford, L.; Smith, J. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol. Head Neck Surg. 2005, 133, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Gonzalez, A.; Jackson, I.T.; Miyawaki, T.; Barakat, K.; DiNick, V. Clinical outcome in cranioplasty: critical review in long-term follow-up. J. Craniofac Surg. 2003, 14, 144–153. [Google Scholar]
- Zins, J.E.; Moreira-Gonzalez, A.; Papay, F.A. Use of calcium- based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast. Reconstr. Surg. 2007, 120, 1332–1342. [Google Scholar] [PubMed]
- Losee, J.E.; Karmacharya, J.; Gannon, F.H.; et al. Reconstruction of the immature craniofacial skeleton with a carbonated calcium phosphate bone cement: interaction with bioresorb- able mesh. J. Craniofac Surg. 2003, 14, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Guerra, A.B.; Bidros, R.S.; Trahan, C.; Baratta, R.; Metzinger, S.E. A comparison of resistance to fracture among four commercially available forms of hydroxyapatite cement. Ann. Plast. Surg. 2005, 55, 87–92. [Google Scholar] [CrossRef]
- Ducic, Y. Titanium mesh and hydroxyapatite cement cranioplasty: a report of 20 cases. J. Oral. Maxillofac. Surg. 2002, 60, 272–276. [Google Scholar]
- Cho, Y.R.; Gosain, A.K. Biomaterials in craniofacial recon- struction. Clin. Plast. Surg. 2002, 31, 377–385. [Google Scholar] [CrossRef]
- Kitsugi, T.; Yamamuro, T.; Kokubo, T. Bonding behavior of a glass-ceramic containing apatite and wollastonite in segmen- tal replacement of the rabbit tibia under load-bearing conditions. J. Bone Jt. Surg. Am. 1989, 71, 264–272. [Google Scholar] [CrossRef]
- Gosain, A. KPlastic Surgery Educational Foundation DATA Committee. Bioactive glass for bone replacement in cranio- maxillofacial reconstruction. Plast. Reconstr. Surg. 2004, 114, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, P.; Heikkila¨, J.; Yli-Urpo, A.; Vuorio, E.; Aro, H.T. Histomorphometric and molecular biologic comparison of bioactive glass granules and autogenous bone grafts in augmentation of bone defect healing. J. Biomed. Mater. Res. 1997, 35, 9–17. [Google Scholar] [CrossRef] [PubMed]
- FDA. Services, H., Ed.; NovaBoneAR Marketing Approval; FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- FDA. Services, H., Ed.; NovaBone Putty Marketing Approval; FDA: Silver Spring, MD, USA, 2006. [Google Scholar]
- Cordioli, G.; Mazzocco, C.; Schepers, E.; Brugnolo, E.; Majzoub, Z. Maxillary sinus floor augmentation using bioactive glass granules and autogenous bone with simultaneous implant placement. Clin. Histol. Find. Clin. Oral Implant. Res. 2001, 12, 270–278. [Google Scholar] [CrossRef]
- Tadjoedin, E.S.; de Lange, G.L.; Lyaruu, D.M.; Kuiper, L.; Burger, E.H. High concentrations of bioactive glass material (BioGran) vs. autogenous bone for sinus floor elevation. Clin. Oral Implant. Res. 2002, 13, 428–436. [Google Scholar] [CrossRef]
- Duskova, M.; Smahel, Z.; Vohradnik, M.; et al. Bioactive glass- ceramics in facial skeleton contouring. Aesthetic Plast. Surg. 2002, 26, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Manson, P.N.; Crawley, W.A.; Hoopes, J.E. Frontal cranioplasty: risk factors and choice of cranial vault reconstructive material. Plast. Reconstr. Surg. 1986, 77, 888–904. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Kilgo, M.; Coleman, J.J.I.I.I. Cranial reconstruc- tion with computer-generated hard-tissue replacement patient-matched implants: indications, surgical technique, and long-term follow-up. Plast Reconstr Surg 2002, 109, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; Sadove, A.M.; German, R.Z. Evaluation of HTR polymer as a craniomaxillofacial graft material. Plast. Reconstr. Surg. 1990, 86, 1085–1092. [Google Scholar] [CrossRef]
- Eppley, B.L. Craniofacial reconstruction with computer- generated HTR patient-matched implants: use in primary bony tumor excision. J. Craniofac Surg. 2002, 13, 650–657. [Google Scholar] [CrossRef]
- Eppley, B.L. Biomechanical testing of alloplastic PMMA cranioplasty materials. J. Craniofac Surg. 2005, 16, 140–143. [Google Scholar] [PubMed]
- Yaremchuk, M.J. Facial skeletal reconstruction using porous polyethylene implants. Plast. Reconstr. Surg. 2003, 111, 1818–1827. [Google Scholar] [PubMed]
- Cenzi, R.; Farina, A.; Zuccarino, L.; Carinci, F. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J. Craniofac Surg. 2005, 16, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Menderes, A.; Baytekin, C.; Topcu, A.; Yilmaz, M.; Barutcu, A. Craniofacial reconstruction with high-density porous poly- ethylene implants. J. Craniofac Surg. 2004, 15, 719–724. [Google Scholar] [PubMed]
- Chen, B.; Lin, H.; Zhao, Y.; et al. Activation of demineralized bone matrix by genetically engineered human bone morpho- genetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J. Biomed. Mater. Res. A 2007, 80, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Clokie, C.M.; Sa’ndor, G.K. Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc 2008, 74, 67–72. [Google Scholar] [PubMed]
- Fu, Y.C.; Nie, H.; Ho, M.L.; Wang, C.K.; Wang, C.H. Optimized bone regeneration based on sustained release from three- dimensional fibrous PLGA/HAp composite scaffolds loaded with BMP-2. Biotechnol Bioeng 2008, 99, 996–1006. [Google Scholar]
- Chung, Y.I.; Ahn, K.M.; Jeon, S.H.; Lee, S.Y.; Lee, J.H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Control Release 2007, 121, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Namikawa, T.; Kato, M.; Terai, H.; Taguchi, S.; Takaoka, K. Repair of bone defects in revision hip arthroplasty by implantation of a new bone-inducing material comprised of recombinant human BMP-2, Beta-TCP powder, and a biodegradable polymer: an experimental study in dogs. J. Orthop. Res. 2007, 25, 1042–1051. [Google Scholar] [PubMed]
- Murata, M.; Akazawa, T.; Tazaki, J.; et al. Blood permeability of a novel ceramic scaffold for bone morphogenetic protein-2. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 81, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chen, C.H.; Tsai, C.L.; Lin, I.H.; Hsiue, G.H. Heterobifunctional poly(ethylene glycol)-tethered bone mor- phogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 2007, 13, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Turhani, D.; Weissenbo¨ck, M.; Stein, E.; Wanschitz, F.; Ewers, R. Exogenous recombinant human BMP-2 has little initial effects on human osteoblastic cells cultured on collagen type I coated/noncoated hydroxyapatite ceramic granules. J. Oral. Maxillofac. Surg. 2007, 65, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Okafuji, N.; Shimizu, T.; Watanabe, T.; et al. Tissue reaction to poly (lactic-co-glycolic acid) copolymer membrane in rhBMP used rabbit experimental mandibular reconstruction. Eur. J. Med. Res. 2006, 11, 394–396. [Google Scholar] [PubMed]
- Va¨lima¨ki, V.V.; Yrjans, J.J.; Vuorio, E.I.; Aro, H.T. Molecular biological evaluation of bioactive glass microspheres and adjunct bone morphogenetic protein 2 gene transfer in the enhancement of new bone formation. Tissue Eng. 2005, 11, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kim, J.I.; Kim, J.; et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials 2005, 26, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.B.; Overgaard, S.; Lind, M.; et al. Osteogenic protein-1 increases the fixation of implants grafted with morcellised bone allograft and ProOsteon bone substitute: an exper- imental study in dogs. J. Bone Jt. Surg. Br. 2007, 89, 121–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elsalanty, M.E.; Por, Y.C.; Genecov, D.G.; et al. Recombinant human BMP-2 enhances the effects of materials used for reconstruction of large cranial defects. J. Oral. Maxillofac. Surg. 2008, 66, 277–285. [Google Scholar] [CrossRef]
- Anderson, J.M. The future of biomedical materials. J. Mater. Sci. Mater. Med. 2006, 17, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
© 2008 by the author. The Author(s) 2008.