Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction
2.2. Determination of Phlorotannins
2.3. Animals
2.4. Serum Biochemical Parameter Measurement
2.5. Measurement of Lipid Index
2.6. Serum Proinflammatory Factor and Antioxidant Enzyme Activity Level Measurement
2.7. Measurement of Lipase Activity in the Adipose Tissue
2.8. Mitochondrial Function Assessment
2.9. Histopathological Analysis
2.10. Fecal Microbiota Analysis
2.11. Statistical Analysis
3. Results
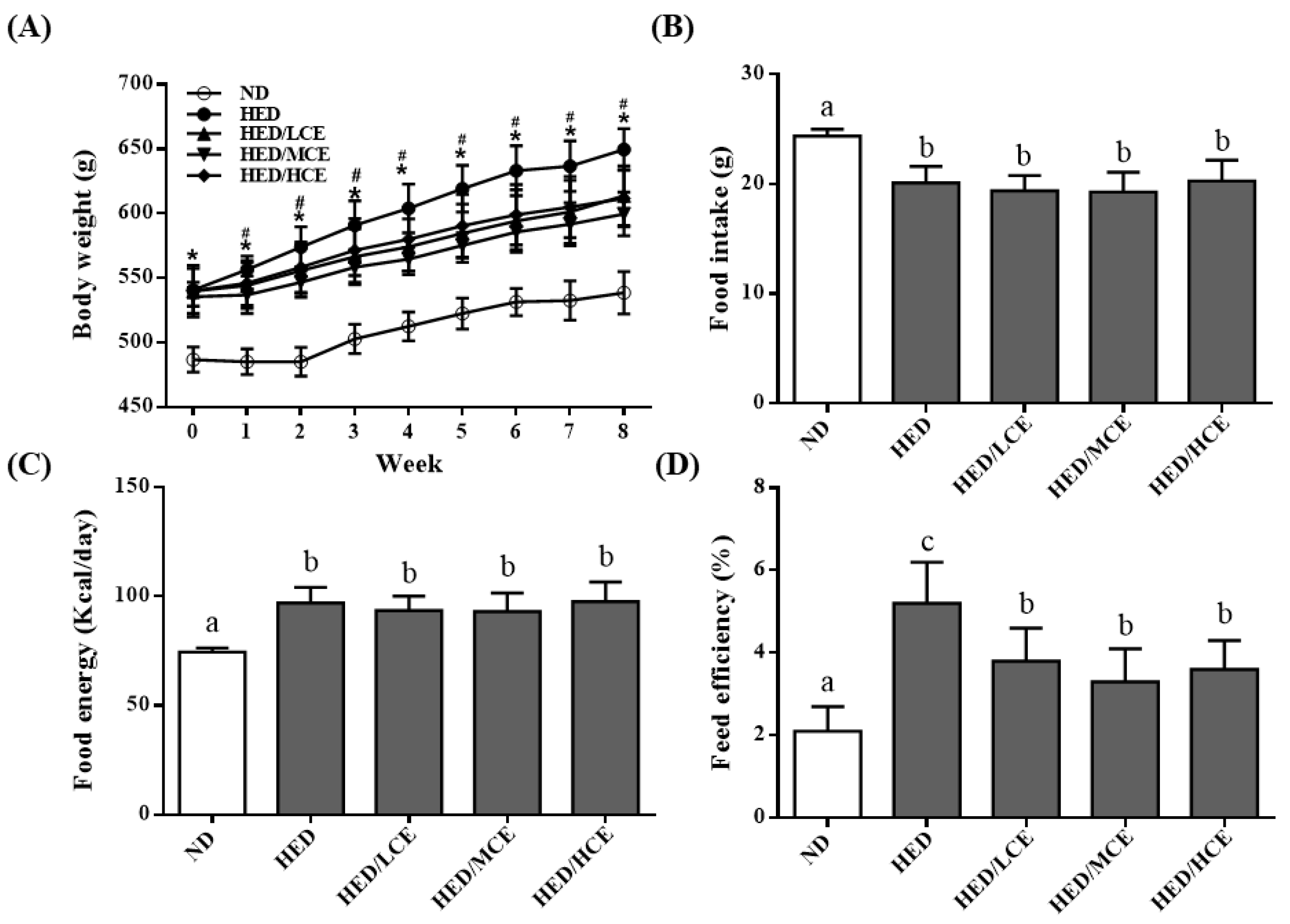
3.1. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Body Weight, Food Intake, Food Energy, and Feed Efficiency of Rats with HED-Induced Hyperlipidemia
3.2. Effects of Phlorotannins-Rich Extracts from A. nodosum and F. vesiculosus on Fat Pads of Rats with HED-Induced Hyperlipidemia
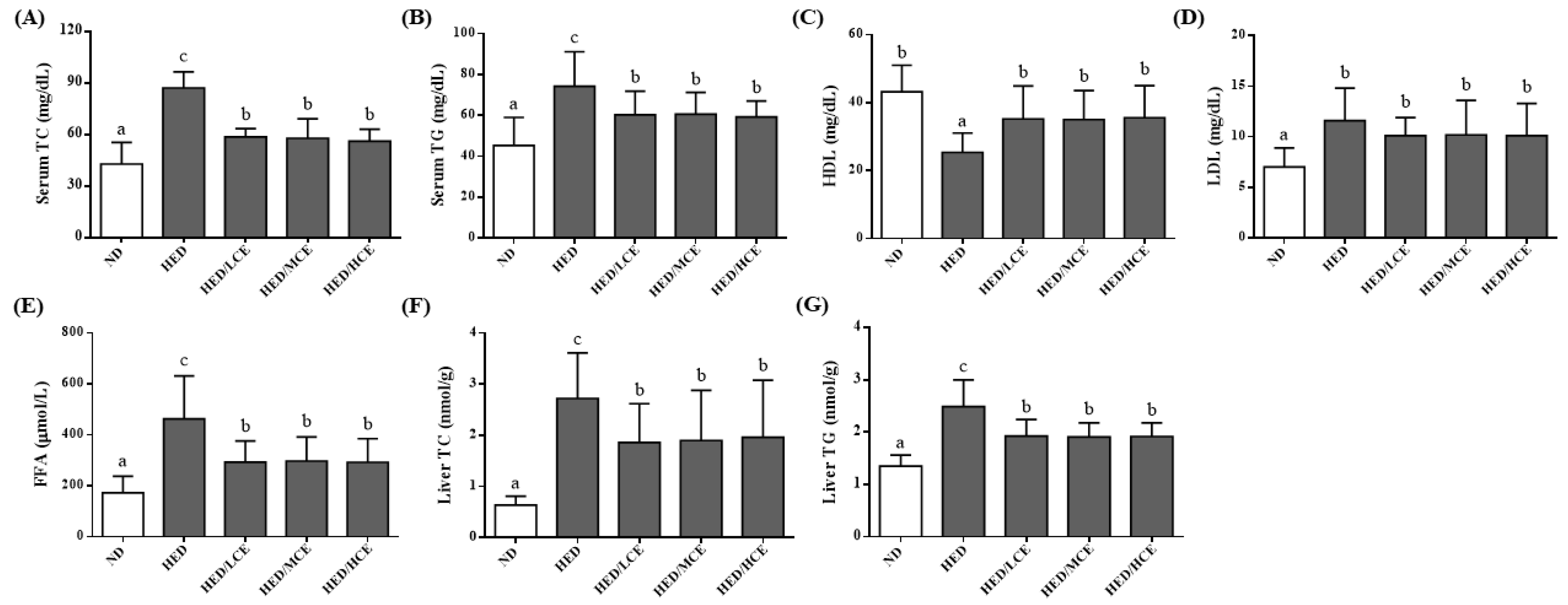
3.3. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Serum Biochemical Parameters of Rats with HED-Induced Hyperlipidemia
3.4. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Lipid Indices of Rats with HED-Induced Hyperlipidemia
3.5. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Proinflammatory Factors and Antioxidant Enzyme Activities in the Serum of Rats with HED-Induced Hyperlipidemia
3.6. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Lipoprotein Lipase Activity and Mitochondrial Function in Rats with HED-Induced Hyperlipidemia
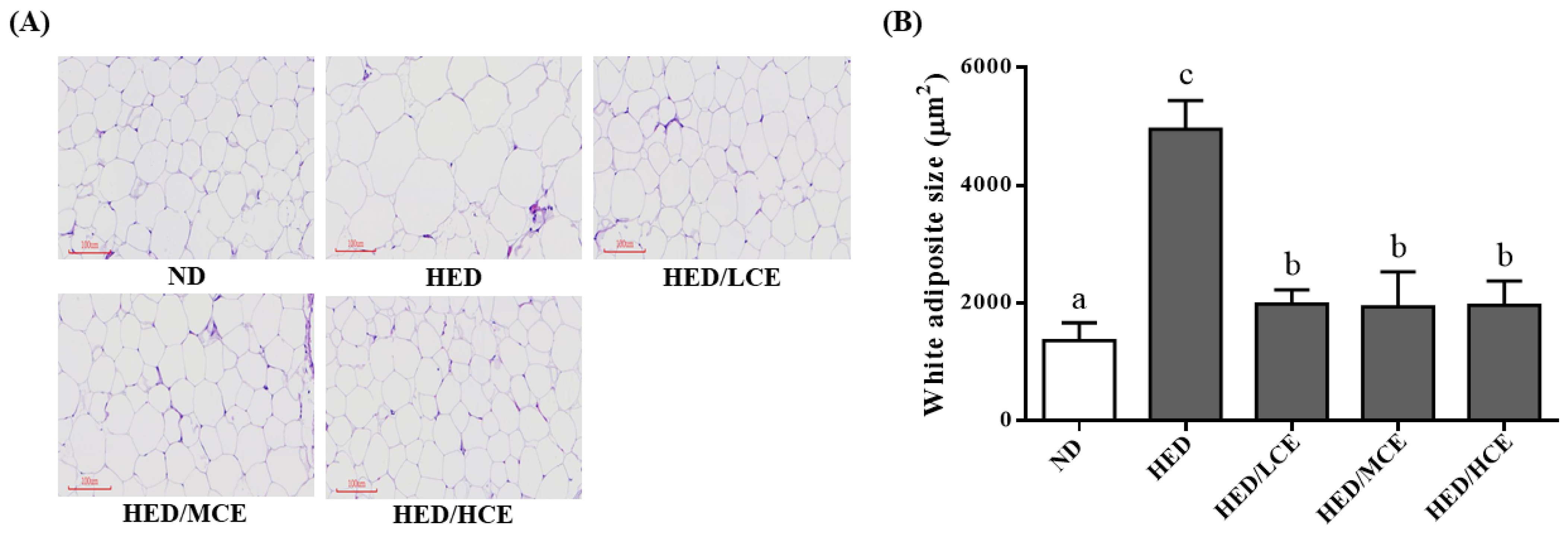
3.7. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Mean Adipocyte Size of Rats with HED-Induced Hyperlipidemia
3.8. Effects of Phlorotannin-Rich Extracts from A. nodosum and F. vesiculosus on Gut Microbiota of Rats with HED-Induced Hyperlipidemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harnafi, H.; Caid, H.S.; Bouanani, N.E.H.; Aziz, M.; Amrani, S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008, 108, 205–212. [Google Scholar] [CrossRef]
- Ghule, B.; Ghante, M.H.; Saoji, A.N.; Yeole, P.G. Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Indian J. Exp. Biol. 2006, 44, 905–909. [Google Scholar] [PubMed]
- Lemhadri, A.; Hajji, L.; Michel, J.-B.; Eddouks, M. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J. Ethnopharmacol. 2006, 106, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jeong, D.M.; Jung, H.J.; Jung, Y.J.; Yokozawa, T.; Choi, J.S. Hypolipidemic effects of Sophora flavescens and its con-stituents in poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Biol. Pharm. Bull. 2008, 31, 73–78. [Google Scholar] [CrossRef]
- Taskin, E.; Ozturk, M.; Kurt, O. Turkiye’nin yararlanilabilir denizel alg potansiyeli. XI. Ulus. Su Urunleri Sempozyumu 2001, 4, 225–232. [Google Scholar]
- Joe, M.-J.; Kim, S.-N.; Choi, H.-Y.; Shin, W.-S.; Park, G.-M.; Kang, D.-W.; Kim, Y.K. The Inhibitory Effects of Eckol and Dieckol from Ecklonia stolonifera on the Expression of Matrix Metalloproteinase-1 in Human Dermal Fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Heo, S.-J.; Kim, J.-P.; Jung, W.-K.; Lee, N.-H.; Kang, H.-S.; Jun, E.-M.; Park, S.-H.; Kang, S.-M.; Lee, Y.-J.; Park, P.-J.; et al. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar]
- Roy, M.C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotan-nins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Kang, K.; Park, Y.; Hwang, H.J.; Kim, S.H.; Lee, J.G.; Shin, H.-C. Antioxidative properties of brown algae polyphenolics and their perspectives as chemopreventive agents against vascular risk factors. Arch. Pharmacal Res. 2003, 26, 286–293. [Google Scholar] [CrossRef]
- Shin, H.C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteo-arthritis from Ecklonia cava. Arch. Pharmacal Res. 2006, 29, 165–171. [Google Scholar] [CrossRef]
- Braden, K.W.; Blanton, J.R., Jr.; Allen, V.G.; Pond, K.R.; Miller, M.F. Ascophyllum nodosum supplementation: A preharvest in-tervention for reducing Escherichia coli O157:H7 and Salmonella spp. in feedlot steers. J. Food Prot. 2004, 67, 1824–1828. [Google Scholar] [CrossRef] [PubMed]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.-H.; Kim, M.-M.; Kim, S.-K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorganic. Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Wijesekara, I.; Kim, S.-K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from laminariaceae ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A(y) mice. Plant Foods Hum. Nutr. 2008, 63, 163–169. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, C.; Liu, Q.; Xiao, H.; Zhao, A.; Hu, Y.; Liu, L.; Zhao, L. Effect of phlorotannins from Sargassum thunbergii on blood lipids regulation in mice with high-fat diet. Nat. Prod. Res. 2012, 26, 2117–2120. [Google Scholar]
- Yoon, N.Y.; Kim, H.R.; Chung, H.Y.; Choi, J.S. Anti-hyperlipidemic effect of an edible brown algae, Ecklonia stolonifera, and its constituents on poloxamer 407-induced hyperlipidemic and cholesterol-fed rats. Arch. Pharmacal Res. 2008, 31, 1564–1571. [Google Scholar] [CrossRef]
- Nurrahma, B.A.; Tsao, S.P.; Wu, C.H.; Yeh, T.H.; Hsieh, P.S.; Panunggal, B.; Huang, H.Y. Probiotic supplementation facili-tates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front. Aging Neurosci. 2021, 13, 668775. [Google Scholar] [CrossRef]
- Chen, H.-L.; Tung, Y.-T.; Tsai, C.-L.; Lai, C.-W.; Lai, Z.-L.; Tsai, H.-C.; Lin, Y.-L.; Wang, C.-H.; Chen, C.-M. Kefir improves fatty liver syndrome by inhibiting the lipogenesis pathway in leptin-deficient ob/ob knockout mice. Int. J. Obes. 2013, 38, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Y.; Li, N.N.; Liang, Y.R. Effects of Ilex latifolia and Camellia sinensis on cholesterol and circulating immune complexes in rats fed with a high-cholesterol diet. Phytother. Res. 2013, 27, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.; Douglas, T.D.; Maiyoh, G.K.; Adeli, K.; Theriault, A.G. Green tea leaf extract improves lipid and glucose homeosta-sis in a fructose-fed insulin-resistant hamster model. J. Ethnopharmacol. 2006, 104, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Chang, Y.-Y.; Yang, D.-J.; Tzang, B.-S.; Chen, Y.-C. Beneficial effects of noni (Morinda citrifolia L.) juice on livers of high-fat dietary hamsters. Food Chem. 2013, 140, 31–38. [Google Scholar] [CrossRef]
- Jung, U.J.; Park, Y.B.; Kim, S.R.; Choi, M.-S. Supplementation of Persimmon Leaf Ameliorates Hyperglycemia, Dyslipidemia and Hepatic Fat Accumulation in Type 2 Diabetic Mice. PLoS ONE 2012, 7, e49030. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Kahn, B.B. Glucose Transporters and Insulin Action—Implications for Insulin Resistance and Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 248–257. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A randomised crossover placebo-controlled trial investigating the effect of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) on postchallenge plasma glucose and insulin levels in men and women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Nicolucci, A.; Rossi, M.C.; Petrelli, M. Effectiveness of Ascophyllum nodosum and Fucus vesiculosus on Metabolic Syndrome Components: A Real-World, Observational Study. J. Diabetes Res. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Akondi, B.; Korukanti, V.; Ponnam, H. Evaluation of antiobesity activity of Fucus vesiculosus. Indian J. Res. Homoeopath. 2013, 7, 126. [Google Scholar] [CrossRef]
- Elsenberg, S. Lipoproteins and lipoprotein metabolism. A dynamic evaluation of the plasma fat transport system. Klin. Woch-Enschr. 1983, 61, 119–132. [Google Scholar]
- Eisenberg, S. High density lipoprotein metabolism. J. Uptd. Res. 1984, 25, 1017–1058. [Google Scholar]
- Nikkilä, E.A.; Taskinen, M.-R.; Sane, T. Plasma high-density lipoprotein concentration and subfraction distribution in relation to triglyceride metabolism. Am. Heart J. 1987, 113, 543–548. [Google Scholar] [CrossRef]
- Tikkanen, M.J.; Nlkkila, E.A. Regulation of hepatic lipase and serum lipoproteins by sex steroids. Am. Heart J. 1987, 113, 562–567. [Google Scholar] [CrossRef]
- Patsch, J.R.; Prasad, S.; Gotto, A.M.; Patsch, W. High density lipoprotein2. Relationship of the plasma levels of this lipoprotein species to its composition, to the magnitude of postprandial lipemia, and to the activities of lipoprotein lipase and hepatic lipase. J. Clin. Investig. 1987, 80, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kasim, S.E.; Tseng, K.; Jen, K.-L.C.; Khilnani, S. Significance of Hepatic Triglyceride Lipase Activity in the Regulation of Serum High Density Lipoproteins in Type II Diabetes Mellitus. J. Clin. Endocrinol. Metab. 1987, 65, 183–187. [Google Scholar] [CrossRef]
- Applebaum-Bowden, D.; Haffner, S.M.; Hazzard, W.R. The dyslipoproteinemia of anabolic steroid therapy: Increase in he-patic triglyceride lipase precedes the decrease in high density lipoprotein cholesterol. Metabolism 1987, 36, 949–952. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61, 223. [Google Scholar] [CrossRef]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Le Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and anti-tumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- O’Sullivan, A.; O’Callaghan, Y.; O’Grady, M.; Queguineur, B.; Hanniffy, D.; Troy, D.; Kerry, J.; O’Brien, N. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- Keyrouz, R.; Abasq, M.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; ArGall, E.; Hauchard, D. Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef]
- Vodouhè, M.; Marois, J.; Guay, V.; Leblanc, N.; Weisnagel, S.J.; Bilodeau, J.F.; Jacques, H. Marginal impact of brown seaweed Ascophyllum nodosum and Fucus vesiculosus extract on metabolic and inflammatory response in overweight and obese predia-betic subjects. Mar. Drugs 2022, 20, 174. [Google Scholar] [CrossRef]



| ND | HED | HED/LCE | HED/MCE | HED/HCE | |
|---|---|---|---|---|---|
| Epididymal fat (g) | 5.97 ± 1.47 a | 16.82 ± 3.76 c | 12.18 ± 2.72 b | 12.27 ± 2.36 b | 12.26 ± 2.35 b |
| Perirenal fat (g) | 6.81 ± 1.81 a | 26.30 ± 4.85 c | 20.59 ± 3.59 b | 20.54 ± 2.86 b | 20.33 ± 3.96 b |
| Mesenteric fat (g) | 5.09 ± 0.99 a | 16.41 ± 3.42 c | 12.18 ± 2.93 b | 12.18 ± 2.45 b | 12.17 ± 2.35 b |
| White adipose tissue (g) | 17.87 ± 4.15 a | 59.53 ± 10.10 c | 44.95 ± 5.73 b | 44.99 ± 6.41 b | 44.76 ± 6.55 b |
| Brown adipose tissue (g) | 0.47 ± 0.16 a | 0.53 ± 0.09 a | 0.72 ± 0.16 b | 0.72 ± 0.19 b | 0.74 ± 0.18 b |
| BAT/WAT (%) | 2.7 ± 1.1 a | 0.9 ± 0.2 c | 1.6 ± 0.5 b | 1.7 ± 0.7 b | 1.7 ± 0.4 b |
| Body fat (%) | 3.32 ± 0.77 a | 9.24 ± 1.63 c | 7.34 ± 1.06 b | 7.51 ± 1.12 b | 7.33 ± 1.14 b |
| ND | HED | HED/LCE | HED/MCE | HED/HCE | |
|---|---|---|---|---|---|
| FBS (g/dL) | 86.1 ± 15.0 a | 114.3 ± 5.4 b | 80.8 ± 11.1 a | 81.2 ± 8.5 a | 84.5 ± 14.0 a |
| Insulin (pg/mL) | 144.1 ± 10.9 a | 86.0 ± 18.8 c | 112.1 ± 10.0 b | 113.9 ± 6.8 b | 114.5 ± 9.4 b |
| AST (U/L) | 142.5 ± 23.5 a | 202.3 ± 30.1 c | 172.7 ± 29.8 b | 171.6 ± 14.9 b | 170.6 ± 23.7 b |
| ALT(U/L) | 43.6 ± 17.1 a | 51.5 ± 15.4 b | 47.7 ± 11.1 a | 46.1 ± 17.0 a | 41.4 ± 8.5 a |
| Uric acid (mg/dL) | 1.0 ± 0.4 | 1.3 ± 0.3 | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.0 ± 0.4 |
| Creatinine (mg/dL) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 |
| Na+ (mmol/L) | 140.3 ± 3.9 | 139.8 ± 2.1 | 138.0 ± 2.2 | 138.2 ± 3.9 | 139.3 ± 1.8 |
| K+ (mmol/L) | 5.0 ± 1.2 | 5.1 ± 1.1 | 5.1 ± 1.3 | 5.1 ± 1.2 | 5.1 ± 0.7 |
| Ketone body | N.D. | N.D. | N.D. | N.D. | N.D. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tung, Y.-T.; Wu, C.-H.; Chen, W.-C.; Pan, C.-H.; Chen, Y.-W.; Tsao, S.-P.; Chen, C.-J.; Huang, H.-Y. Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats. Nutrients 2022, 14, 4665. https://doi.org/10.3390/nu14214665
Tung Y-T, Wu C-H, Chen W-C, Pan C-H, Chen Y-W, Tsao S-P, Chen C-J, Huang H-Y. Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats. Nutrients. 2022; 14(21):4665. https://doi.org/10.3390/nu14214665
Chicago/Turabian StyleTung, Yu-Tang, Chieh-Hsi Wu, Wen-Chao Chen, Chun-Hsu Pan, Yi-Wen Chen, Shu-Ping Tsao, Chia-Jung Chen, and Hui-Yu Huang. 2022. "Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats" Nutrients 14, no. 21: 4665. https://doi.org/10.3390/nu14214665
APA StyleTung, Y.-T., Wu, C.-H., Chen, W.-C., Pan, C.-H., Chen, Y.-W., Tsao, S.-P., Chen, C.-J., & Huang, H.-Y. (2022). Ascophyllum nodosum and Fucus vesiculosus Extracts Improved Lipid Metabolism and Inflammation in High-Energy Diet–Induced Hyperlipidemia Rats. Nutrients, 14(21), 4665. https://doi.org/10.3390/nu14214665







