Suppression of Anti-Inflammatory Mediators in Metabolic Disease May Be Driven by Overwhelming Pro-Inflammatory Drivers
Abstract
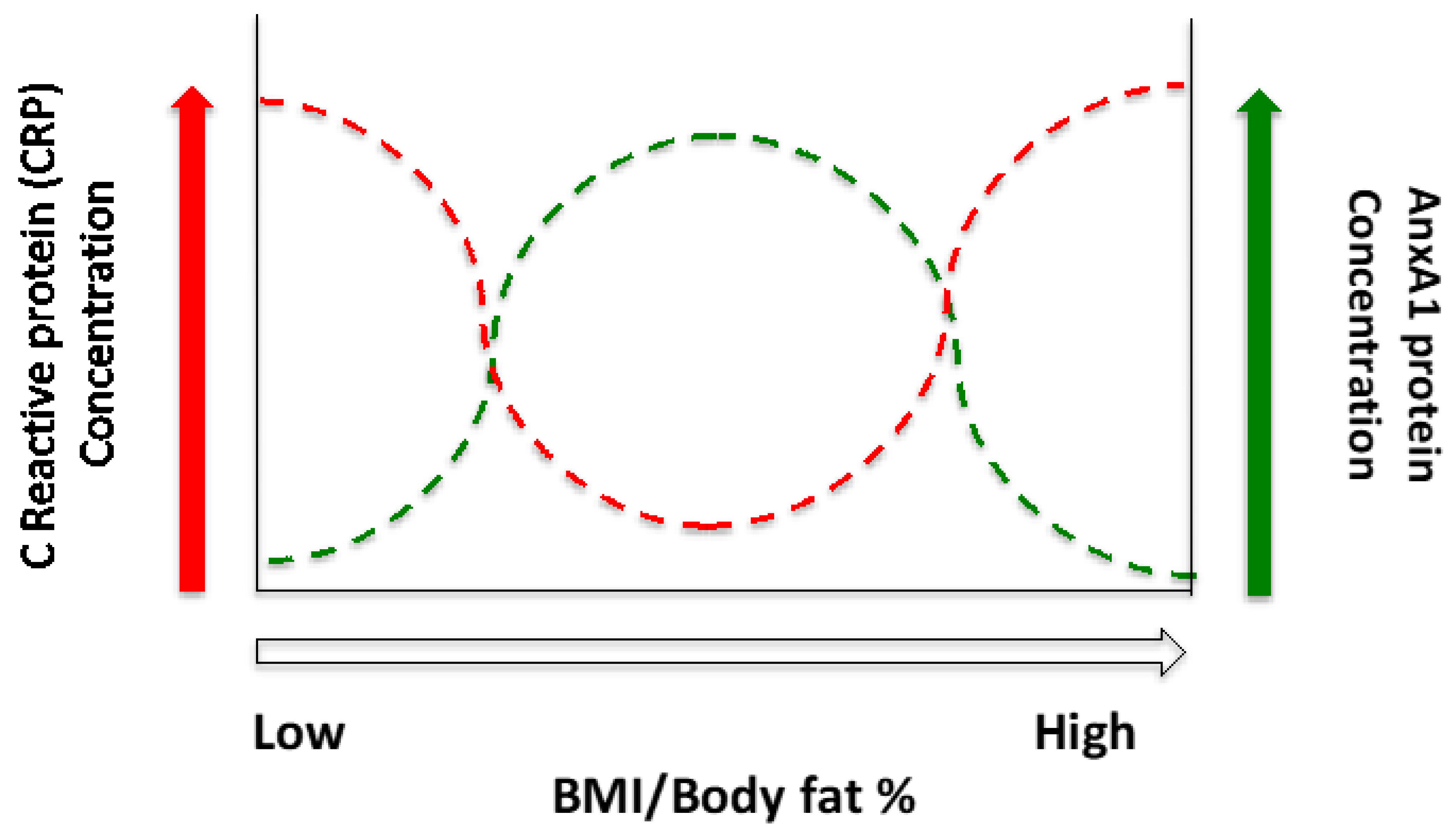
1. Introduction
2. Materials and Methods
2.1. Bariatric Surgery-Subject Recruitment
2.2. Bariatric Surgery-Patient Enrolment and Follow Up
2.3. Bariatric Surgery-Study Design
2.4. Plasma Biomarker Measurement
2.5. Lipodystrophy Patients
2.6. Primary Human Adipocyte Cell Culture
2.7. RNA Extraction
2.8. cDNA Synthesis
2.9. Real-Time qPCR
2.10. Statistical Analysis
3. Results
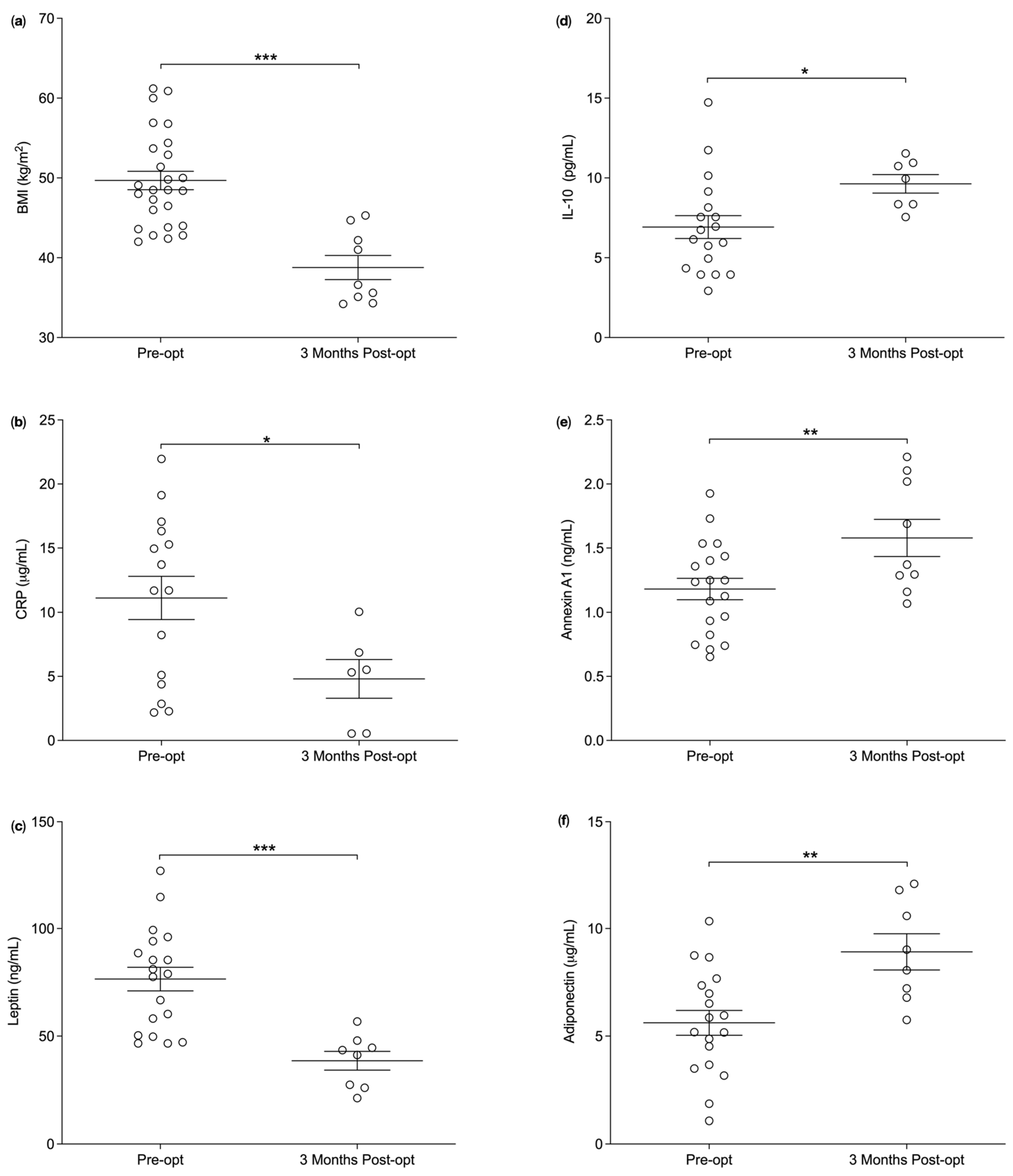
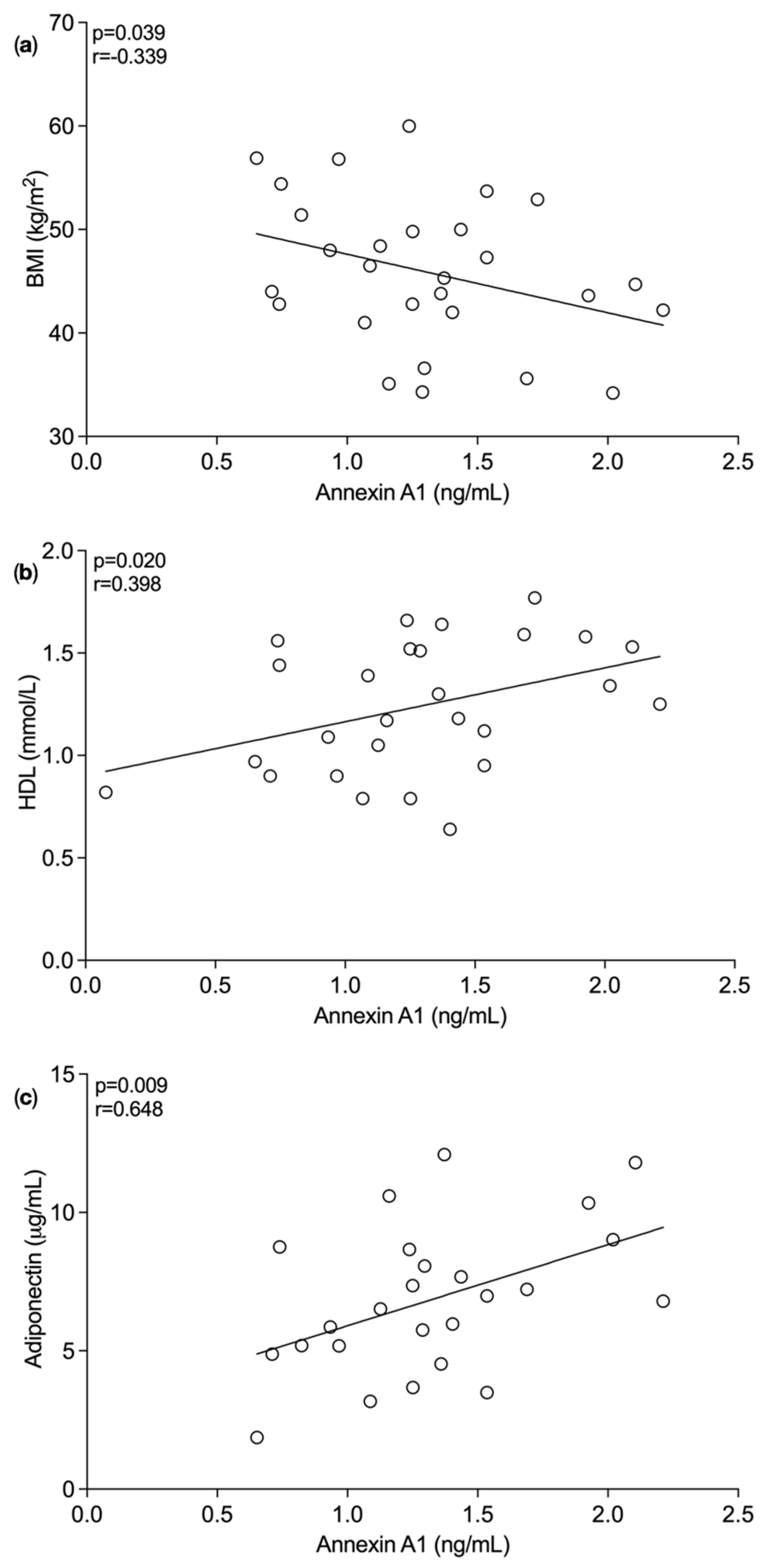
3.1. Plasma Annexin A1 Levels Pre and Post Bariatric Surgery
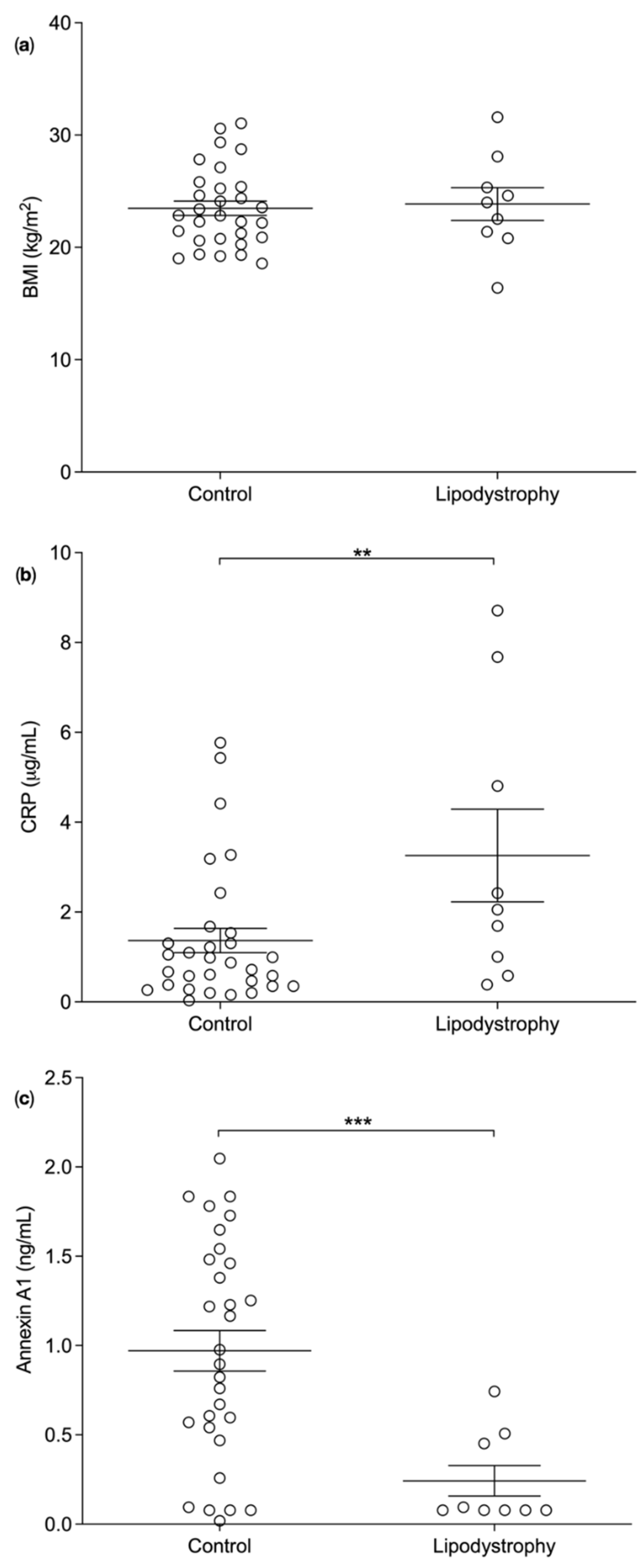
3.2. Plasma Biomarker Levels in Lipodystrophy Patients versus BMI Matched Controls
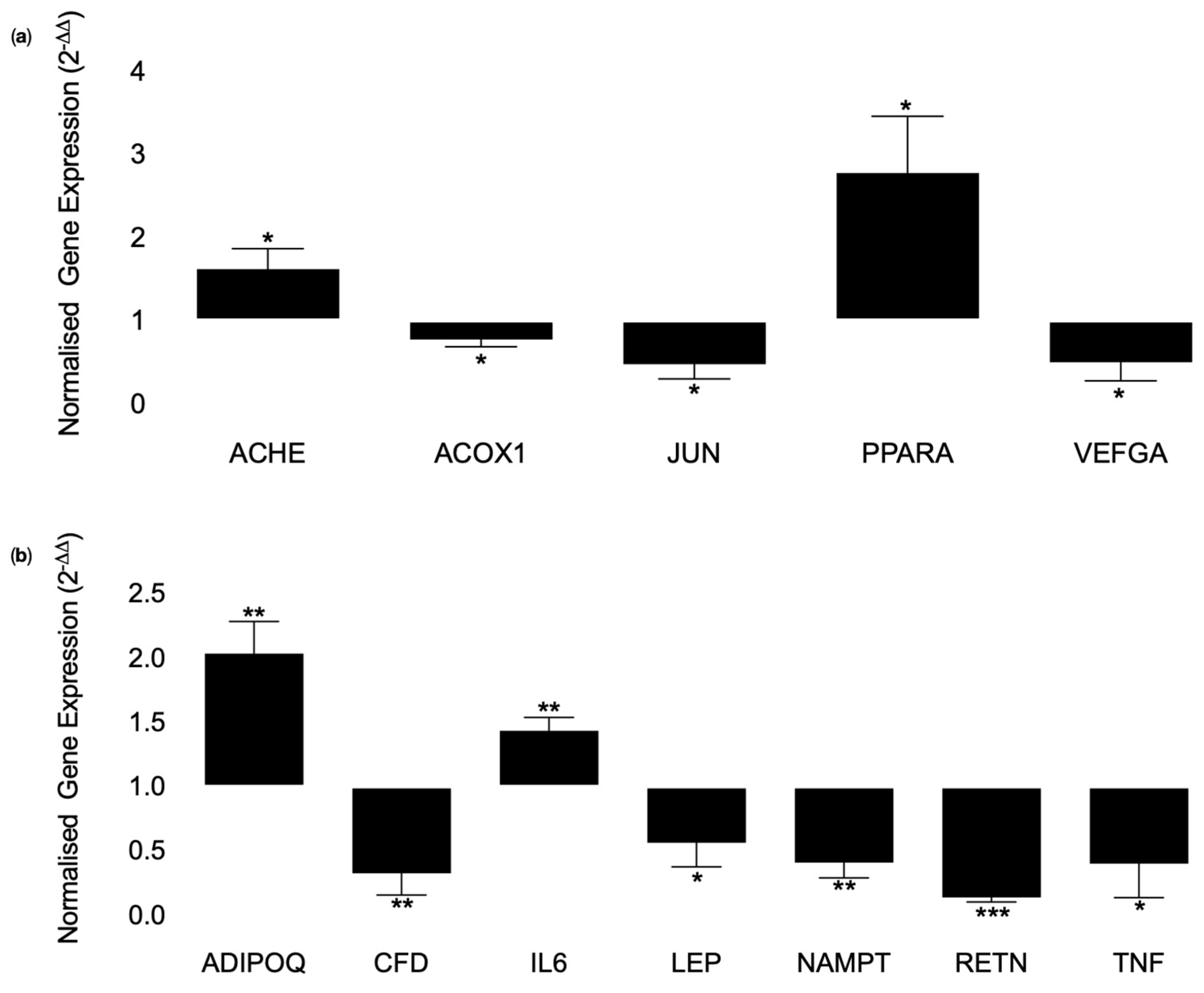
3.3. Cell Culture Experiments Using Human Primary Adipocytes (SGBS Cells)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Singh, G.M.; Lu, Y.; Danaei, G.; Lin, J.K.; Finucane, M.M.; Bahalim, A.N.; McIntire, R.K.; Gutierrez, H.R.; Cowan, M.; et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012, 10, 22. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Ronti, T.; Lupattelli, G.; Mannarino, E. The endocrine function of adipose tissue: An update. Clin. Endocrinol. 2006, 64, 355–365. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagai, R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov. Med. 2009, 8, 55–60. [Google Scholar] [PubMed]
- Ye, L.; Kleiner, S.; Wu, J.; Sah, R.; Gupta, R.K.; Banks, A.S.; Cohen, P.; Khandekar, M.J.; Boström, P.; Mepani, R.J.; et al. TRPV4 Is a Regulator of Adipose Oxidative Metabolism, Inflammation, and Energy Homeostasis. Cell 2012, 151, 96–110. [Google Scholar] [CrossRef]
- Goran, M.I.; Alderete, T.L. Targeting Adipose Tissue Inflammation to Treat the Underlying Basis of the Metabolic Complications of Obesity. Nestle Nutr. Inst. Workshop Ser. 2012, 73, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, A.W.; Carey, F.; Forder, R.; Rothwell, N.J. The role of lipocortin-1 in dexamethasone-induced suppression of PGE2 and TNF alpha release from human peripheral blood mononuclear cells. Br. J. Pharmacol. 1996, 117, 1449–1456. [Google Scholar] [CrossRef]
- Perretti, M.; Croxtall, J.D.; Wheller, S.K.; Goulding, N.J.; Hannon, R.; Flower, R.J. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat. Med. 1996, 2, 1259–1262. [Google Scholar] [CrossRef] [PubMed]
- Scannell, M.; Flanagan, M.B.; Destefani, A.; Wynne, K.; Cagney, G.; Godson, C.; Maderna, P. Annexin-1 and Peptide Derivatives Are Released by Apoptotic Cells and Stimulate Phagocytosis of Apoptotic Neutrophils by Macrophages. J. Immunol. 2007, 178, 4595–4605. [Google Scholar] [CrossRef]
- Sena, A.; Grishina, I.; Thai, A.; Goulart, L.; Macal, M.; Fenton, A.; Li, J.; Prindiville, T.; Oliani, S.M.; Dandekar, S.; et al. Dysregulation of Anti-Inflammatory Annexin A1 Expression in Progressive Crohns Disease. PLoS ONE 2013, 8, e76969. [Google Scholar] [CrossRef]
- Patel, H.B.; Kornerup, K.N.; Sampaio, A.L.; D’Acquisto, F.; Seed, M.P.; Girol, A.P.; Gray, M.; Pitzalis, C.; Oliani, S.M.; Perretti, M. The impact of endogenous annexin A1 on glucocorticoid control of inflammatory arthritis. Ann. Rheum. Dis. 2012, 71, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Montero-Melendez, T.; McArthur, S.; Perretti, M. Annexin A1 N-Terminal Derived Peptide Ac2-26 Exerts Chemokinetic Effects on Human Neutrophils. Front. Pharmacol. 2012, 3, 28. [Google Scholar] [CrossRef]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Benabdelkamel, H.; Masood, A.; Moustafa, A.; Sallam, R.; Bassas, A.; Duncan, M. Proteomic analysis of mature adipocytes from obese patients in relation to aging. Exp. Gerontol. 2013, 48, 1196–1203. [Google Scholar] [CrossRef]
- Akasheh, R.T.; Pini, M.; Pang, J.; Fantuzzi, G. Increased Adiposity in Annexin A1-Deficient Mice. PLoS ONE 2013, 8, e82608. [Google Scholar] [CrossRef] [PubMed]
- Kosicka, A.; Cunliffe, A.D.; Mackenzie, R.; Zariwala, M.G.; Perretti, M.; Flower, R.J.; Renshaw, D. Attenuation of plasma annexin A1 in human obesity. FASEB J. 2013, 27, 368–378. [Google Scholar] [CrossRef]
- Pietrani, N.T.; Ferreira, C.N.; Rodrigues, K.F.; Perucci, L.O.; Carneiro, F.S.; Bosco, A.A.; Oliveira, M.C.; Pereira, S.S.; Teixeira, A.L.; Alvarez-Leite, J.I.; et al. Proresolving protein Annexin A1: The role in type 2 diabetes mellitus and obesity. Biomed. Pharmacother. 2018, 103, 482–489. [Google Scholar] [CrossRef]
- Purvis, G.S.; Collino, M.; Loiola, R.A.; Baragetti, A.; Chiazza, F.; Brovelli, M.; Sheikh, M.H.; Collotta, D.; Cento, A.; Mastrocola, R.; et al. Identification of AnnexinA1 as an Endogenous Regulator of RhoA, and Its Role in the Pathophysiology and Experimental Therapy of Type-2 Diabetes. Front. Immunol. 2019, 10, 571. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and Adipose Tissue Function and Dysfunction in Obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Falasca, K.; Ucciferri, C.; Manzoli, L.; Mancino, P.; Pizzigallo, E.; Conti, P.; Vecchiet, J. Metabolic Syndrome and Cardiovascular Risk in HIV-Infected Patients with Lipodystrophy. Int. J. Immunopathol. Pharmacol. 2007, 20, 519–527. [Google Scholar] [CrossRef]
- Bays, H.E.; Toth, P.P.; Kris-Etherton, P.M.; Abate, N.; Aronne, L.J.; Brown, W.V.; Gonzalez-Campoy, J.M.; Jones, S.R.; Kumar, R.; La Forge, R.; et al. Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. J. Clin. Lipidol. 2013, 7, 304–383. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Dash, S.; Xiao, C.; Lewis, G.F. Effects of bariatric surgery on hepatic and intestinal lipoprotein particle metabolism. Curr. Opin. Lipidol. 2016, 27, 14–18. [Google Scholar] [CrossRef]
- Heffron, S.; Parikh, A.; Volodarskiy, A.; Ren-Fielding, C.; Schwartzbard, A.; Nicholson, J.; Bangalore, S. Changes in Lipid Profile of Obese Patients Following Contemporary Bariatric Surgery: A Meta-Analysis. Am. J. Med. 2016, 129, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Kong, J.; Jin, J.; Kong, J.; He, Y.; Dong, S.; Ji, L.; Liu, D.; He, D.; Kong, L.; et al. A novel anti-inflammatory mechanism of high density lipoprotein through up-regulating annexin A1 in vascular endothelial cells. Biochim. Biophys. Acta 2016, 1861, 501–512. [Google Scholar] [CrossRef]
- Cheuk, B.L.Y.; Cheng, S.W.K. Annexin A1 Expression in Atherosclerotic Carotid Plaques and its Relationship with Plaque Characteristics. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 364–371. [Google Scholar] [CrossRef]
- Drechsler, M.; de Jong, R.; Rossaint, J.; Viola, J.R.; Leoni, G.; Wang, J.M.; Grommes, J.; Hinkel, R.; Kupatt, C.; Weber, C.; et al. Annexin A1 counteracts chemokine-induced arterial myeloid cell recruitment. Circ. Res. 2015, 116, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.H.; Laguna-Fernandez, A.; Gonzalez-Diez, M.; Paulsson-Berne, G.; Hansson, G.K.; Back, M. The role of the FPR2/ALX receptor in atherosclerosis development and plaque stability. Cardiovasc. Res. 2015, 105, 65–74. [Google Scholar] [CrossRef]
- Wood, I.S.; de Heredia, F.P.; Wang, B.; Trayhurn, P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc. Nutr. Soc. 2009, 68, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Gunnett, C.A.; Heistad, D.D.; Faraci, F.M. Interleukin-10 protects nitric oxide-dependent relaxation during diabetes: Role of superoxide. Diabetes 2002, 51, 1931–1937. [Google Scholar] [CrossRef][Green Version]
- Jung, S.H.; Park, H.S.; Kim, K.-S.; Choi, W.H.; Ahn, C.W.; Kim, B.T.; Kim, S.M.; Lee, S.Y.; Ahn, S.M.; Kim, Y.K.; et al. Effect of weight loss on some serum cytokines in human obesity: Increase in IL-10 after weight loss. J. Nutr. Biochem. 2008, 19, 371–375. [Google Scholar] [CrossRef]
- Netto, B.D.M.; Bettini, S.C.; Clemente, A.P.G.; Ferreira, J.P.D.C.; Boritza, K.; Souza, S.D.F.; Von der Heyde, M.E.; Earthman, C.P.; Dâmaso, A.R. Roux-en-Y Gastric Bypass Decreases Pro-inflammatory and Thrombotic Biomarkers in Individuals with Extreme Obesity. Obes. Surg. 2015, 25, 1010–1018. [Google Scholar] [CrossRef]
- Wolf, A.M.; Wolf, D.; Rumpold, H.; Enrich, B.; Tilg, H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem. Biophys. Res. Commun. 2004, 323, 630–635. [Google Scholar] [CrossRef]
- Ferlazzo, V.; D’Agostino, P.; Milano, S.; Caruso, R.; Feo, S.; Cillari, E.; Parente, L. Anti-inflammatory effects of annexin-1: Stimulation of IL-10 release and inhibition of nitric oxide synthesis. Int. Immunopharmacol. 2003, 3, 1363–1369. [Google Scholar] [CrossRef]
- Hussaina, I.; Garg, A. Lipodystrophy syndromes. Dermatol. Clin. 2008, 26, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Garg, A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: Case reports and review of the literature. Medicine 2003, 82, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W., 2nd; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef]
- Herrero, L.; Shapiro, H.; Nayer, A.; Lee, J.; Shoelson, S.E. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc. Natl. Acad. Sci. USA 2010, 107, 240–245. [Google Scholar] [CrossRef]
- Goulding, N.J.; Jefferiss, C.M.; Pan, L.; Rigby, W.F.C.; Guyre, P.M. Specific binding of lipocortin-1 (annexin I) to monocytes and neutrophils is decreased in rheumatoid arthritis. Arthritis Rheum. 1992, 35, 1395–1397. [Google Scholar] [CrossRef]
- Goulding, N.J.; Podgorski, M.R.; Hall, N.D.; Flower, R.J.; Browning, J.L.; Pepinsky, R.B.; Maddison, P.J. Autoantibodies to recombinant lipocortin-1 in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 1989, 48, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Warne, J.P.; John, C.D.; Christian, H.C.; Morris, J.F.; Flower, R.J.; Sugden, D.; Solito, E.; Gillies, G.E.; Buckingham, J.C. Gene deletion reveals roles for annexin A1 in the regulation of lipolysis and IL-6 release in epididymal adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1264–E1273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Aeberli, D.; Dacumos, A.; Xue, J.R.; Morand, E. Annexin-1 Regulates Macrophage IL-6 and TNF via Glucocorticoid-Induced Leucine Zipper. J. Immunol. 2009, 183, 1435–1445. [Google Scholar] [CrossRef]
- Zeng, J.; Deng, S.; Wang, Y.; Li, P.; Tang, L.; Pang, Y. Specific Inhibition of Acyl-CoA Oxidase-1 by an Acetylenic Acid Improves Hepatic Lipid and Reactive Oxygen Species (ROS) Metabolism in Rats Fed a High Fat Diet. J. Biol. Chem. 2017, 292, 3800–3809. [Google Scholar] [CrossRef]
- Huang, J.; Jia, Y.; Fu, T.; Viswakarma, N.; Bai, L.; Rao, M.S.; Zhu, Y.; Borensztajn, J.; Reddy, J.K. Sustained activation of PPARα by endogenous ligands increases hepatic fatty acid oxidation and prevents obesity in ob/ob mice. FASEB J. 2012, 26, 628–638. [Google Scholar] [CrossRef]
- Shiomi, Y.; Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Nakayama, R.; Orikawa, Y.; Yoshioka, Y.; Tanaka, K.; Ueki, K.; Kadowaki, T. A Novel Peroxisome Proliferator-activated Receptor (PPAR)α Agonist and PPARγ Antagonist, Z-551, Ameliorates High-fat Diet-induced Obesity and Metabolic Disorders in Mice. J. Biol. Chem. 2015, 290, 14567–14581. [Google Scholar] [CrossRef] [PubMed]
- Guerre-Millo, M.; Gervois, P.; Raspé, E.; Madsen, L.; Poulain, P.; Derudas, B.; Herbert, J.-M.; Winegar, D.A.; Willson, T.M.; Fruchart, J.-C.; et al. Peroxisome Proliferator-activated Receptor α Activators Improve Insulin Sensitivity and Reduce Adiposity. J. Biol. Chem. 2000, 275, 16638–16642. [Google Scholar] [CrossRef]
- Tsuchida, A.; Yamauchi, T.; Takekawa, S.; Hada, Y.; Ito, Y.; Maki, T.; Kadowaki, T. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: Comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 2005, 54, 3358–3370. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Bujo, H.; Unoki, H.; Saito, Y. Effect of PPARalpha activation of macrophages on the secretion of inflammatory cytokines in cultured adipocytes. Eur. J. Pharmacol. 2007, 561, 206–213. [Google Scholar] [CrossRef]
- Atgie, C.; D’Allaire, F.; Bukowiecki, L.J. Role of beta1- and beta3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. Am. J. Physiol. 1997, 273 Pt 1, C1136–C1142. [Google Scholar] [CrossRef]
- Patil, M.; Sharma, B.K.; Elattar, S.; Chang, J.; Kapil, S.; Yuan, J.; Satyanarayana, A. Id1 Promotes Obesity by Suppressing Brown Adipose Thermogenesis and White Adipose Browning. Diabetes 2017, 66, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Bakker, A.H.F.; Van Dielen, F.M.H.; Greve, J.W.M.; Adam, J.A.; Buurman, W.A. Preadipocyte Number in Omental and Subcutaneous Adipose Tissue of Obese Individuals. Obes. Res. 2004, 12, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; Kassem, D.H.; El-Demerdash, E.; Amin, A.I. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism 2011, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Oki, K.; Yamane, K.; Kamei, N.; Nojima, H.; Kohno, N. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin. Endocrinol. 2007, 67, 796–800. [Google Scholar] [CrossRef]
- Sissons, J.G.P.; West, R.J.; Fallows, J.; Williams, D.G.; Boucher, B.J.; Amos, N.; Peters, D.K. The Complement Abnormalities of Lipodystrophy. N. Engl. J. Med. 1976, 294, 461–465. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Van De Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Kern, P.A.; Saghizadeh, M.; Ong, J.M.; Bosch, R.J.; Deem, R.; Simsolo, R.B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J. Clin. Investig. 1995, 95, 2111–2119. [Google Scholar] [CrossRef]
- Gualillo, O.; González-Juanatey, J.R.; Lago, F. The emerging role of adipokines as mediators of cardiovascular function: Physiologic and clinical perspectives. Trends Cardiovasc. Med. 2007, 17, 275–283. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, L.; Zhao, W.; Rigas, B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: Anticancer effects in vitro and in vivo. Cancer Res. 2010, 70, 2379–2388. [Google Scholar] [CrossRef]
- Sheikh, M.H.; Solito, E. Annexin A1: Uncovering the Many Talents of an Old Protein. Int. J. Mol. Sci. 2018, 19, 1045. [Google Scholar] [CrossRef]
- Matthews, V.B.; Allen, T.L.; Risis, S.; Chan, M.H.S.; Henstridge, D.C.; Watson, N.; Zaffino, L.A.; Babb, J.R.; Boon, J.; Meikle, P.J.; et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 2010, 53, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Hasenfuss, S.C.; Bakiri, L.; Thomsen, M.K.; Williams, E.G.; Auwerx, J.; Wagner, E.F. Regulation of steatohepatitis and PPARgamma signaling by distinct AP-1 dimers. Cell Metab. 2014, 19, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Halazonetis, T.D.; Georgopoulos, K.; Greenberg, M.E.; Leder, P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 1988, 55, 917–924. [Google Scholar] [CrossRef]
- Wang, X.; Ron, D. Stress-Induced Phosphorylation and Activation of the Transcription Factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef]
- Eferl, R.; Ricci, R.; Kenner, L.; Zenz, R.; David, J.-P.; Rath, M.; Wagner, E.F. Liver Tumor Development: C-Jun Antagonizes the Proapoptotic Activity of p53. Cell 2003, 112, 181–192. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Albrektsen, T.; Fleckner, J. The Transcription Factor Fos-Related Antigen 1 Is Induced by Thiazolidinediones During Differentiation of 3T3-L1 Cells. Mol. Pharmacol. 2001, 59, 567–575. [Google Scholar] [CrossRef]
- Cao, Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat. Rev. Drug Discov. 2010, 9, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Shao, Z.; Yang, Y.; Wu, D.; Zhou, X.; Yuan, H. Annexin 1 protects against apoptosis induced by serum deprivation in transformed rat retinal ganglion cells. Mol. Biol. Rep. 2012, 39, 5543–5551. [Google Scholar] [CrossRef]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-Inducible Factor 1α Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef]
- Fatima, L.A.; Campello, R.S.; Santos, R.D.S.; Freitas, H.S.; Frank, A.P.; Machado, U.F.; Clegg, D.J. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Sci. Rep. 2017, 7, 16716. [Google Scholar] [CrossRef]
- Sun, K.; Asterholm, I.W.; Kusminski, C.M.; Bueno, A.C.; Wang, Z.V.; Pollard, J.W.; Brekken, R.A.; Scherer, P.E. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. USA 2012, 109, 5874–5879. [Google Scholar] [CrossRef]
- Metz, C.N.; Tracey, K.J. It takes nerve to dampen inflammation. Nat. Immunol. 2005, 6, 756–757. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 13, RA214–RA221. [Google Scholar] [PubMed]
- Shenhar-Tsarfaty, S.; Sherf-Dagan, S.; Berman, G.; Webb, M.; Raziel, A.; Keidar, A.; Goitein, D.; Sakran, N.; Zwang, E.; Shapira, I.; et al. Obesity-related acetylcholinesterase elevation is reversed following laparoscopic sleeve gastrectomy. Int. J. Obes. 2018, 43, 297–305. [Google Scholar] [CrossRef]
- Svensson, M.K.; Jansson, P.-A.; Persson, A.L.; Sjöstrand, M.; Eriksson, J.W. Atropine Improves Insulin Sensitivity in Both Lean and Abdominally Obese Subjects. J. Clin. Endocrinol. Metab. 2011, 96, E1843–E1847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ashrafian, H.; Athanasiou, T.; Li, J.V.; Bueter, M.; Ahmed, K.; Nagpal, K.; Holmes, E.; Darzi, A.; Bloom, S.R. Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes. Rev. 2011, 12, e257–e272. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics-Bariatric Surgery Study | |||
|---|---|---|---|
| Pre-Opt | Post-Opt | ||
| Number of participants | n = 26 | n = 11 | |
| Gender | |||
| Males | 4 | 0 | |
| Females | 22 | 11 | |
| Age, years | |||
| 48 ± 1.9 | 51 ± 2.7 | ||
| Type of Surgery | |||
| Sleeve Gastrectomy | 13 | 5 | |
| Roux-en-Y Gastric Bypass | 13 | 6 | |
| Type 2 diabetes mellitus | |||
| Diabetics | 10 | 4 | |
| Non-Diabetics | 16 | 7 | |
| Anthropometric Measures and LIPID PROFILES | |||
|---|---|---|---|
| Pre-Opt | Post-Opt | ||
| Weight (kg) | 131.5 ± 6.4 (n = 26) | 105.9 ± 5.3 * (n = 9) | |
| Lipid Profiles | |||
| Triglycerides (mmol/L) | 1.3 ± 0.1 (n = 25) | 1.4 ± 0.3 (n = 9) | |
| Total Cholesterol (mmol/L) | 4.5 ± 0.1 (n = 25) | 4.5 ± 0.4 (n = 9) | |
| HDL (mmol/L) | 1.2 ± 0.1 (n = 25) | 1.3 ± 0.1 (n = 8) | |
| LDL (mmol/L) | 2.7 ± 0.1 (n = 25) | 2.5 ± 0.2 (n = 9) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, S.; Zariwala, M.G.; Mackenzie, R.; Turner, M.; Nell, T.; Bellary, S.; Renshaw, D. Suppression of Anti-Inflammatory Mediators in Metabolic Disease May Be Driven by Overwhelming Pro-Inflammatory Drivers. Nutrients 2022, 14, 2360. https://doi.org/10.3390/nu14112360
Sajid S, Zariwala MG, Mackenzie R, Turner M, Nell T, Bellary S, Renshaw D. Suppression of Anti-Inflammatory Mediators in Metabolic Disease May Be Driven by Overwhelming Pro-Inflammatory Drivers. Nutrients. 2022; 14(11):2360. https://doi.org/10.3390/nu14112360
Chicago/Turabian StyleSajid, Sehar, Mohammed Gulrez Zariwala, Richard Mackenzie, Mark Turner, Theo Nell, Srikanth Bellary, and Derek Renshaw. 2022. "Suppression of Anti-Inflammatory Mediators in Metabolic Disease May Be Driven by Overwhelming Pro-Inflammatory Drivers" Nutrients 14, no. 11: 2360. https://doi.org/10.3390/nu14112360
APA StyleSajid, S., Zariwala, M. G., Mackenzie, R., Turner, M., Nell, T., Bellary, S., & Renshaw, D. (2022). Suppression of Anti-Inflammatory Mediators in Metabolic Disease May Be Driven by Overwhelming Pro-Inflammatory Drivers. Nutrients, 14(11), 2360. https://doi.org/10.3390/nu14112360






