Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Samples Collection, Preparing, and Extraction of Essential Oils
2.3. Gas Chromatography/Mass Spectrometry (GC-MS)
2.4. Free Radical Scavenging Activity
2.5. Antimicrobial Activity
2.6. Cell Culture and Cytotoxicity Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Rosmarinus officinalis Essential Oils
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
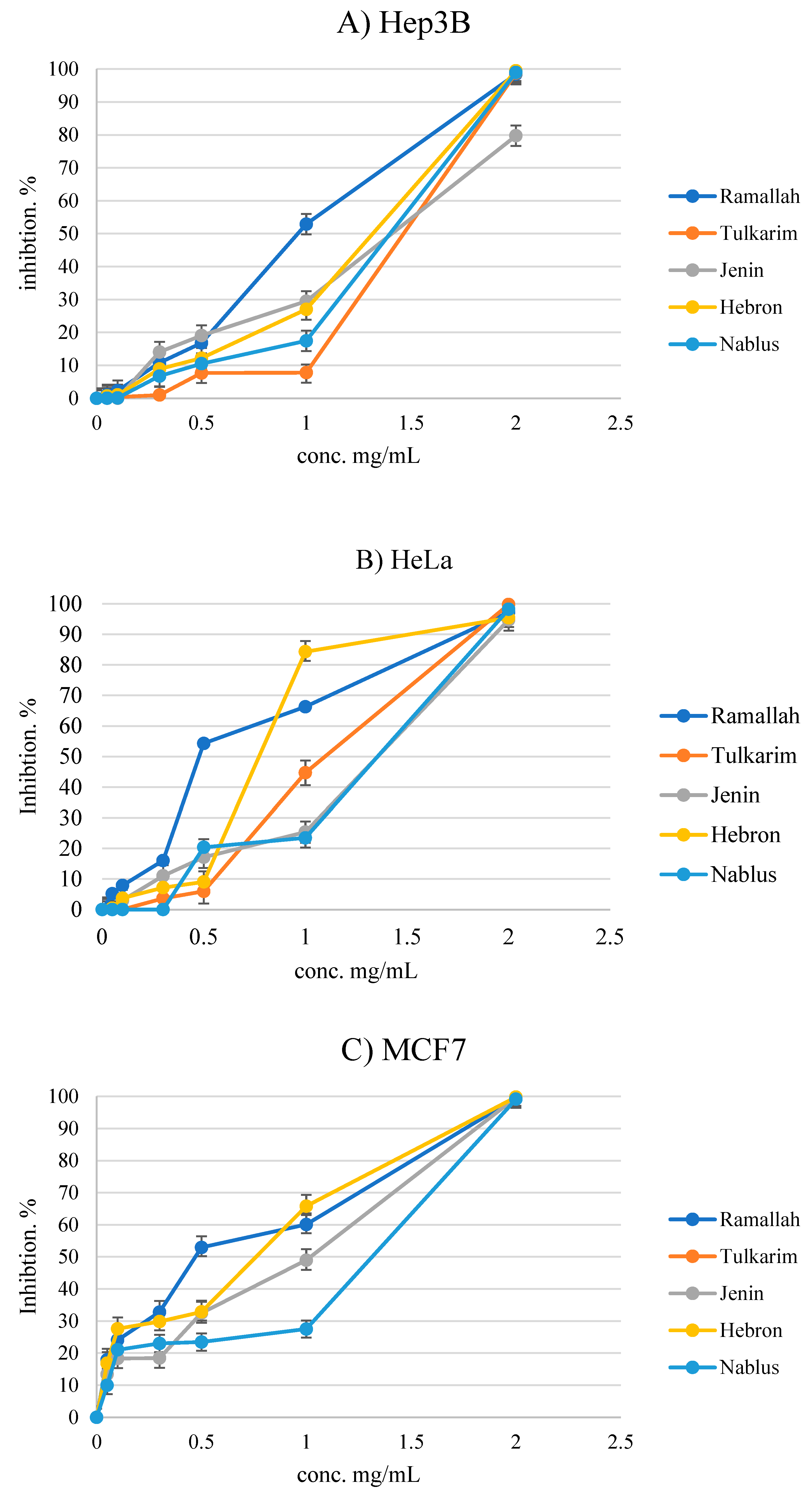
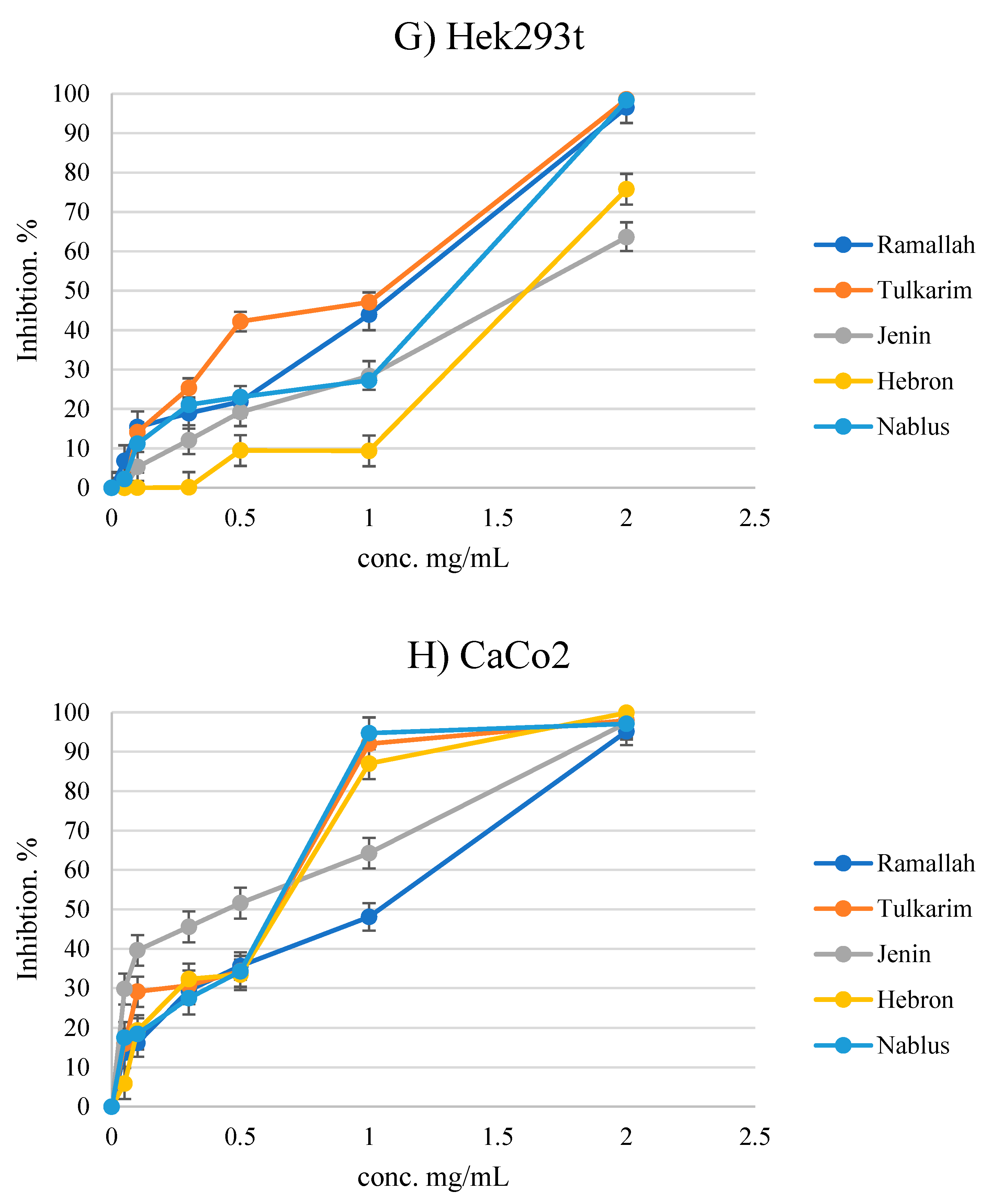
3.4. Antiproliferative Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100. [Google Scholar]
- Hosseinzadeh, H.; Karimi, G.; Nobakht, N. Effects of Rosmarinus officinalis L. aerial parts essential oil on intact memory and scopolamine-induced learning deficits in rats performing the morris water maze task. J. Med. Plants 2004, 3, 51–57. [Google Scholar]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Capra, J.C.; Dalmarco, J.B.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 642–650. [Google Scholar] [CrossRef]
- Sasaki, K.; El Omri, A.; Kondo, S.; Han, J.; Isoda, H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013, 238, 86–94. [Google Scholar] [CrossRef]
- Hou, C.-W.; Lin, Y.-T.; Chen, Y.-L.; Wang, Y.-H.; Chou, J.-L.; Ping, L.-Y.; Jeng, K.-C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012, 15, 257–263. [Google Scholar] [CrossRef]
- González-Trujano, M.; Peña, E.; Martínez, A.; Moreno, J.; Guevara-Fefer, P.; Deciga-Campos, M.; López-Muñoz, F. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Bakırel, T.; Bakırel, U.; Keleş, O.Ü.; Ülgen, S.G.; Yardibi, H. In vivo assessment of antidiabetic and antioxidant activities of rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef]
- Benincá, J.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Fröde, T.S. Analysis of the anti-inflammatory properties of Rosmarinus officinalis L. in mice. Food Chem. 2011, 124, 468–475. [Google Scholar] [CrossRef]
- Pietrocola, F.; Pedro, B.-S.; Manuel, J. Targeting autophagy to counteract obesity-associated oxidative stress. Antioxidants 2021, 10, 102. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 October 2022).
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease; Hindawi: London, UK, 2017. [Google Scholar]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, T.I.; Mandalakis, M.; Krigas, N.; Vézignol, T.; Lazari, D.; Katharios, P.; Dailianis, T.; Antonopoulou, E. Comparative evaluation of essential oils from medicinal-aromatic plants of Greece: Chemical composition, antioxidant capacity and antimicrobial activity against bacterial fish pathogens. Molecules 2020, 25, 148. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Rivera-Chavira, B.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Differential antimicrobial effect of essential oils and their main components: Insights based on the cell membrane and external structure. Membranes 2021, 11, 405. [Google Scholar] [CrossRef]
- Nuță, D.C.; Limban, C.; Chiriță, C.; Chifiriuc, M.C.; Costea, T.; Ioniță, P.; Nicolau, I.; Zarafu, I. Contribution of essential oils to the fight against microbial biofilms—A Review. Processes 2021, 9, 537. [Google Scholar] [CrossRef]
- Lagha, R.; Ben Abdallah, F.; Al-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, X.; Wang, Q.; Lu, T.; Deng, T.; Tian, M.; Zhou, Y. Antioxidant, antibacterial, enzyme inhibitory, and anticancer activities and chemical composition of Alpinia galanga flower essential oil. Pharmaceuticals 2022, 15, 1069. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, X.; Wang, H.; Zhang, M.; Tian, M. Chemical composition, anticancer activities and related mechanisms of the essential oil from Alpinia coriandriodora rhizome. Ind. Crops Prod. 2022, 176, 114328. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Samer Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind. Crops Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Al-Masri, M.; Shanab, B.A.; Hussein, F.; Qneibi, M.; Eldin, A.N.; Yassin, T.; Khawaja, M. Phytochemical and antibacterial assessment of Rhagadiolus stellatus Plant in Jerusalem Area-Palestine. Pal. Med. Pharm. J. 2017, 2, 35–44. [Google Scholar]
- Yeddes, W.; Aidi Wannes, W.; Hammami, M.; Smida, M.; Chebbi, A.; Marzouk, B.; Saidani Tounsi, M. Effect of environmental conditions on the chemical composition and antioxidant activity of essential oils from Rosmarinus officinalis L. growing wild in Tunisia. J. Essent. Oil Bear. Plants 2018, 21, 972–986. [Google Scholar] [CrossRef]
- Celiktas, O.Y.; Kocabas, E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Chalchat, J.-C.; Garry, R.-P.; Michet, A.; Benjilali, B.; Chabart, J. Essential oils of rosemary (Rosmarinus officinalis L.). The chemical composition of oils of various origins (Morocco, Spain, France). J. Esent. Oil. Res. 1993, 5, 613–618. [Google Scholar] [CrossRef]
- Socaci, S.A.; Tofana, M.; Socaciu, C. GC-MS analysis of rosemary essential oil. Bull. UASVM Agric. 2008, 65, 405–409. [Google Scholar]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flav. Frag. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Akrout, A.; Hajlaoui, H.; Mighri, H.; Najjaa, H.; Jani, H.E.; Zaidi, S.; Neffati, M. Chemical and biological characteristics of essential oil of Rosmarinus officinalis cultivated in Djerba. J. Essent. Oil Bear. Plants 2010, 13, 398–411. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chemist. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis essential oil: Antiproliferative, antioxidant and antibacterial activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef]
- Ferreira, H. Assessment Report on Rosmarinus officinalis L., aetheroleum and Rosmarinus officinalis L., Folium; Committee on Herbal Medicinal Products (HMPC): London, UK, 2010. [Google Scholar]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of Rosmarinus officinalis (rosemary)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2018, 37, 12S–50S. [Google Scholar] [CrossRef]
- Santos, P.; Avanço, G.; Nerilo, S.; Marcelino, R.; Janeiro, V.; Valadares, M.; Machinski, M. Assessment of cytotoxic activity of rosemary (Rosmarinus officinalis L.), turmeric (Curcuma longa L.), and ginger (Zingiber officinale R.) essential oils in cervical cancer cells (HeLa). Sci. World J. 2016, 2016, 9273078. [Google Scholar] [CrossRef]
- Miladi, H.; Slama, R.B.; Mili, D.; Zouari, S.; Bakhrouf, A.; Ammar, E. Essential oil of Thymus vulgaris L. and Rosmarinus officinalis L.: Gas chromatography-mass spectrometry analysis, cytotoxicity and antioxidant properties and antibacterial activities against foodborne pathogens. Nat. Sci. 2013, 5, 729–739. [Google Scholar]
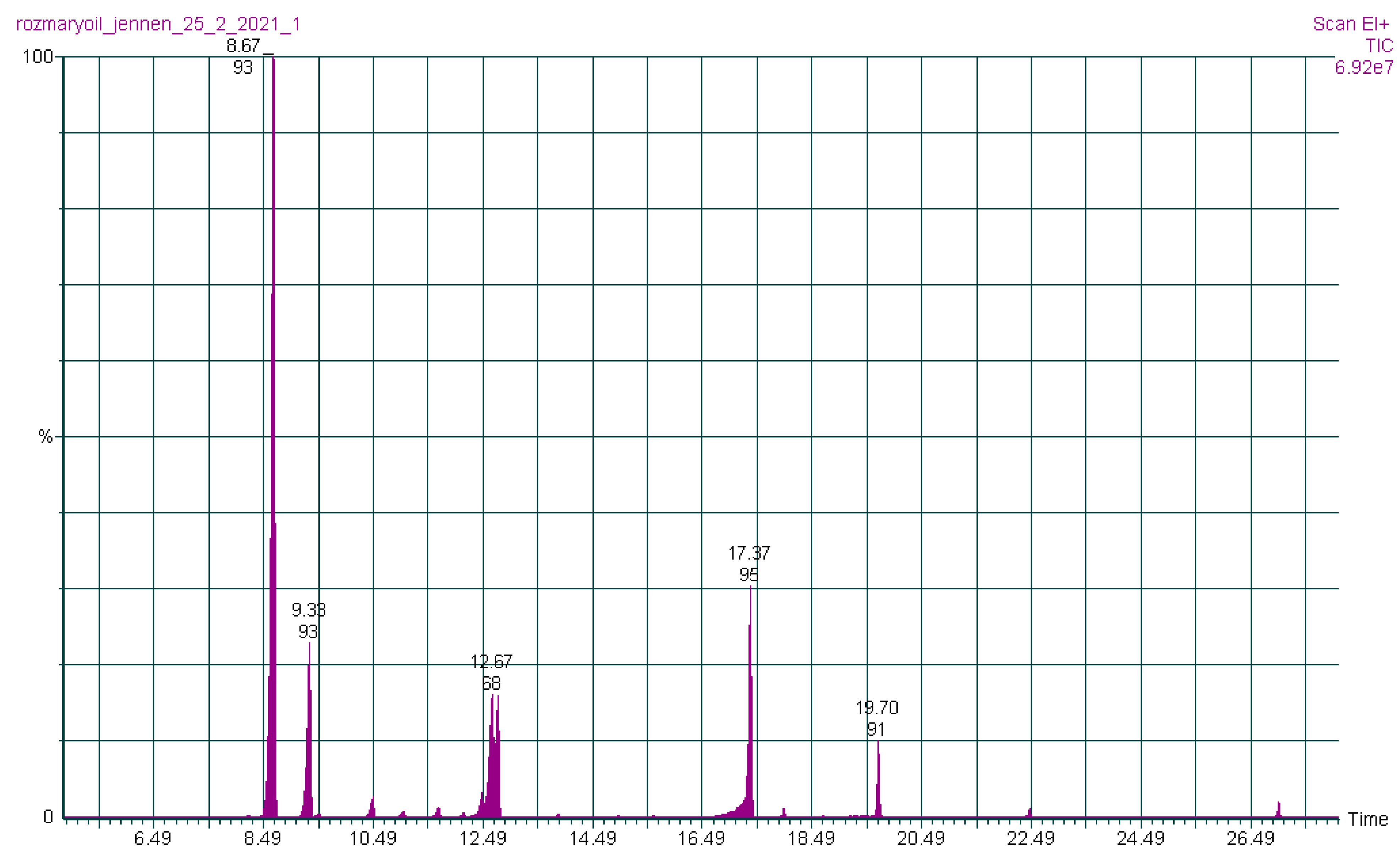
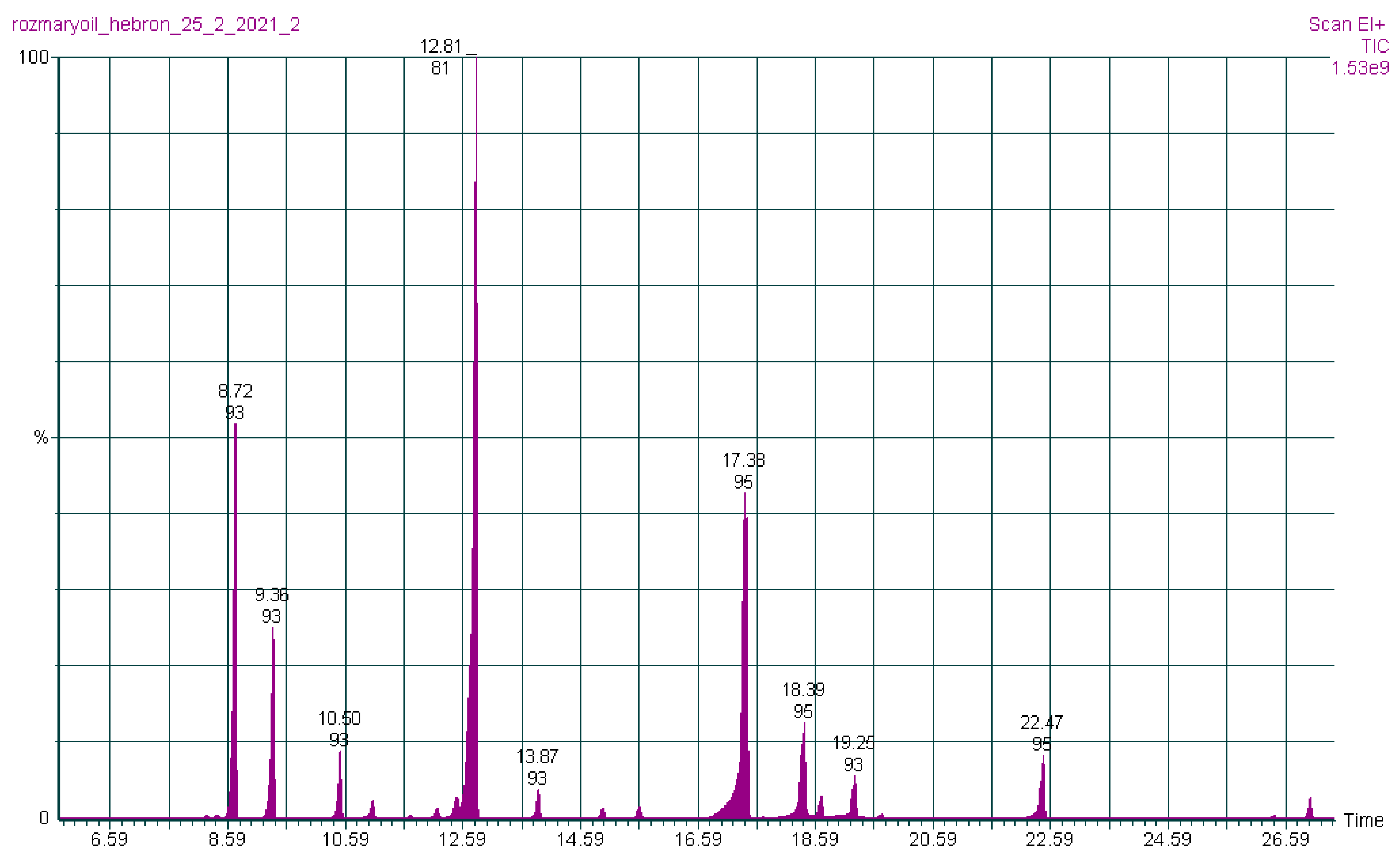



| Essential Oil Content (%) | |||||||
|---|---|---|---|---|---|---|---|
| Name | RIcalculated | Hebron | Jenin | Ramallah | Nablus | Tulkarm | m/z of Fragments |
| Tricyclene | 921 | 0.09 | 0.07 | 0.14 | 0.13 | 0.13 | 136, 121, 105, 93, 77, 67, 55 |
| α-Thujene | 926 | 0.10 | 0.002 | - | - | - | 136, 121, 1075, 93, 77, 65, 53 |
| α-Pinene | 933 | 13.07 | 51.36 | 26.90 | 25.29 | 29.18 | 136, 121, 105, 93, 77, 67, 53 |
| Camphene | 949 | 6.67 | 11.62 | 7.45 | 9.57 | 5.81 | 136, 121, 105, 93, 77, 67, 53 |
| Thuja-2,4(10)-diene | 953 | - | - | 0.11 | 0.05 | 0.21 | 134, 119, 105, 91, 78, 65 |
| Unknown | 965 | - | 1.16 | - | - | - | 119, 105, 93, 79, 67 |
| β-Pinene | 976 | 2.46 | 0.30 | 0.85 | 2.19 | 0.58 | 136, 121, 107, 93, 79, 69, 53 |
| Myrcene | 990 | 0.69 | 0.56 | 0.72 | 0.73 | - | 136, 93, 79, 69, 53 |
| α-Phellandrene | 1005 | 0.10 | - | 2.24 | 1.99 | - | 136, 93, 77, 65 |
| Unknown | 1006 | - | 0.18 | - | - | 0.11 | 136, 121, 91, 77, 63 |
| α-Terpinene | 1016 | 0.40 | 1.48 | 1.01 | 0.66 | 0.39 | 136, 121, 105, 93, 77, 65 |
| p-Cymene | 1024 | - | 10.12 | 1.39 | - | 1.82 | 134, 119, 103, 91, 77, 65 |
| Limonene | 1029 | - | 3.02 | - | - | - | 136, 121, 107, 93, 79, 68, 53 |
| 1,8-Cineole | 1033 | 37.82 | 4.81 | 31.09 | 31.09 | 33.28 | 154, 139, 119, 108, 93, 81, 71, 55 |
| Unknown | 1058 | - | 0.11 | - | - | - | 119, 91, 79, 65 |
| γ-Terpinene | 1059 | 1.23 | 0.06 | 0.64 | 0.90 | 0.58 | 136, 121, 105, 93, 77, 65, 53 |
| Terpinolene | 1085 | 0.38 | 0.04 | 0.36 | 0.41 | 0.36 | 136, 121, 105, 93, 79, 67, 58 |
| Linalool | 1101 | 0.54 | - | 0.15 | 0.16 | 0.71 | 136, 121, 107, 93, 79, 71, 55 |
| Chrysanthenone | 1122 | - | - | 0.06 | - | - | 150, 135, 122, 107,91, 70 |
| cis-Menth-2-en-l-ol | 1125 | - | - | 0.06 | 0.23 | 0.02 | 154, 139, 121, 111, 93, 79, 55 |
| Camphor | 1148 | 24.30 | 13.86 | 15.52 | 18.47 | 11.95 | 152, 137, 108, 95, 81, 69, 55 |
| Neoisothujol | 1156 | - | - | 0.05 | 0.06 | 0.06 | 136, 121, 108, 93, 79, 71, 53 |
| Isoborneol | 1162 | - | 0.32 | - | 0.29 | - | 136, 121, 110, 95, 67 |
| Pinocarvone | 1163 | - | 0.02 | 0.04 | - | 0.66 | 150, 135, 122, 108, 91, 81, 53 |
| Borneol | 1174 | 4.76 | - | 3.62 | 2.25 | 5.07 | 154, 139, 121, 110, 95, 79, 67, 55 |
| Terpinene-4-ol | 1181 | 0.94 | - | 0.65 | 0.49 | 0.96 | 154, 136, 121, 107, 93, 77, 71, 55 |
| Unknown | 1188 | - | - | - | 0.07 | 0.21 | 150, 135, 121, 117, 107, 91, 81, 67, 59, 53 |
| α-Terpineol | 1196 | 2.13 | - | 1.66 | 1.05 | 2.69 | 150, 135, 121, 107, 93, 79, 67, 59, 53 |
| Verbenone | 1208 | 0.12 | - | 2.23 | 1.56 | 2.69 | 150, 135, 122, 117, 107, 91, 79, 67, 59, 55 |
| Cuminaldehyde | 1242 | - | - | - | - | 0.07 | 148, 133, 119, 105, 91, 77 |
| Carvone | 1245 | - | - | - | - | 0.04 | 150, 135, 93, 82, 54 |
| Piperitone | 1255 | - | - | - | 0.02 | 0.01 | 152, 137, 110, 95, 82, 67 |
| Isobornyl acetate | 1285 | 3.17 | - | 0.52 | 1.06 | 0.35 | 154, 136, 121, 108, 95, 79, 67, 55 |
| Piperitenone | 1340 | - | - | - | - | 0.04 | 150, 135, 121, 107, 91, 79, 67, 53 |
| α-Ylangene | 1371 | 0.02 | - | - | - | 0.15 | 204, 189, 161, 119, 105, 93, 79, 67, 55 |
| α-Copaene | 1376 | - | - | - | - | 0.01 | 204, 161, 119, 105, 91, 81, 67, 55 |
| Methyl eugenol | 1401 | 0.09 | 0.59 | 0.06 | 0.03 | 0.16 | 178, 163, 147, 107, 91, 77, 65, 51 |
| β-Caryophellene | 1420 | 0.72 | - | 2.14 | 1.06 | 1.04 | 204, 189, 175, 161, 119, 105, 91, 79, 69, 55 |
| Linalool butanoate | 1426 | - | - | - | 0.10 | - | 136, 121, 107, 93, 71, 55 |
| α-Caryopyllene | 1457 | 0.08 | - | 0.23 | - | 0.16 | 204, 147, 135, 121, 107, 93, 80, 67, 53 |
| Germacrene D | 1477 | 0.02 | - | - | - | - | 204, 161, 147, 133, 119, 105, 91, 79, 67, 55 |
| γ-Muurolene | 1476 | - | - | - | - | 0.13 | 204, 161, 147, 133, 119, 105, 91, 79, 67, 55 |
| α-Muurolene | 1500 | 0.01 | - | -- | - | - | 204, 189, 161, 147, 133, 119, 105, 91, 81, 67 |
| β-Bisabolene | 1507 | 0.03 | - | - | - | - | 204, 189, 161, 147, 133, 119, 105, 91, 81, 67 |
| γ -Cadinene | 1514 | - | - | - | - | 0.09 | 204, 161, 147, 133, 119, 105, 91, 79, 67, 55 |
| δ-Cadinene | 1521 | 0.05 | - | - | - | 0.24 | 204, 189, 161, 147, 133, 119, 105, 91, 77, 67 |
| Z-Nerolidol | 1525 | 0.01 | - | - | -- | 0.03 | 189, 161, 147, 136, 121, 107, 93, 81, 69 |
| Caryophyllene oxide | 1586 | - | - | 0.08 | 0.10 | 0.04 | 177, 161, 149, 135, 121, 107, 91, 79, 69, 55 |
| Oil Yield | 1.01 | 1.22 | 1.41 | 1.35 | 1.31 | ||
| Total identified | 99.62 | 98.43 | 99.97 | 99.94 | 99.81 | ||
| Monoterpene hydrocarbons | 24.81 | 78.75 | 41.80 | 41.92 | 39.17 | ||
| Oxygenated monoterpenes | 73.78 | 19.01 | 55.66 | 56.83 | 58.59 | ||
| Sesquiterpene hydrocarbons | 0.93 | - | 2.37 | 1.06 | 1.82 | ||
| Oxygenated Sesquiterpenes | 0.01 | - | 0.08 | 0.10 | 0.07 | ||
| Others | 0.9 | 0.59 | 0.06 | 0.03 | 0.16 | ||
| Samples/Microbe | Bacteria | Fungus | |||||
|---|---|---|---|---|---|---|---|
| Gram-Positives | Gram-Negative | Yeast | |||||
| Clinical Strain | ATCC 25923 | ATCC 25922 | ATCC 13883 | ATCC 8427 | ATCC 9027 | ATCC 90028 | |
| MRSA | S. aureus | E. coli | K. pneumoniae | P. vulgaris | P. aeruginosa | C. albicans | |
| Hebron EO | 50 ± 0.91 | 50 ± 1.11 | R | 50 ± 0.88 | 50 ± 1.09 | R | 25 ± 1.07 |
| Tulkarm EO | 50 ± 1.01 | 25 ± 0.97 | 50 ± 1.02 | 50 ± 1.09 | 25 ± 0.1 | R | 12.5 ± 1.01 |
| Ramallah EO | 25 ± 0.35 | 25 ± 0.35 | 12.5 ± 0.39 | 12.5 ± 0.92 | 12.5 ± 88 | R | 1.56 ± 0.07 |
| Nablus EO | 25 ± 0.47 | 25 ± 0.81 | 12.5 ± 0.71 | 12.5 ± 0.2 | 12.5 ± 0.7 | R | 0.78 ± 0.01 |
| Jenin EO | 12.5 ± 0.2 | 12.5 ± 0.17 | 6.25 ± 0.97 | 6.25 ± 0.33 | 6.25 ± 0.4 | R | 0.78 ± 0.01 |
| Ciprofloxacin | 12.5 ± 0.15 | 0.78 ± 0.01 | 1.56 ± 0.06 | 0.13 ± 0.04 | 15 ± 0.97 | 3.12 ± 0.02 | - |
| Ampicillin | R | 25 ± 0.25 | 3.12 ± 0.59 | 1.25 ± 0.09 | 18 | R | - |
| Fluconazole | - | - | - | - | - | - | 1.56 ± 0.02 |
| IC50 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Heb3B | HeLa | MCF7 | HepG2 | B16F1 | CaCo2 | LX2 Normal | Hek293t Normal | |
| Ramallah | 0.96 ± 0.45 | 0.59 ± 0.18 | 0.53 ± 0.05 | 1.54 ± 0.21 | 0.82 ± 0.05 | 0.79 ± 0.22 | 1.21 ± 0.20 | 1.01 ± 0.28 |
| Tulkarm | 1.64 ± 0.17 | 1.06 ± 0.59 | 0.86 ± 0.47 | 1.32 ± 0.24 | 1.16 ± 0.12 | 0.49 ± 0.04 | 1.00 ± 0.23 | 0.82 ± 0.11 |
| Jenin | 1.35 ± 0.18 | 1.32 ± 0.23 | 1.09 ± 0.25 | >2 | 1.65 ± 0.35 | 0.33 ± 0.12 | 1.21 ± 0.42 | 1.56 ± 0.02 |
| Hebron | 1.33 ± 0.11 | 0.73 ± 0.35 | 0.63 ± 0.33 | 1.47 ± 0.08 | 1.08 ± 0.42 | 0.54 ± 0.09 | 1.12 ± 0.24 | 1.72 ± 0.17 |
| Nablus | 1.47 ± 0.34 | 1.34 ± 0.20 | 1.12 ± 0.19 | >2 | 0.66 ± 0.38 | 0.53 ± 0.05 | 0.54 ± 0.04 | 1.17 ± 0.12 |
| Doxorubicin | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations 2022, 9, 339. https://doi.org/10.3390/separations9110339
Al-Maharik N, Jaradat N, Hawash M, Al-Lahham S, Qadi M, Shoman I, Jaber S, Rahem RA, Hussein F, Issa L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations. 2022; 9(11):339. https://doi.org/10.3390/separations9110339
Chicago/Turabian StyleAl-Maharik, Nawaf, Nidal Jaradat, Mohammed Hawash, Saad Al-Lahham, Mohammad Qadi, Izzeddin Shoman, Shatha Jaber, Raghad Abdel Rahem, Fatimah Hussein, and Linda Issa. 2022. "Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine" Separations 9, no. 11: 339. https://doi.org/10.3390/separations9110339
APA StyleAl-Maharik, N., Jaradat, N., Hawash, M., Al-Lahham, S., Qadi, M., Shoman, I., Jaber, S., Rahem, R. A., Hussein, F., & Issa, L. (2022). Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus officinalis from five Different Sites in Palestine. Separations, 9(11), 339. https://doi.org/10.3390/separations9110339







