Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition
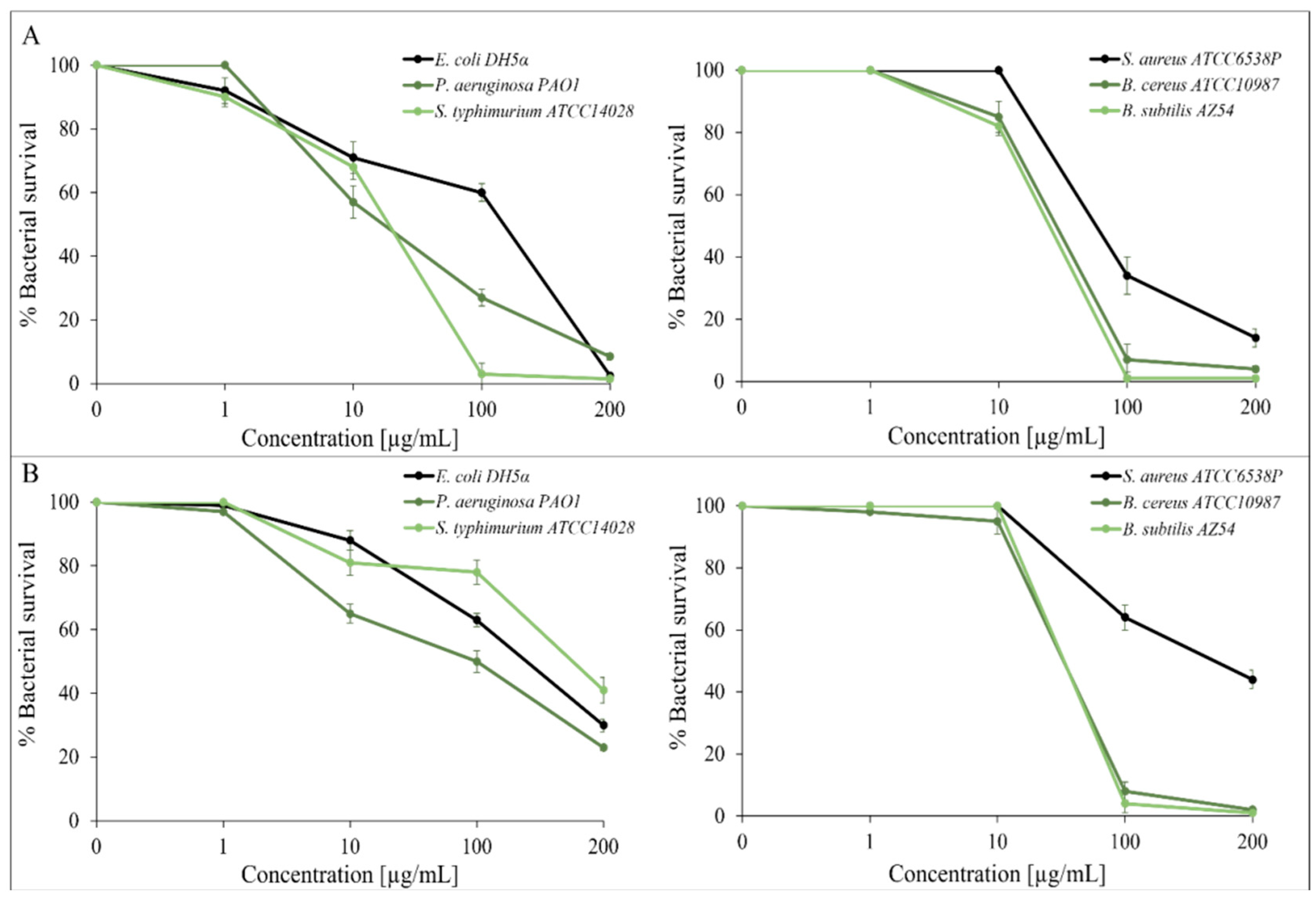
2.2. Essential Oil Antimicrobial Activity
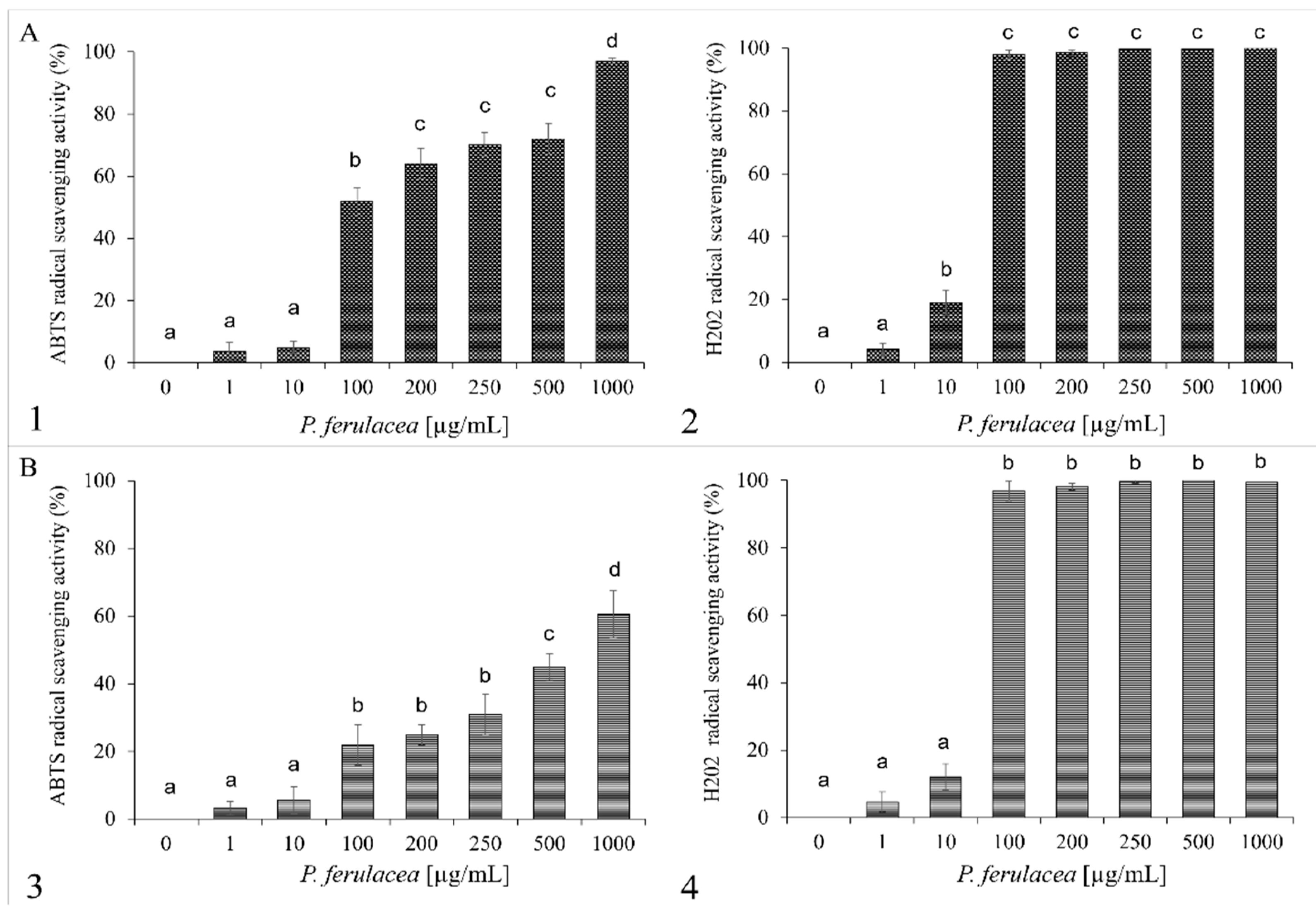
2.3. Essential Oil Antioxidant Activity
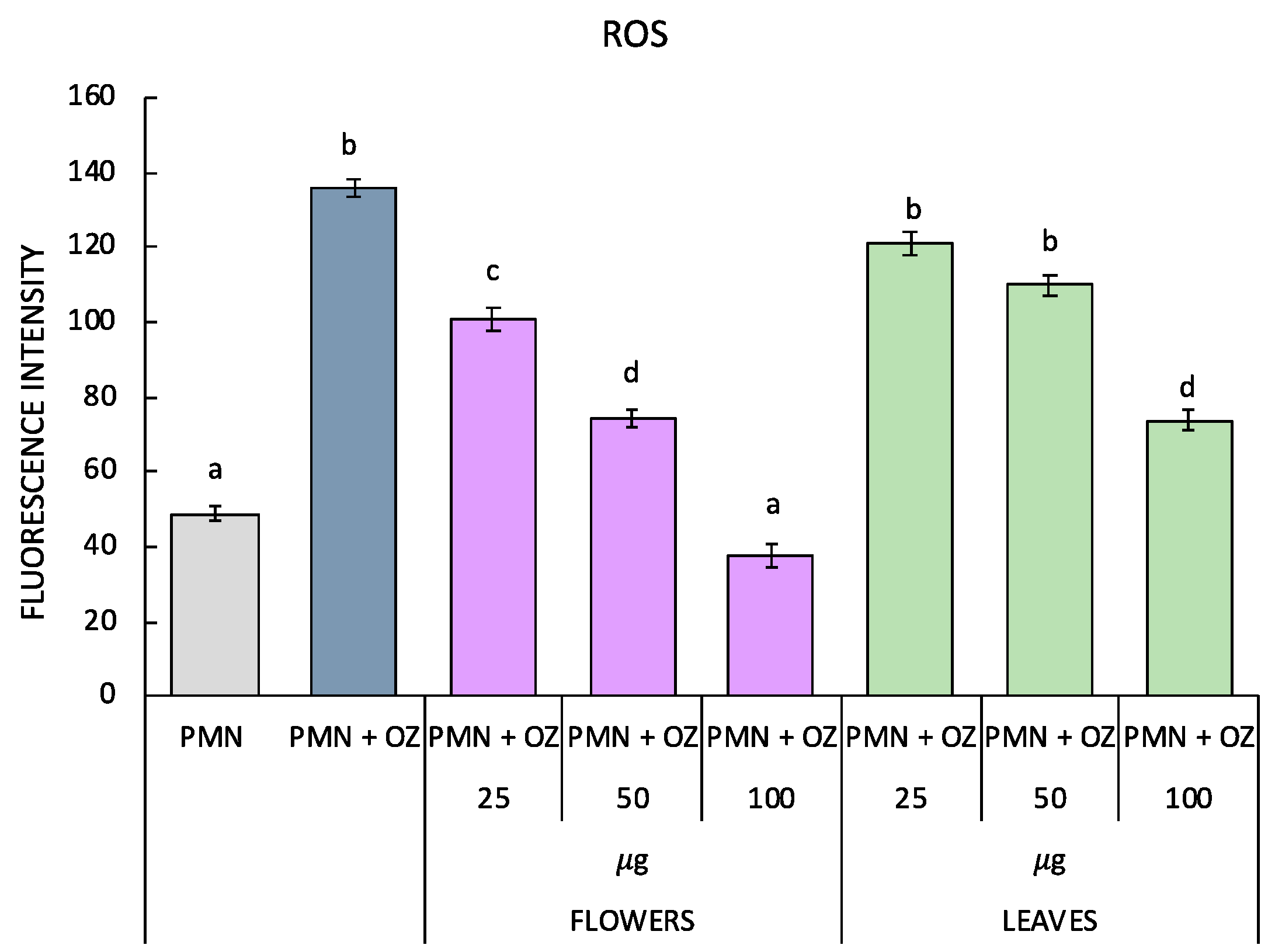
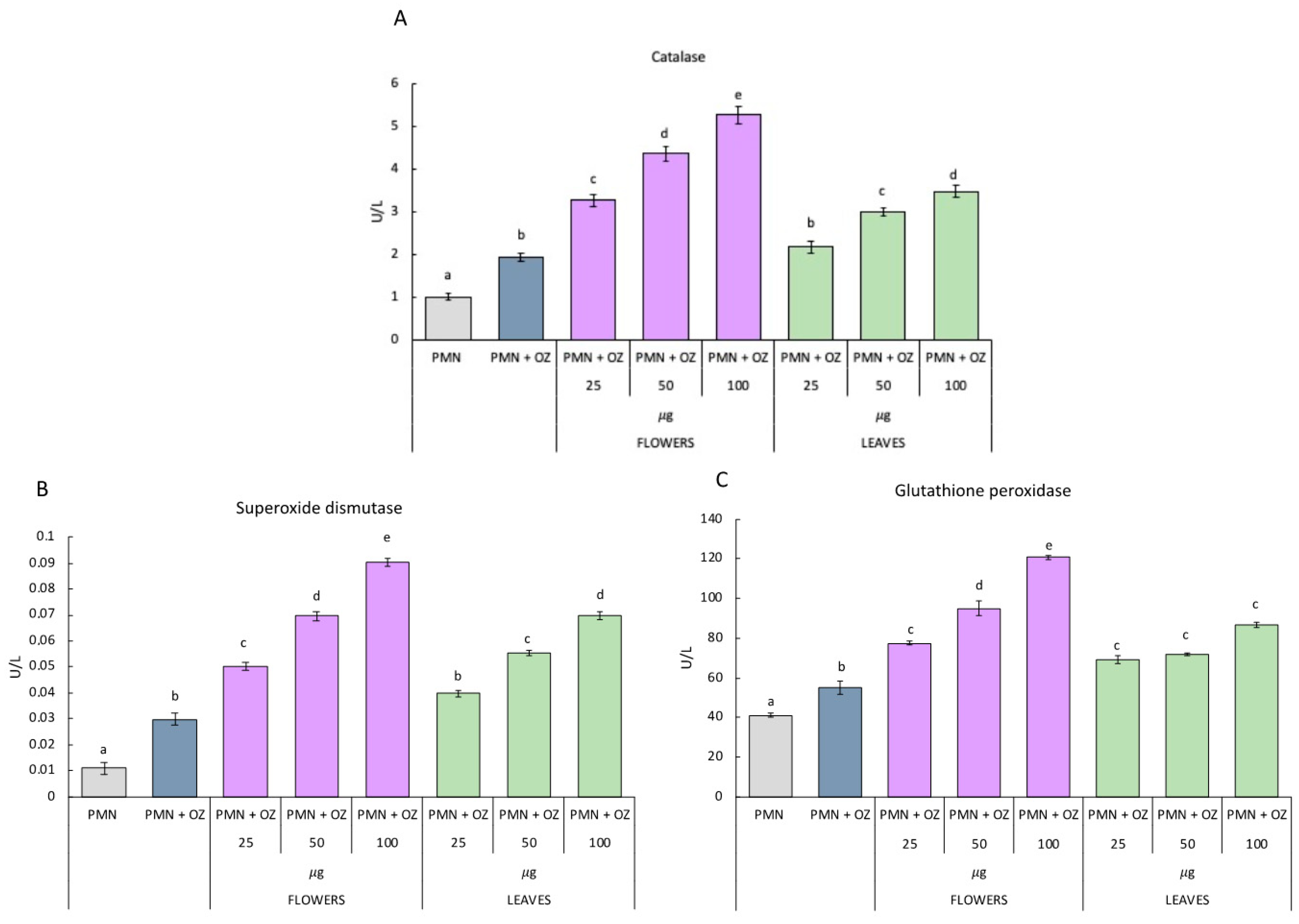
2.4. Antioxidant Enzymes Measured in PMN Cells
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Volatile Components
3.3. GC-MS Analysis
3.4. Bacterial Strains
3.5. Antimicrobial Activity Assay
3.6. Determination of Minimal Inhibitory Concentration
3.7. ABTS Scavenging Capacity Assay
3.8. Hydrogen Peroxide Scavenging Assay
3.9. Antioxidant Enzymes Measured in PMN Cells
3.10. Reactive Oxygen Species ROS Generation
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 12 September 2022).
- Pimenov, M.G.; Tikhomirov, V.N. The taxonomic problems in the genera Prangos Lindl., Cachrys L., Cryptodiscus Schrenk and Hippomarathrum Hoffmanns. et Link (Umbelliferae–Apioideae). Feddes Reper. 1983, 94, 145–164. [Google Scholar]
- Lyskov, D.F.; Degtjareva, G.V.; Samigullin, T.H.; Pimenov, M.G. The revision of Prangos subsections Koelzella and Fedtschenkoana (Apiaceae) with some notes to phylogeny and biogeography of the genus: Molecular and morphological evidences. Plant Syst. Evol. 2017, 303, 815–826. [Google Scholar] [CrossRef]
- Ajani, Y.; Ajani, A.; Cordes, J.M.; Watson, M.F.; Downie, S.R. Phylogenetic analysis of nrDNA ITS reveals relationships within five groups of Iranian Apiaceae sub family Apioideae. Taxon 2008, 57, 383–401. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Kiss, T.; Tòth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The nonvolatile and volatile metabolites of Prangos ferulacea and their biological properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. Composition and biological activities of the essential oil from a Sicilian accession of Prangos ferulacea (L.) Lindl. Nat. Prod. Res. 2021, 35, 733–743. [Google Scholar] [CrossRef]
- Meshkatalsadat, M.H.; Mirzaei, H.H. Chemical compositions of the essential oils of stems, leaves and flowers of Prangos acaulis (DC) Bornm. Pak. J. Biol. Sci. 2007, 10, 2775–2777. [Google Scholar] [CrossRef][Green Version]
- Meshkatalsadat, M.H.; Bamoniri, A.; Batooli, H. The bioactive and volatile constituents of Prangos acaulis (DC) Bornm extracted using hydrodistillation and nano scale injection techniques. Dig. J. Nanomater. Biostruct. 2010, 5, 263–266. [Google Scholar]
- Mirzaei, H.H.; Meshkatalsadat, M.H.; Soheilivand, S. Determination of essential oil composition of Prangos acaulis (DC) Bornm obtained by hydrodistillation and supercritical fluid extraction methods. J. Appl. Sci. 2007, 7, 2535–2538. [Google Scholar] [CrossRef][Green Version]
- Moharramipour, S.; Taghizadeh, A.; Meshkatalsadat, M.H.; Fathipour, Y.; Talebi, A.A. Repellent activity and persistence of essential oil extracted from Prangos acaulis to three stored-product beetles. Am.-Eurasian. J. Sustain. Agric. 2009, 3, 202–204. [Google Scholar]
- Rustaiyan, A.; Mazloomifar, H.; Masoudi, S.; Aghjani, Z. Volatile oils of Ducrosia assadii Alava. and Prangos acaulis (DC.) Bornm. from Iran. J. Essent. Oil Res. 2006, 18, 682–684. [Google Scholar] [CrossRef]
- Velikorodov, A.V.; Pilipenko, V.N.; Pilipenko, T.A.; Malyi, S.V. Studying the chemical composition of essential oil received from fruits of Prangos odontalgica wild-growing in Astrakhan Region. Khimiya Rastit. Syr’ya 2019, 3, 95–101. [Google Scholar]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Raimondo, F.A. Distribuzione es ecologia di Cachrys ferulacea (L.) Calestani interessante foraggera dei pascoli alto montani della Sicilia. Boll. Stud. Inform. Bot. Giardin. Colon. 1974, 26, 116–129. [Google Scholar]
- Dagdelen, S.; Bilenler, T.; Durmaz, G.; Gokbulut, I.; Hayaloglu, A.A.; Karabulut, I. Volatile composition, antioxidant and antimicrobial activities of herbal plants used in the manufacture of Van Herby (OTLU) cheese. J. Food Process. Pres. 2014, 38, 1716–1725. [Google Scholar] [CrossRef]
- Özcan, M.M.; Dursun, N.; Arslan, D. Some nutritional properties of Prangos ferulacea (L.) Lindl and Rheum ribes L. stems growing wild in Turkey. Int. J. Food Sci. Nutr. 2007, 58, 162–167. [Google Scholar] [CrossRef]
- Kafash-Farkhad, N.; Asadi-Samani, M.; Khaledifar, B. A review on secondary metabolites and pharmacological effects of Prangos ferulacea (L.) Lindl. Life Sci. J. 2013, 10, 360–367. [Google Scholar]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.E.; Mozafari, M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res. Pharm. Sci. 2012, 8, 137–144. [Google Scholar]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of essential oil and evaluation of its bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 19, 1–6. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the essential oils of Thymus vulgaris L. and Crithmum maritimum L. as biocidal on two Tholu Bommalu Indian leather puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, V.; Badalamenti, N.; Bruno, M. Chemical composition of the essential oil from different vegetative parts of Foeniculum vulgare subsp piperitum (Ucria) Coutinho (Umbelliferae) growing wild in Sicily. Nat. Prod. Res. 2021, 36, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Maggio, A.; Badalamenti, N.; Bruno, M.; D’Angelo, G.D.; D’Anneo, A. Essential oil of Foeniculum vulgare subsp. piperitum fruits exerts an anti-tumor effect in triple-negative breast cancer cells. Mol. Med. Rep. 2022, 26, 243. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.P.B.; Neto, A.F.S.; Nascimento, P.M.; Campos, A.F.; Silva, L.C.R.C.; et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Maggi, F.; Giuliani, C.; Fico, G.; Ricciutelli, M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Cianfaglione, K.; Tirillini, B.; et al. Secondary metabolites, secretory structures and biological activity of water celery (Apium nodiflorum (L.) Lag.) growing in central Italy. Plant Biosyst. 2018, 153, 325–335. [Google Scholar] [CrossRef]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of cinnamaldehyde, ocimene, camphene, curcumin and farnesene on Candida albicans. Adv. Microbiol. 2016, 6, 627–643. [Google Scholar] [CrossRef]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responsesagainst aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef]
- Afshar, F.H.; Maggi, F.; Iannarelli, R.; Cianfaglione, K.; Isman, M.B. Comparative toxicity of Helosciadium nodiflorum essential oils and combinations of their main constituents against the cabbage looper, Trichoplusia ni (Lepidoptera). Ind. Crops Prod. 2017, 98, 46–52. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Ricciutelli, M.; Lupidi, G.; Maggi, F. Efficacy of the volatile oil from water celery (Helosciadium nodiflorum, Apiaceae) against the filariasis vector Culex quinquefasciatus, the housefly Musca domestica and the African cotton leafworm Spodoptera littoralis. Chem. Biodivers. 2017, 14, e1700376. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Volatile β-ocimene can regulate developmental performance of peach aphid Myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Seidi Damyeh, M.; Niakousari, M.; Saharkhiz, M.J. Ultrasound pretreatment impact on Prangos ferulacea Lindl. and Satureja macrosiphonia Bornm. essential oil extraction and comparing their physicochemical and biological properties. Ind. Crops Prod. 2016, 87, 105–115. [Google Scholar] [CrossRef]
- Sumer Ercan, F.; Bas, H.; Koc, M.; Pandir, D.; Oztemiz, S. Insecticidal activity of essential oil of Prangos ferulacea (Umbelliferae) against Ephestia kuehniella (Lepidoptera: Pyralidae) and Trichogramma embryophagum (Hymenoptera: Trichogrammatidae). Turk. J. Agric. For. 2013, 37, 719–725. [Google Scholar] [CrossRef]
- Bertoli, A.; Pistelli, L.; Morelli, I.; Spinelli, G.; Manunta, A. Constituents of Cachrys ferulacea oils. J. Essent. Oil Res. 1998, 10, 533–536. [Google Scholar] [CrossRef]
- Hojjati, M.; Barzegar, H. Chemical composition and biological activities of lemon (Citrus limon) leaf essential oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef]
- Tan, N.; Yazici-Tutunis, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef]
- Yazici, T.S.; Tan, N.; Mericli, F.; Özsoy, N.; Tan, E. Biological activities of endemic Prangos hulusii. Planta Med. 2013, 79, PN113. [Google Scholar] [CrossRef]
- Mneimne, M.; Baydoun, S.; Nemer, N.; Arnold Apostolides, N. Chemical vomposition and evaluation of antimicrobial activity of essential oils isolated from Achillea kotschyi Boiss. subsp. kotschyi (Asteraceae) of Lebanon. Pharm. Chem. J. 2016, 3, 91–98. [Google Scholar]
- Sarma, A.D.; Mallick, A.R.; Ghosh, A.K. Free radicals and their role in different clinical conditions: An overview. Int. J. Pharm. Sci. Res. 2010, 1, 185–192. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Coruh, N.U.R.S.E.N.; Celep, A.S.; Özgökçe, F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007, 100, 1237–1242. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Bisht, R.; Kandari Negi, S. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. essential oil. Iran. J. Pharm. Res. 2012, 11, 68–70. [Google Scholar]
- Dhami, A.; Singh, A.; Palariya, D.; Kumar, R.; Prakash, O.; Rawat, D.S.; Pant, A.K. α-Pinene rich bark essential oils of Zanthoxylum armatum DC. from three different altitudes of Uttarakhand, India and their antioxidant, in vitro anti-inflammatory and antibacterial activity. J. Essent. Oil Bear. Plants 2019, 22, 660–674. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 10.3. 2020. Determination of Essential Oils in Herbal Drugs, 2.8.12., 307. Available online: https://www.edqm.eu/ (accessed on 12 October 2022).
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, A.; Pizzo, E.; Di Maro, A.; Varcamonti, M.; D’Alessio, G. The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease. FEBS J. 2010, 277, 1921–1928. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) n-heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Petruk, G.; Donadio, G.; Lanzilli, M.; Isticato, R.; Monti, D.M. Alternative use of Bacillus subtilis spores: Protection against environmental oxidative stress in human normal keratinocytes. Sci. Rep. 2018, 8, 1745. [Google Scholar] [CrossRef]
- Beers, R.; Sizer, I. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Pala, D.; Silva, C.T.; de Souza, M.O.; do Amaral, J.F.; Vieira, R.A.L.; de Freitas Folly, G.A.; Volp, A.C.P.; de Freitas, R.N. Açai (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 2016, 32, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.; Saha, P.; Sarkar, A.; Mukhopadhyay, D.; Bauri, A.K.; Kumar, D.; Das, P.; Chattopadhyay, S.; Chatterjee, M. Malabaricone-A induces a redox imbalance that mediates apoptosis in U937 cell line. PLoS ONE 2012, 7, e36938. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Zanfardino, A. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira Island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 30, 3955. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha piperita var. mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef]
| No. | Compounds a | LRI b | LRI c | Flowers d | Leaves d |
|---|---|---|---|---|---|
| 1 | α-Pinene | 1002 | 1017 | 4.28 | 2.26 |
| 2 | 3-Thujene | 1024 | 1030 | 5.79 | 0.70 |
| 3 | Camphene | 1032 | 1037 | 0.21 | 0.13 |
| 4 | β-Pinene | 1087 | 1099 | 0.42 | 0.09 |
| 5 | Sabinene | 1111 | 1115 | 20.18 | 10.11 |
| 6 | 3-Carene | 1149 | 1158 | 0.99 | 0.87 |
| 7 | α-Phellandrene | 1170 | 1174 | 0.66 | 0.09 |
| 8 | β-Myrcene | 1172 | 1176 | 1.64 | 0.87 |
| 9 | α-Terpinene | 1176 | 1179 | 3.03 | 0.33 |
| 10 | Limonene | 1187 | 1193 | 3.32 | 1.51 |
| 11 | Sylvestrene | 1201 | 1205 | 1.17 | 0.14 |
| 12 | γ-Terpinene | 1233 | 1238 | 8.09 | - |
| 13 | (E)-β-Ocimene | 1239 | 1243 | - | 4.62 |
| 14 | (Z)-β-Ocimene | 1244 | 1246 | 44.39 | 64.91 |
| 15 | α-Bourbonene | 1525 | 1528 | - | 0.13 |
| 16 | 4-Terpineol | 1605 | 1611 | 2.08 | - |
| 17 | Caryophyllene | 1607 | 1612 | - | 7.65 |
| 18 | α-Amorphene | 1669 | 1675 | - | 0.72 |
| 19 | Humulene | 1682 | 1687 | - | 0.20 |
| 20 | γ-Muurolene | 1699 | 1704 | - | 0.31 |
| 21 | Germacrene D | 1703 | 1706 | - | 0.58 |
| 22 | Cedrene | 1807 | - | 0.28 | - |
| Monoterpene Hydrocarbons | - | 94.17 | 86.63 | ||
| Oxygenated Monoterpenes | - | 2.08 | - | ||
| Sesquiterpene Hydrocarbons | - | 0.28 | 9.59 | ||
| Total | - | 96.53 | 96.22 |
| Strains | P. ferulacea Flowers (µg/mL) | P. ferulacea Leaves (µg/mL) |
|---|---|---|
| E. coli DH5α | 200 | >200 |
| P. aeruginosa PAO1 | >200 | >200 |
| S. tiphymurium ATCC14028 | 100 | >200 |
| S. aureus ATCC6538P | >200 | >200 |
| B. cereus ATCC10987 | 100 | 100 |
| B. subtilis AZ54 | 100 | 100 |
| Sample | IC50 of H2O2 (µg/mL) | Sample | IC50 of ABTS (µg/mL) |
|---|---|---|---|
| P. ferulacea flowers | 60 | P. ferulacea flowers | 100 |
| P. ferulacea leaves | 50 | P. ferulacea leaves | 500 |
| Resveratrol | 0.05 | Ascorbic acid | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalamenti, N.; Maresca, V.; Di Napoli, M.; Bruno, M.; Basile, A.; Zanfardino, A. Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils. Molecules 2022, 27, 7430. https://doi.org/10.3390/molecules27217430
Badalamenti N, Maresca V, Di Napoli M, Bruno M, Basile A, Zanfardino A. Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils. Molecules. 2022; 27(21):7430. https://doi.org/10.3390/molecules27217430
Chicago/Turabian StyleBadalamenti, Natale, Viviana Maresca, Michela Di Napoli, Maurizio Bruno, Adriana Basile, and Anna Zanfardino. 2022. "Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils" Molecules 27, no. 21: 7430. https://doi.org/10.3390/molecules27217430
APA StyleBadalamenti, N., Maresca, V., Di Napoli, M., Bruno, M., Basile, A., & Zanfardino, A. (2022). Chemical Composition and Biological Activities of Prangos ferulacea Essential Oils. Molecules, 27(21), 7430. https://doi.org/10.3390/molecules27217430









