Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of the Bioactive and Antioxidant Potential of Spent Coffee Grounds
2.2. Analysis of Bioactive Compounds by UHPLC-PDA
Validation of the µ-SPEed/UHPLC-PDA Methodology
2.3. Application of the µSPEed/UHPLC-PDA Method in the Spent Coffee Grounds Samples
2.4. Possible Applications for the Bioactive Compounds Identified in the Spent Coffee Grounds Samples
3. Materials and Methods
3.1. Reagents and Standards
3.2. Sample Preparation
3.3. Evaluation of the Bioactive and Antioxidant Potential of Spent Coffee Grounds
3.3.1. µ-QuEChERS Extraction
3.3.2. Total Phenolic Content
3.3.3. 2,2-Diphenyl-1-picrylhydrazyl Scavenging Assay
3.3.4. 2,29-Azinobis-(3-ethylbenzothiazoline-6-sulfonic Acid) Assay
3.4. Analysis of Bioactive Compounds by UHPLC-PDA
3.4.1. Sample Preparation
3.4.2. Extraction of Bioactive Compounds via µ-SPEed
3.4.3. UHPLC-PDA Analysis
3.4.4. Validation of the µ-SPEed/UHPLC-PDA Methodology
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mitraka, G.C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. [Google Scholar] [CrossRef]
- International Coffee Organization. Coffee Market Report; International Coffee Organization: London, UK, 2021. [Google Scholar]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, Composition, and Application of Coffee and Its Industrial Residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Crossley, O.P.; Thorpe, R.B.; Peus, D.; Lee, J. Phosphorus recovery from process waste water made by the hydrothermal carbonisation of spent coffee grounds. Bioresour. Technol. 2020, 301, 122664. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Battista, F.; Zuliani, L.; Rizzioli, F.; Fusco, S.; Bolzonella, D. Biodiesel, biogas and fermentable sugars production from Spent coffee Grounds: A cascade biorefinery approach. Bioresour. Technol. 2021, 342, 125952. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gomez-Mejia, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Bravo, J.; Juániz, I.; Monente, C.; Caemmerer, B.; Kroh, L.W.; de Peña, M.-P.; Cid, C. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Fiore, A. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: A study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2017, 243, 835–847. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Catelani, T.A.; Páscoa, R.N.M.J.; Santos, J.R.; Pezza, L.; Pezza, H.R.; Lima, J.L.F.C.; Lopes, J.A. A Non-invasive Real-Time Methodology for the Quantification of Antioxidant Properties in Coffee During the Roasting Process Based on Near-Infrared Spectroscopy. Food Bioprocess Technol. 2017, 10, 630–638. [Google Scholar] [CrossRef]
- Baeza, G.; Sarriá, B.; Bravo, L.; Mateos, R. Exhaustive Qualitative LC-DAD-MSn Analysis of Arabica Green Coffee Beans: Cinnamoyl-glycosides and Cinnamoylshikimic Acids as New Polyphenols in Green Coffee. J. Agric. Food Chem. 2016, 64, 9663–9674. [Google Scholar] [CrossRef]
- Zhu, M.; Long, Y.; Ma, Y.; Chen, Y.; Yu, Q.; Xie, J.; Li, B.; Tian, J. Comparison of chemical and fatty acid composition of green coffee bean (Coffea arabica L.) from different geographical origins. LWT 2021, 140, 110802. [Google Scholar] [CrossRef]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. Technol. 2021, 59, 102254. [Google Scholar] [CrossRef]
- Andrade, K.S.; Gonalvez, R.T.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, M.D.; Buntić, A.V.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Novoa, L.; Pavon-Perez, J.; Henriquez-Aedo, K.; Aranda, M. Chemometric Optimization of QuEChERS Extraction Method for Polyphenol Determination in Beers by Liquid Chromatography with Ultraviolet Detection. Food Anal. Methods 2019, 12, 448–457. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef]
- Wrona, M.; Blasco, S.; Becerril, R.; Nerin, C.; Sales, E.; Asensio, E. Antioxidant and antimicrobial markers by UPLC®–ESI-Q-TOF-MSE of a new multilayer active packaging based on Arctostaphylos uva-ursi. Talanta 2019, 196, 498–509. [Google Scholar] [CrossRef]
- Song, X.-C.; Canellas, E.; Asensio, E.; Nerín, C. Predicting the antioxidant capacity and total phenolic content of bearberry leaves by data fusion of UV–Vis spectroscopy and UHPLC/Q-TOF-MS. Talanta 2020, 213, 120831. [Google Scholar] [CrossRef]
- Woraratphoka, J.; Intarapichet, K.-O.; Indrapichate, K. Phenolic compounds and antioxidative properties of selected wines from the northeast of Thailand. Food Chem. 2007, 104, 1485–1490. [Google Scholar] [CrossRef]
- Vinci, G.; D’ascenzo, F.; Maddaloni, L.; Prencipe, S.A.; Tiradritti, M. The Influence of Green and Black Tea Infusion Parameters on Total Polyphenol Content and Antioxidant Activity by ABTS and DPPH Assays. Beverages 2022, 8, 18. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Tundis, R.; Loizzo, M.R. Reuse of Food Waste: The Chemical Composition and Health Properties of Pomelo (Citrus maxima) Cultivar Essential Oils. Molecules 2022, 27, 3273. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Assessing the activity of natural food antioxidants. In Oxidation in Foods and Beverages and Antioxidant Applications; Elsevier: Amsterdam, The Netherlands, 2010; pp. 332–367. ISBN 9781845696481. [Google Scholar]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Feinberg, M. Validation of analytical methods based on accuracy profiles. J. Chromatogr. A 2007, 1158, 174–183. [Google Scholar] [CrossRef]
- Chu, Y.-F. Coffee: Emerging Health Effects and Disease Prevention; Institute of Food Technologists: Chicago, IL, USA, 2012; p. 324. [Google Scholar]
- Moon, J.-K.; Yoo, H.S.; Shibamoto, T. Role of Roasting Conditions in the Level of Chlorogenic Acid Content in Coffee Beans: Correlation with Coffee Acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Alonso-Salces, R.M.; Serra, F.; Remero, F.; Heberger, K. Botanical and geographical characterization of green coffee (Coffea arabica and Coffea canephora): Chemometric evaluation of phenolic and methylxanthine contents. J. Agric. Food Chem. 2009, 57, 4224–4235. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind. Crops Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of bioactive compounds during roasting process of coffee beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Kokubo, Y.; Iso, H.; Saito, I.; Yamagishi, K.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Tsugane, S. The Impact of Green Tea and Coffee Consumption on the Reduced Risk of Stroke Incidence in Japanese Population: The Japan public health center-based study cohort. Stroke 2013, 44, 1369–1374. [Google Scholar] [CrossRef]
- Baratloo, A.; Rouhipour, A.; Forouzanfar, M.M.; Safari, S.; Amiri, M.; Negida, A. The Role of Caffeine in Pain Management: A Brief Literature Review. Anesthesiol. Pain Med. 2016, 6, e33193. [Google Scholar] [CrossRef]
- dos Santos Lins, P.V.; Henrique, D.C.; Ide, A.H.; de Paiva e Silva Zanta, C.L.; Meili, L. Evaluation of caffeine adsorption by MgAl-LDH/biochar composite. Environ. Sci. Pollut. Res. 2019, 26, 31804–31811. [Google Scholar] [CrossRef]
- Luo, L.; Lane, M.E. Topical and transdermal delivery of caffeine. Int. J. Pharm. 2015, 490, 155–164. [Google Scholar] [CrossRef]
- Visconti, M.J.; Haidari, W.; Feldman, S.R. Therapeutic use of caffeine in dermatology: A literature review. J. Dermatol. Dermatol. Surg. 2020, 24, 18. [Google Scholar] [CrossRef]
- Šimůnková, K.; Reinprecht, L.; Nábělková, J.; Hýsek, S.; Kindl, J.; Borůvka, V.; Lišková, T.; Šobotník, J.; Pánek, M. Caffeine—Perspective natural biocide for wood protection against decaying fungi and termites. J. Clean. Prod. 2021, 304, 127110. [Google Scholar] [CrossRef]
- Hollingsworth, R.G.; Armstrong, J.W.; Campbell, E. Caffeine as a novel toxicant for slugs and snails. Ann. Appl. Biol. 2003, 142, 91–97. [Google Scholar] [CrossRef]
- Tomac, I.; Šeruga, M.; Labuda, J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chem. 2020, 325, 126787. [Google Scholar] [CrossRef]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Conney, A.H.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Manchand, P.S.; Laskin, J.D.; Huang, M.-T. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv. Enzym. Regul. 1991, 31, 385–396. [Google Scholar] [CrossRef]
- Chen, W.-C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Effect of topical application of chlorogenic acid on excision wound healing in rats. Planta Medica 2013, 79, 616–621. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of chlorogenic acid with β-cyclodextrins: Inclusion effects, antioxidative properties and potential application in grape juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Jiao, W.; Shu, C.; Li, X.; Cao, J.; Fan, X.; Jiang, W. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Silva, T.; Oliveira, C.; Borges, F. Caffeic acid derivatives, analogs and applications: A patent review (2009–2013). Expert Opin. Ther. Patents 2014, 24, 1257–1270. [Google Scholar] [CrossRef]
- Hallan, S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Podsędek, A.; Zyzelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009, 228, 913–922. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. An improved and miniaturized analytical strategy based on μ-QuEChERS for isolation of polyphenols. A powerful approach for quality control of baby foods. Microchem. J. 2018, 139, 110–118. [Google Scholar] [CrossRef]
- Figueira, J.; Porto-Figueira, P.; Berenguer, C.; Pereira, J.; Câmara, J. Evaluation of the Health-Promoting Properties of Selected Fruits. Molecules 2021, 26, 4202. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Casado, N.; Perestrelo, R.; Silva, C.L.; Sierra, I.; Câmara, J.S. Comparison of high-throughput microextraction techniques, MEPS and μ-SPEed, for the determination of polyphenols in baby food by ultrahigh pressure liquid chromatography. Food Chem. 2019, 292, 14–23. [Google Scholar] [CrossRef]
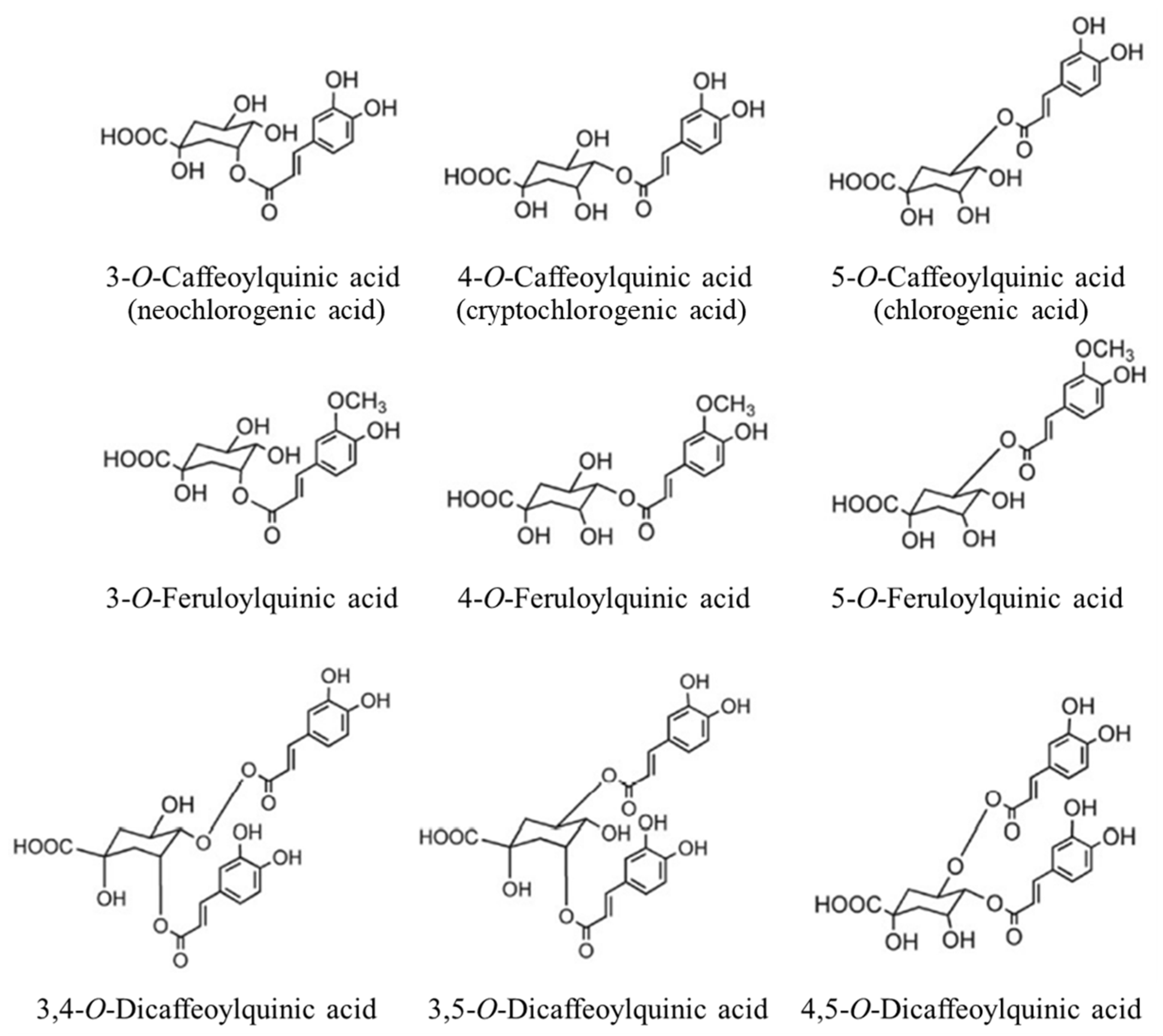

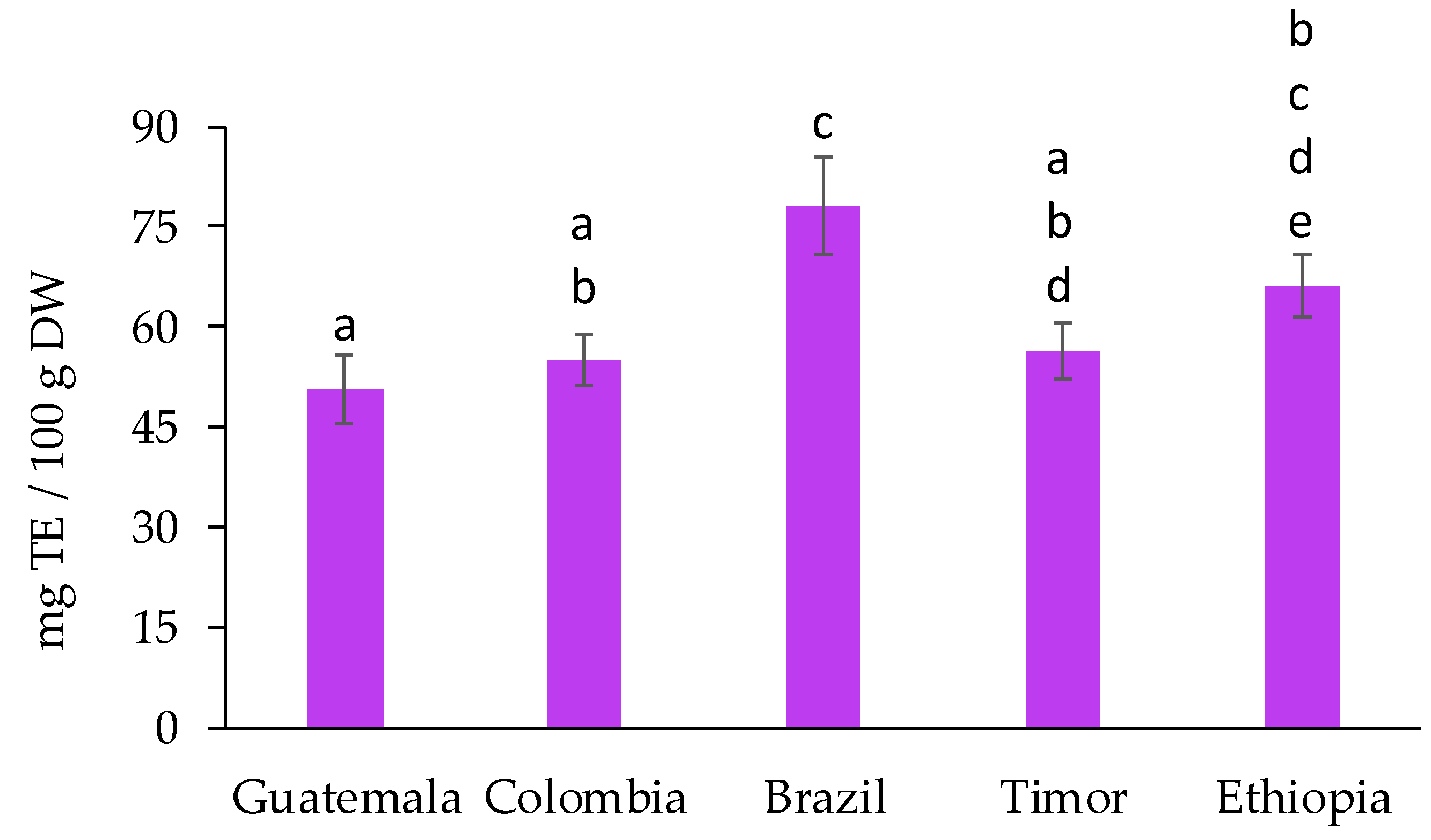
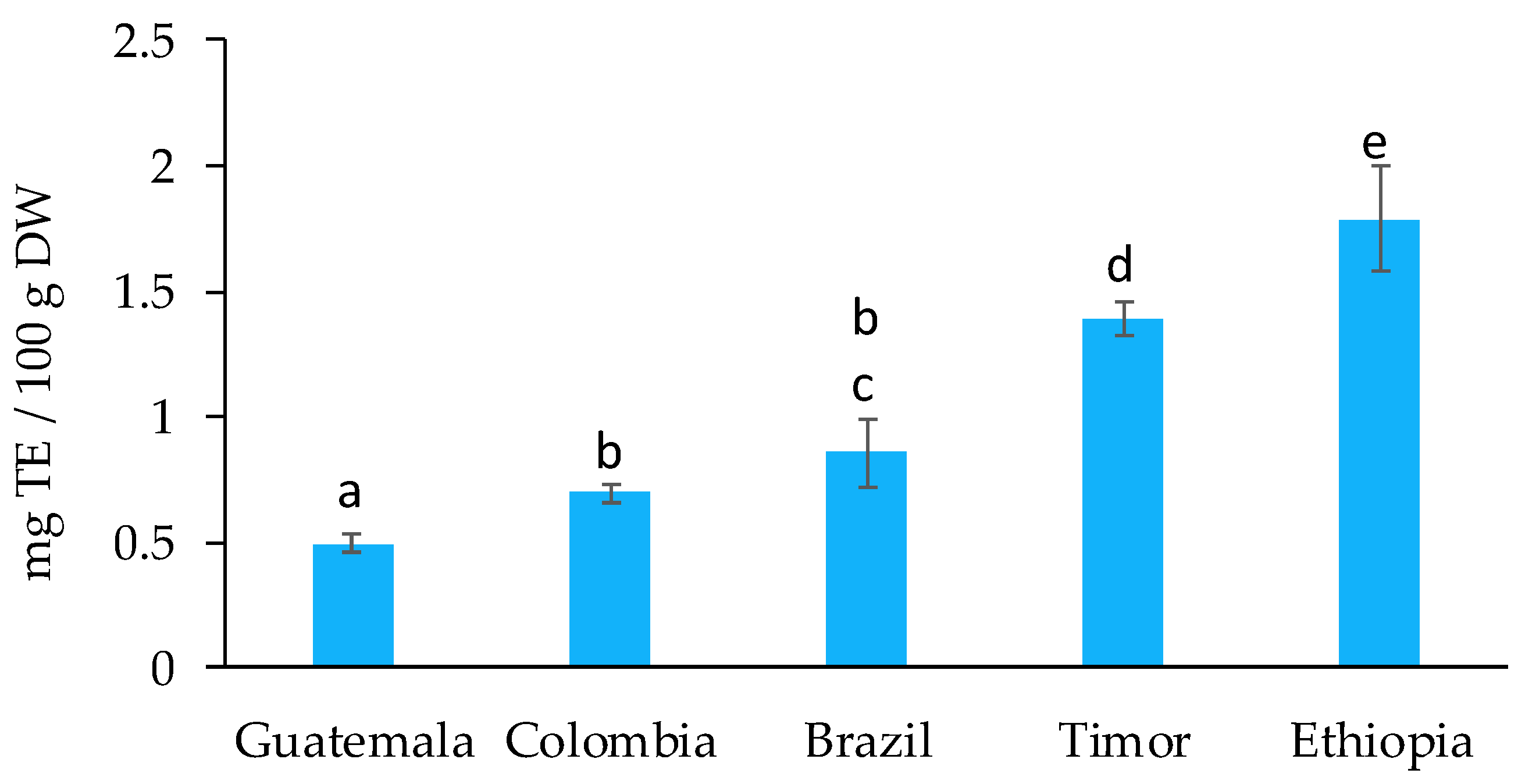

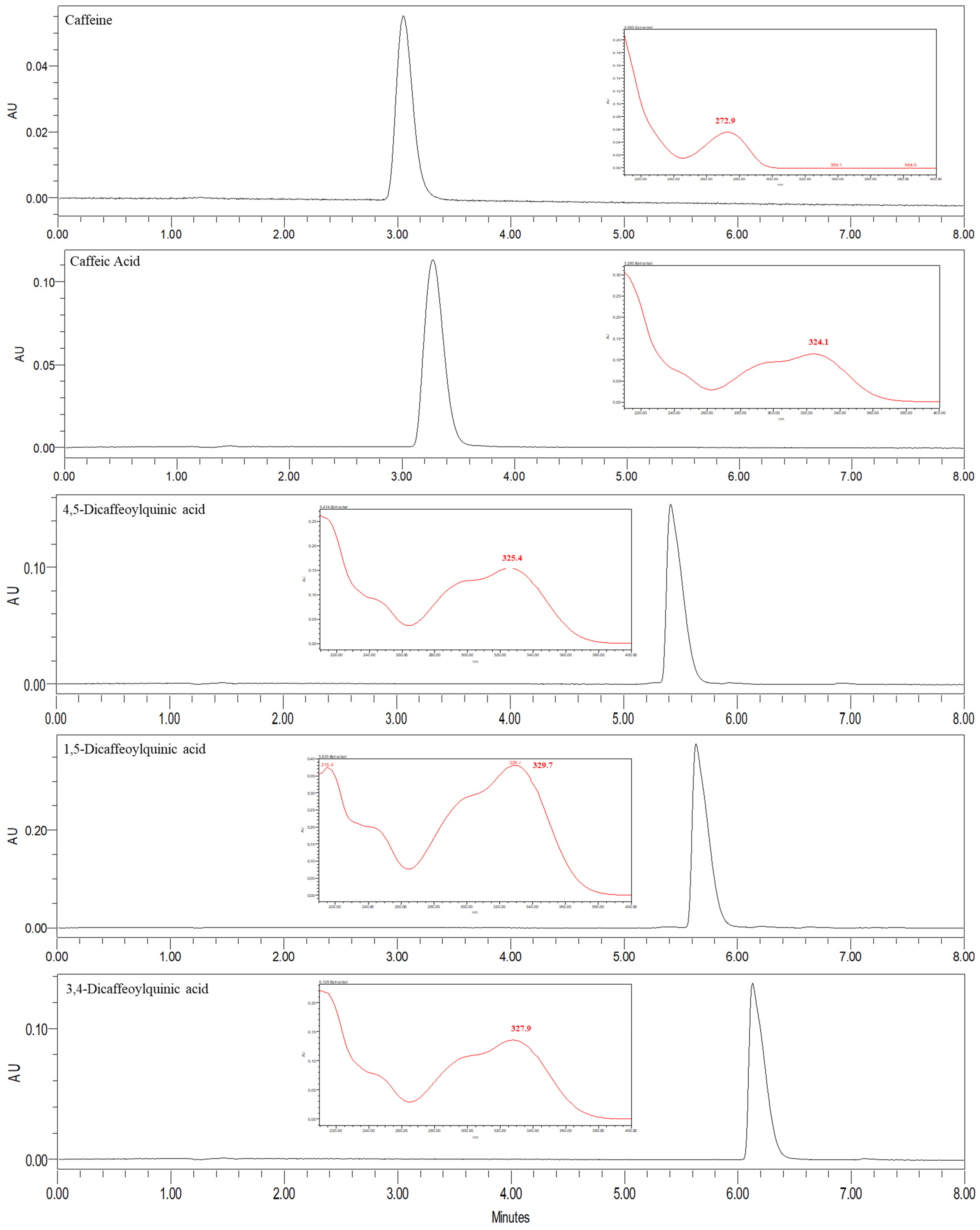
| RT (min) | Analyte | λmax (nm) | Linear Range (µg/mL) | Calibration Curve | LOD (ng/mL) | LOQ (ng/mL) | |
|---|---|---|---|---|---|---|---|
| 2.45 | 5-CQA | 326 | 0.2–75 | Equation | y = 72508x + 57908 | 10.3 | 32.4 |
| R2 | 0.998 | ||||||
| 2.85 | Caffeine | 273 | 0.2–75 | Equation | y = 67275x − 23743 | 29.9 | 998.5 |
| R2 | 0.999 | ||||||
| 3.94 | Caffeic acid | 326 | 0.2–75 | Equation | y = 128542x + 57103 | 31.1 | 102.9 |
| R2 | 0.999 | ||||||
| Analyte | Spiking Level (µg/mL) | Precision (RSD%) | Recovery ± RSD | |
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| 5-CQA | 0.2 | 1.47 | 15.72 | 98.5 ± 9.5 |
| 25 | 6.21 | 8.47 | 112 ± 2.7 | |
| 75 | 6.06 | 6.19 | 108 ± 2.6 | |
| Caffeine | 0.2 | 4.68 | 15.64 | 94.5 ± 4.9 |
| 25 | 2.90 | 12.94 | 101 ± 4.9 | |
| 75 | 2.69 | 6.88 | 120 ± 1.5 | |
| Caffeic acid | 0.2 | 4.23 | 11.26 | 112 ± 5.9 |
| 25 | 1.78 | 10.45 | 110 ± 7.3 | |
| 75 | 1.52 | 7.91 | 109 ± 8.0 | |
| RT (min) | Analyte | λmax (nm) | Concentration (mg/100 g DW) ± Standard Deviation | ||||
|---|---|---|---|---|---|---|---|
| Guatemala | Colombia | Brazil | Timor | Ethiopia | |||
| 1.83 | 3-CQA * | 326 | 104.1 ± 20.7 a | 23.9 ± 4.0 b | 8.3 ± 1.5 c | 24.8 ± 2.2 b | 53.8 ± 5.30 e |
| 2.45 | 5-CQA | 326 | 385.3 ± 24.4 a | 348.9 ± 26.3 a | 167.3 ± 10.5 b | 330.6 ± 29.6 a | 247.7 ± 11.8 c |
| 2.85 | Caffeine | 273 | 391.9 ± 27.3 a | 386.8 ± 25.6 a | 194.1 ± 4.8 b | 381.1 ± 9.3 a | 198.6 ± 6.8 c |
| 3.94 | Caffeic acid | 326 | 3.7 ± 0.7 | <LOQ | <LOQ | <LOQ | <LOQ |
| 5.41 | 4,5-diCQA * | 326 | 11.0 ± 1.1 a | 8.8 ± 1.34 b,a | 3.6 ± 0.3 c | 7.5 ± 0.5 b | 4.5 ± 0.2 c |
| 5.71 | 1,5-diCQA * | 326 | 2.3 ± 0.5 | <LOQ | <LOQ | <LOQ | <LOQ |
| 6.12 | 3,4-diCQA * | 326 | 7.0 ± 0.7 a | 4.8 ± 0.7 b | 0.6 ± 0.06 c | 3.5 ± 0.3 d | 1.6 ± 0.2 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. https://doi.org/10.3390/molecules27217504
Andrade C, Perestrelo R, Câmara JS. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules. 2022; 27(21):7504. https://doi.org/10.3390/molecules27217504
Chicago/Turabian StyleAndrade, Carolina, Rosa Perestrelo, and José S. Câmara. 2022. "Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization" Molecules 27, no. 21: 7504. https://doi.org/10.3390/molecules27217504
APA StyleAndrade, C., Perestrelo, R., & Câmara, J. S. (2022). Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules, 27(21), 7504. https://doi.org/10.3390/molecules27217504






