Abstract
In this work, nine new bromophenol derivatives were designed and synthesized. The alkylation reactions of (2-bromo-4,5-dimethoxyphenyl)methanol (7) with substituted benzenes 8–12 produced new diaryl methanes 13–17. Targeted bromophenol derivatives 18–21 were synthesized via the O-Me demethylation of diaryl methanes with BBr3. Moreover, the synthesized bromophenol compounds were tested with some metabolic enzymes such as acetylcholinesterase (AChE), carbonic anhydrase I (CA I), and II (CA II) isoenzymes. The novel synthesized bromophenol compounds showed Ki values that ranged from 2.53 ± 0.25 to 25.67 ± 4.58 nM against hCA I, from 1.63 ± 0.11 to 15.05 ± 1.07 nM against hCA II, and from 6.54 ± 1.03 to 24.86 ± 5.30 nM against AChE. The studied compounds in this work exhibited effective hCA isoenzyme and AChE enzyme inhibition effects. The results show that they can be used for the treatment of glaucoma, epilepsy, Parkinson’s as well as Alzheimer’s disease (AD) after some imperative pharmacological studies that would reveal their drug potential.
1. Introduction
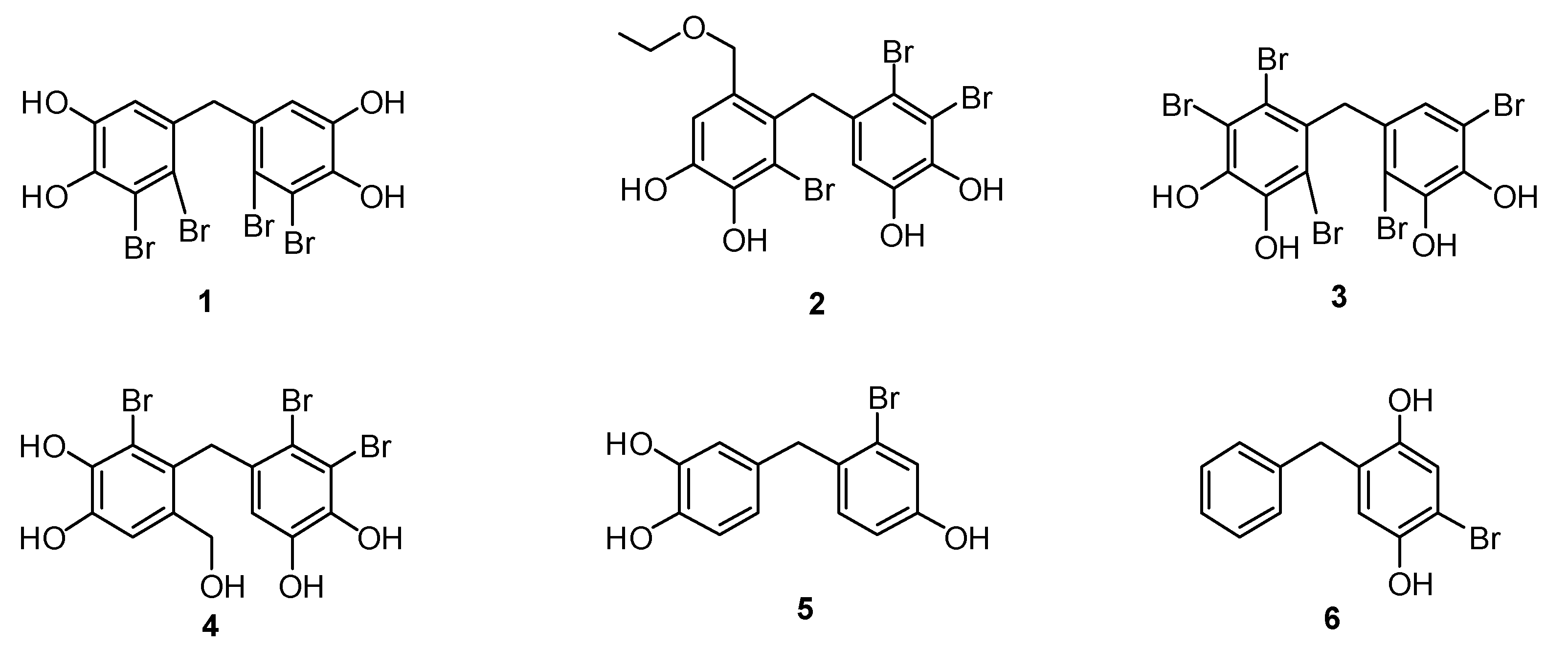
Nature is an important source in drug development research [1]. Marine life is one of the sources that produce naturally occurring bromophenols. In the last decades, there have been many studies on the isolation of bromophenols from marine algae [2,3,4], sponges [5,6], ascidians [7], and corals [8]. In these studies, all these natural bromophenols showed important biological activities. For instance, 5,5′-methylenebis(3,4-dibromobenzene-1,2-diol) (1), isolated from the marine algae Rhodomela confervoides and Leathesia nana showed anti-cancer activity [9]. In another research work, the isolation of 3,4-dibromo-5-(2-bromo-6-(ethoxymethyl)-3,4-dihydroxybenzyl)benzene-1,2-diol (2) from red alga (R. confervoides) and its antidiabetic activity were reported [10]. Naturally occurring 3,4,6-tribromo-5-(2,5-dibromo-3,4-dihydroxybenzyl)benzene-1,2-diol (3), derived from the red alga Symphyocladia latiuscula, has been proven to inhibit the aldose reductase enzyme [11]. The isolation from the red algae V. lanosa of 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(hydroxymethyl)benzyl)benzene-1,2-diol (4) together with glucose 6-phosphate dehydrogenase and their antioxidant properties has also been addressed [12]. In addition, compound 1 has been reported to have isocitrate lyase [13], cytotoxicity [14], antimicrobial [15], and feeding deterrent [16] properties. Moreover, it has been reported that compounds 2 and 4 have antibacterial activities [17] (Figure 1).
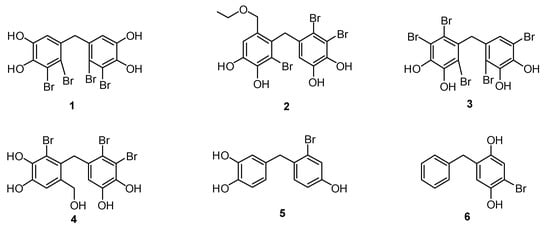
Figure 1.
Some important natural and synthetic bromophenols.
In our ongoing project on the total synthesis and biological evaluation of natural bromophenols and their derivatives, we have already reported the first synthesis of bromophenols 1 [18], 2 [19], and 3 [20]. In these studies, the antioxidant properties of 1, the CA inhibition effects of 2 and 3 were described [18,19,20]. From our early studies, we concluded that not only naturally occurring bromophenols but also their synthetic derivatives, including 4-(2-bromo-4-hydroxybenzyl)benzene-1,2-diol (5) and 2-benzyl-5-bromobenzene-1,4-diol (6), exhibit CA, AChE, and BChE inhibitory properties [21,22,23] (Figure 1).
Carbonic anhydrases (CAs) catalyze the reversible hydration of water and carbon dioxide (CO2) to protons (H+) and bicarbonate ions (HCO3−) [24,25,26]. They take part in a variety of physiological functions, such as ion transport, fatty acid metabolism, bone resorption, pH regulation, and gas exchange. Furthermore, edema and glaucoma occur when the activity of CAs reaches abnormal levels [27,28,29]. Sulfonamides are used as CA inhibitors [30], including N-substituted phthalazine sulfonamides [31], sulphonamide Schiff bases [32], imidazolinone-based benzenesulfonamides and thiourea-substituted benzenesulfonamides [33], imidazolinone-based benzenesulfonamides [34], pyrazoline benzensulfonamides [35,36,37], hetaryl sulfonamides [38], phenolic sulfonamides [39], and quinazolin-sulfonamide [40]. However, various sulfonamides unspecifically block all CA isoforms, which results in adverse side effects. The development of non-sulphonamide-based CAIs is necessary because a sizable section of the population cannot be treated with sulphonamides due to sulfa allergies [41].
By hydrolyzing the neurotransmitter acetylcholine (ACh), the enzyme acetylcholinesterase (AChE) modulates cholinergic transmission at the synaptic level [42,43]. AChE affects cell adhesion, proliferation, and differentiation; the formation of tumors, apoptosis, and amyloid protein deposition in organs as well as AChE are all important cholinergic functions [44,45,46]. Abnormal levels of AChE are associated widely with neurodegenerative disorders such as myasthenia gravis, Parkinson’s disease (PD), and Alzheimer’s disease (AD). Currently, oral active AChE inhibitors that only provide palliative, symptomatic relief are the mainstay of treatment for AD [47,48,49].
The construction or extension of chemical libraries is very important for the development of novel lead compounds in the field of drug design and discovery. Therefore, in this study, we synthesized some novel bromophenols and evaluated their hCA I, hCA II, and AChE inhibitory properties.
2. Results and Discussion
2.1. Chemistry
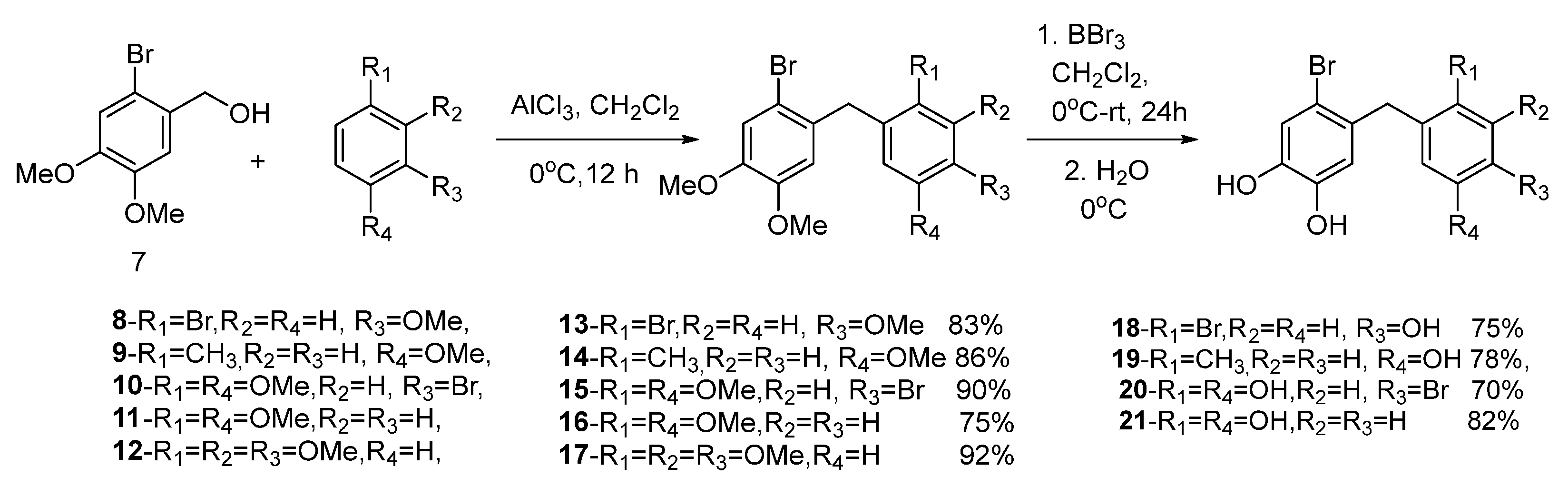
In this study, novel bromophenol derivatives 18–21 were synthesized in two steps. To synthesize desired diaryl methane compounds 13–17, compound 7 was first synthesized according to the procedure described by Crombie and Josephs [50]. The alkylation of substituted benzenes is a very important reaction for the synthesis of novel alkyl benzenes. The synthesis of diaryl methanes can be achieved via the reaction of benzylalcohol with substituted benzenes in the presence of AlCl3 [51]. The application of this methodology to (2-bromo-4,5-dimethoxyphenyl)methanol (7) and benzene derivatives 8–12 in CH2Cl2 (DCM) in the presence of AlCl3 afforded novel compounds 13–16 and a known compound 17 [52], with good yields (75–92%). The O-Me demethylation of arylmethyl ethers with BBr3 is an important strategy for the synthesis of bioactive phenols [21]. Therefore, the targeted novel bromophenols 18–21 were synthesized from the demethylation reaction of 13–16 with BBr3 in DCM, with the yields ranging from 73 to 82% (Scheme 1). The structures of all the compounds described in this paper were characterized by IR, elemental analysis, and the 1H and 13C-NMR techniques.
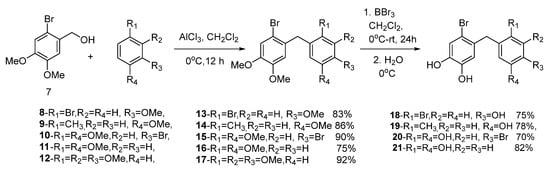
Scheme 1.
The synthesis of novel bromophenol derivatives.
2.2. Biochemistry
Since abnormal levels or behaviors of the majority of the sixteen hCA isoenzymes have frequently been linked to several human diseases [53,54,55]. These CA isoforms are intensively found in different tissues and are involved in many important mechanisms such as electrolyte secretion, cell differentiation, bone resorption, calcification, pH and CO2 homeostasis, gluconeogenesis, and neurotransmission in mammals [56,57,58]. Hence, many pharmaceutical uses have notable goals for a variety of CA isoforms, including antiglaucoma drugs, anticonvulsant factors/diagnostic, diuretics, antiobesity, and antitumor tools [59,60]. For instance, inhibitors of the hCAs IX and XII isozymes have been used as antitumor and antimetastatic agents [61,62].
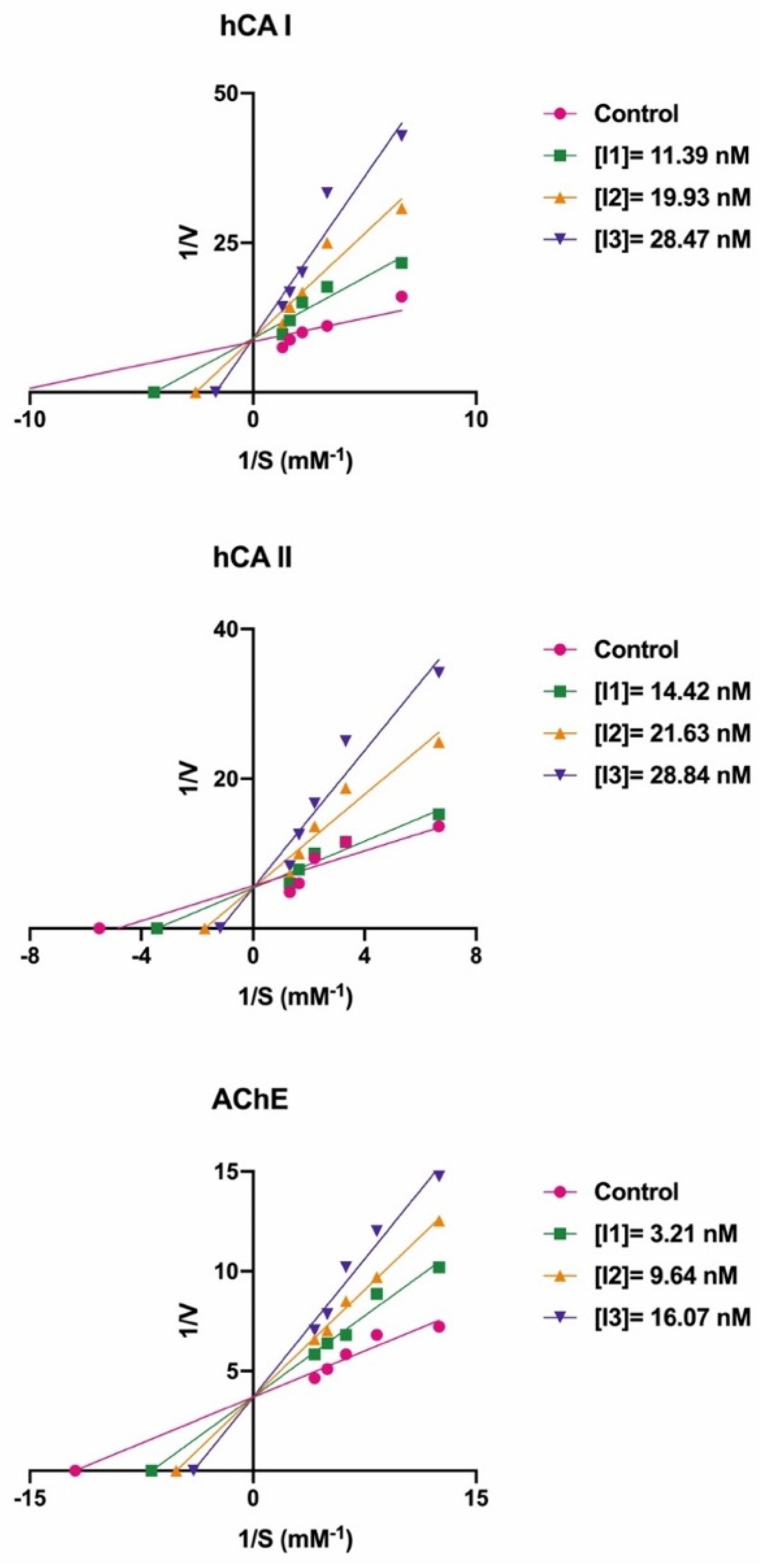
High amounts of the hCA I isoform have been found in the red blood cells and the gastrointestinal tract of mammals. The inhibition of this enzyme can be a key component in the treatment of conditions or diseases, including cerebral and retinal edema [63,64]. The enzyme results are given in Table 1 and Figure 2. In the current study, all the novel, synthesized a series of bromophenols (13–21) efficiently inhibited the hCA I isozyme, with IC50 values ranging from 12.38 to 38.50 nM and Ki values ranging from 2.53 ± 0.25 to 25.67 ± 4.58 nM. The compound 1-bromo-4,5-dimethoxy-2-(5-methoxy-2-methylbenzyl)benzene (14) demonstrated the best inhibition (Ki: 2.53 ± 0.25 nM) (Figure 2). However, the Ki values of novel compounds (13–21) towards hCA I were decreased as follows: 14 (2.53 ± 0.25 nM) > 15 (9.35 ± 1.88 nM) > 21 (11.00 ± 3.83 nM) > 18 (12.49 ± 0.66 nM) > 16 (12.80 ± 0.52 nM) > 20 (13.37 ± 2.29 nM) > 17 (18.76 ± 4.97 nM) > 19 (20.35 ± 2.92 nM) > 13 (25.67 ± 4.58 nM). Similarly, all the novel, synthesized a series of bromophenols (13–21) demonstrated competitive inhibition against hCA I isozyme. According to Table 1, the binding of the bromo group to the 4th position of compound 16 caused a 1.37-fold decrease in the Ki value, which increased the inhibition efficiency (15, Ki: 9.35 ± 1.88 nM). In compound 16, methyl bonding (14) instead of the methoxy group showed a 5.06-fold greater effect on inhibition. The methoxy group instead of the -OH group in the compounds were more effective in inhibiting hCA I. For example, when compounds 14 and 19 are compared with each other, there is an 8.04-fold difference in the inhibition value.

Table 1.
Inhibition parameters of the novel synthesized bromophenols (13–21) against AChE, hCA I, and hCA II enzymes.
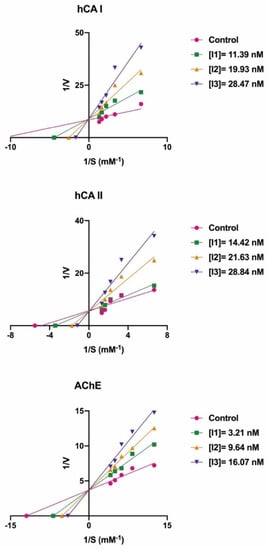
Figure 2.
The Lineweaver–Burk plots of novel bromophenol compounds (14 for hCA I, 13 for hCA II, and 21 for AChE).
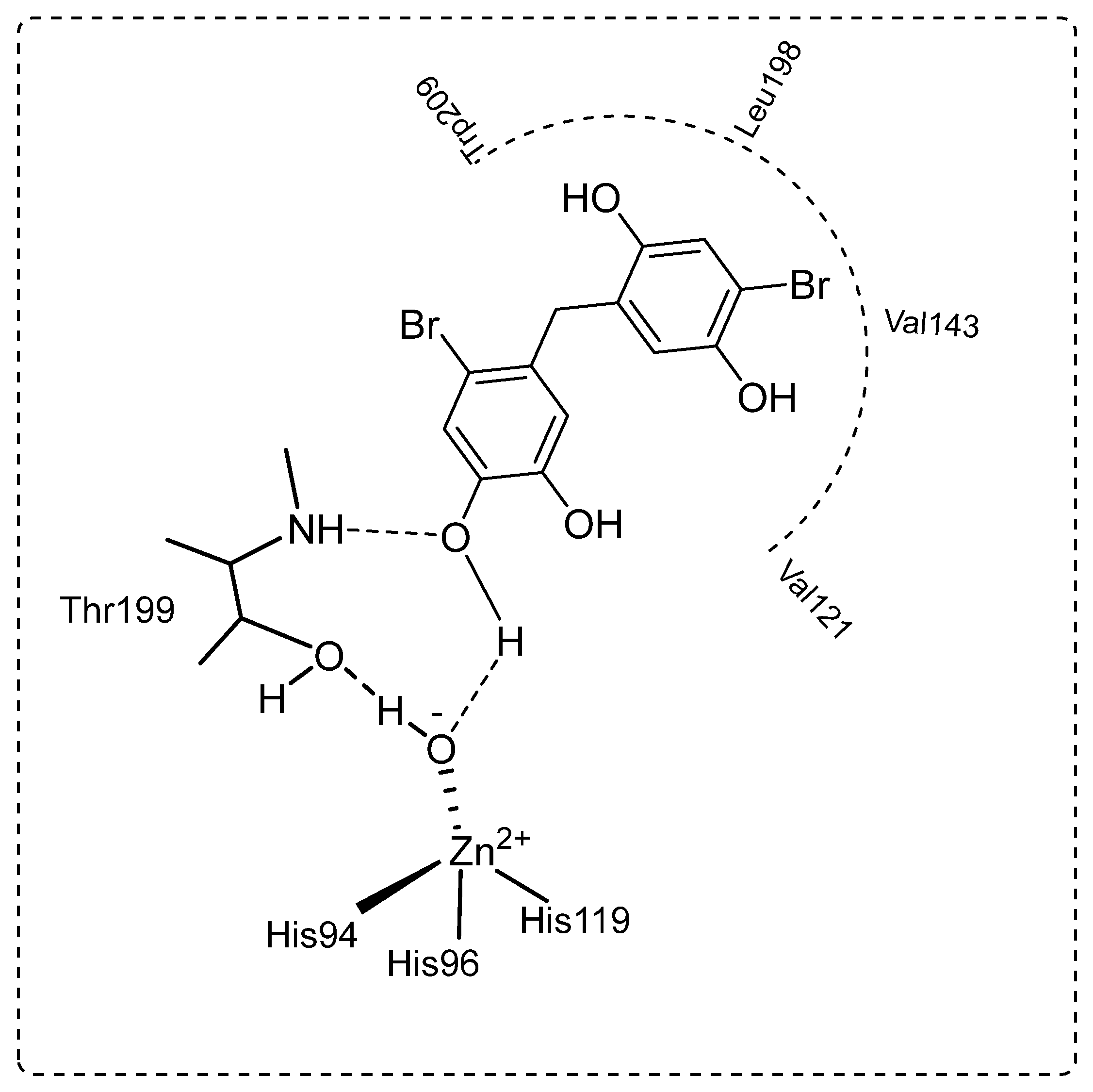
The dominant cytosolic hCA II isoform plays a critical function in disorders such as glaucoma [65]. In fact, the production of HCO3− acts as a method to introduce water and Na+ ions into the eye, increasing intraocular pressure. As a result, hCA II isozyme inhibition lowers HCO3− generation and eye pressure [66,67]. In the current study, bromophenols (13–21) effectively inhibited hCA II with IC50s ranging from 7.45 to 27.72 nM and Kis ranging from 1.63 ± 0.11 to 15.05 ± 1.07 nM. Compound 13 demonstrated the best inhibition effects (Ki: 1.63 ± 0.11 nM) (Figure 2). When the Ki values of the studied compounds (13–21) were evaluated against hCA II, the following order was found: 13 (1.63 ± 0.11 nM) > 15 (2.62 ± 0.13 nM) > 14 (4.28 ± 0.86 nM) > 21 (4.97 ± 0.59 nM) > 20 (6.21 ± 1.01 nM) > 16 (7.77 ± 0.57 nM) > 18 (9.15 ± 1.36 nM) > 17 (10.33 ± 1.88 nM) > 19 (15.05 ± 1.07 nM). Furthermore, all the novel, synthesized a series of bromophenols (13–21) exhibited competitive inhibition against the physiologically dominant hCA II isoenzyme. The proposed interaction between the most powerful bromophenols (20) and the CA II isoforms is illustrated in Figure 3. Bromophenol (20) has two dihydroxy benzyl rings. A second hydrogen bond was modeled between the oxygen atom, which attached to the -OH group at the phenol moiety in the ortho-position, and the amide NH of Thr199, a universally conserved amino acid residue in CAs. Thus, phenolic compounds and derivatives bind non-classically to CA, providing clues for the identification of new types of CA inhibitors. Such inhibition mechanisms of phenolic compounds, including bromophenols, are known [68,69]. As shown in Table 1, the attachment of three methoxy groups caused a decrease in the hCA II inhibition value. The methoxy group at positions 2, 3, and 4 may have created a steric hindrance in enzyme inhibition. As in the hCA I isoform, the presence of the methoxy group instead of the hydroxyl group in the compounds was more effective in inhibiting hCA II. When the compounds of 19 and 21 were compared with each other, the presence of the hydroxyl group instead of the methyl group caused a 3.03-fold increase in the inhibition value. A similar situation was observed in hCA I inhibition. This may be because the hydroxyl group is more electronegative than the methyl group.
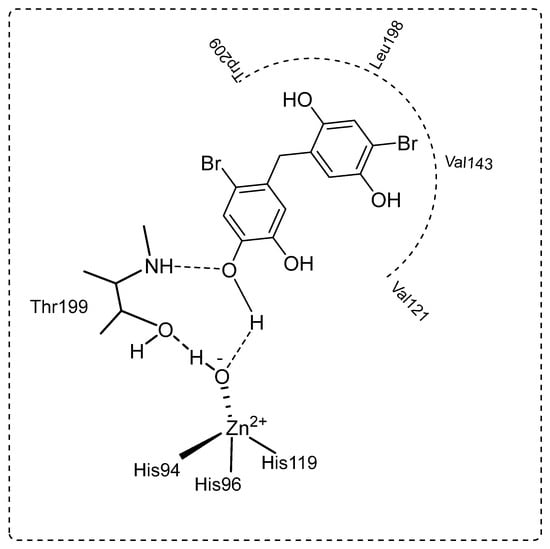
Figure 3.
The proposed binding mechanism between the CA II isoenzyme and 4-bromo-5-(4-bromo-2,5-dihydroxybenzyl)benzene-1,2-diol (20) by anchoring to the zinc ion (Zn2+) coordinated water (H2O)/hydroxide ion (-OH).
ACh is used as a neurotransmitter component, and AChE is a crucial enzyme that catalyzes ACh breakdown. This enzyme has been linked to therapeutic targets for AD [70,71]. The hypothesis was put forth to explain AD that synaptic depression is hampered because the cholinergic neuron cells impede ACh hydrolysis [72,73]. ACh hydrolysis is hindered because of AChE inhibition. As a result, the development of AChE enzyme inhibitor drugs and/or modulators is of great interest because it is currently one of the main goals in the fight against AD [74,75]. In the current study, bromophenols (13–21) effectively inhibited AChE with IC50s ranging from 8.35 to 21.00 nM and Kis ranging from 6.54 ± 1.03 to 24.86 ± 5.30 nM. The inhibitor effects of the studied compounds (13–21) against AChE were decreased as follows: 21 (6.54 ± 1.03 nM) > 18 (7.92 ± 1.38 nM) > 20 (8.32 ± 0.69 nM) > 13 (11.04 ± 0.61 nM) > 14 (11.62 ± 2.75 nM) > 16 (16.27 ± 2.98 nM) > 19 (17.43 ± 3.15 nM) > 17 (21.04 ± 4.72 nM) > 15 (24.86 ± 5.30 nM). In addition, all the novel synthesized a series of bromophenols (13–21) showed competitive inhibition against the cholinergic enzyme of AChE. As shown in Table 1, in the methoxy-bonded compound groups, the fact that the methyl group (14) is attached instead of the bromine ion (13) did not cause any change in inhibition. When compounds 15 and 16 are compared, the addition of the bromine group to the 4th position caused a rise in the inhibition value. The presence of the -OCH3 groups in the middle position without the bromine group was more effective in AChE inhibition (15, Ki: 24.86 ± 5.30 nM; 16, Ki: 16.27 ± 2.98 nM). As in hCA I and II, the presence of the methoxy group instead of the hydroxyl group in the compounds was more effective in inhibiting AChE. When compounds 19 and 21 were compared with each other, the presence of the hydroxyl group instead of the methyl group caused a 2.67-fold increase in the inhibition value.
3. Materials and Methods
3.1. General
Commercially purchased chemicals were used without further purification. Solvents were used after distillation or after drying with various drying agents. The melting points were determined using a capillary melting equipment and were not corrected (Buechi 530). A PerkinElmer spectrophotometer was used to collect IR spectra (Lancashire, Great Britain) from liquids in 0.1 mm cells. On a 400 (100) MHz (Varian, Danbury, CT) and 400 (100) MHz (Bruker, Fallanden, Switzerland) spectrometers, the 1H and 13C NMR spectra were collected; d was in ppm, with Me4Si as the internal standard. On a Leco CHNS-932 apparatus (St. Joseph, Missouri, USA), elemental analyses were performed. The silica gel was used for column chromatography (60-mesh, Merck, Darmstadt, Germany). PLC stands for preparative thick-layer chromatography, which used 1 mm of silica gel (60 PF, Merck, Darmstadt, Germany) on glass plates. The synthesized compounds’ 1HNMR and 13C NMR spectra are provided as Supplementary Materials.
3.2. Chemistry
The synthesis of compound 7 was performed according to procedure of Crombie and Joseph [50]. The synthesis of compounds 8–12 were carried out according to the method given in the literature [51]. The compound 1-(2-bromo-4,5-dimethoxybenzyl)-2,3,4-trimethoxy benzene (17) was synthesized differently in this work [52].
3.2.1. General Synthesis Procedure for the Synthesis of Compounds 13–17
The compound 2-Bromo-4,5-dimethoxybenzenemethanol (7) (5 mmol), the corresponding benzene derivatives (8–12) (5 mmol), and AlCl3 (7 mmol) were dissolved in 30 mL of dry CH2Cl2. The solution was cooled to 0 °C in an ice bath and stirred for 24 h. The reaction mixture was quenched by ice-cold water (20 mL) to remove unreacted AlCl3. The organic phase was separated, and the water phase was extracted with CH2Cl2 (2 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4, and the solvent was evaporated. Then, the crude products were separated on a silica gel column by using hexane/EtOAc to obtain the pure products.
3.2.2. 1-Bromo-2-(2-bromo-4-methoxybenzyl)-4,5-dimethoxybenzene (13)
Yield: 83%, Rf: 0.53, cream solid. M.p. 84–86 °C. 1H-NMR (400 MHz, CDCl3) δ: 7.15 (1H, d, J = 2.6 Hz, Ar-H), 7.06 (1H, s, Ar-H), 6.89 (1H, d, J = 8.5 Hz, Ar-H), 6.77 (1H, dd, J = 8.5 Hz, 2.6 Hz, Ar-H), 6.57 (1H, s, Ar-H), 4.06 (2H, s, C-H), 3.87 (3H, s, OCH3), 3.78 (3H, s, OCH3), 3.75 (3H, s, OCH3). 13C-NMR (100 MHz, CDCl3) δ: 158.6 (OC), 148.2 (OC), 138.7 (OC), 131.2 (C), 131.2 (C), 130.7 (CH), 124.8 (C-Br), 118.0 (CH), 115.6 (C-Br), 114.7 (CH), 113.6 (CH), 113.5 (CH), 56.2 (OCH3), 56.0 (OCH3), 55.5 (OCH3), 40.6 (CH2). IR (cm−1, CH2Cl2): 3080, 3001, 2906, 2837, 1603,1567, 1504, 1463, 1435, 1379, 1341, 1256, 1218, 1162, 1031, 961, 845. Anal. Calcld for C16H16Br2O3; C, 46.18; H,3.88. Found: C, 45.88; H, 3.90.
3.2.3. 1-Bromo-4,5-dimethoxy-2-(5-methoxy-2-methylbenzyl)benzene (14)
Yield: 86%, Rf: 0.53, white solid. M.p. 93–95 °C. 1H NMR (400 MHz, CDCl3) δ: 7.03 (1H, s, Ar-H), 6.99 (1H, dd, J = 8.4, 1.6 Hz, Ar-H), 6.78 (1H, s, Ar-H), 6.76 (1H, d, J = 8.4 Hz, Ar-H), 6.67 (1H, s, Ar-H), 3.98 (2H, s, C-H), 3.85 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.74 (3H, s, OCH3), 2.22 (3H, s, CH3),13C NMR (100 MHz, CDCl3) δ: 155.2 (OC), 148.3 (OC), 147.8 (OC), 132.2 (C), 130.6 (C), 129.7 (CH), 128.0 (C), 127.7 (CH), 115.4 (C-Br), 114.7 (CH), 113.8 (CH), 110.2 (CH), 56.2 (OCH3), 55.9 (OCH3), 55.5 (OCH3),35.4 (CH2), 20.6 (CH3). Anal. Calcld for C17H19Br2O3; 58.13; H, 5.45. Found: C, 57.65; H, 5.39.
3.2.4. 1-Bromo-2-(4-bromo-2,5-dimethoxybenzyl)-4,5-dimethoxybenzene (15)
Yield: 90%, Rf: 0.43, white solid. M.p. 104–106 °C. 1H NMR (400 MHz, CDCl3) δ: 7.06 (1H, s, Ar-H), 7.03 (1H, s, Ar-H), 6.67 (1H, s, Ar-H), 6.64 (1H, s, Ar-H), 3.96 (2H, s, C-H), 3.85 (3H, s, OCH3), 3.80 (3H, s, OCH3), 3.76 (3H, s, OCH3), 3.74 (3H, s, OCH3), 13C NMR (100 MHz, CDCl3) δ: 151.7 (OC), 150.1 (OC), 148.4 (OC), 148.1 (OC), 131.3 (C), 128.6 (C), 115.8 (C-Br), 115.5, 114.7 (CH), 114.6 (CH), 113.6 (CH), 109.2 (CH), 56.9 (OCH3), 56.1 (2.OCH3), 56.0 (OCH3), 35.4 (CH2). IR (cm−1, CH2Cl2): 2934, 2838, 1602, 1449, 1463, 1378, 1257, 1212, 1163, 1034, 852. Anal. Calcld for C17H18Br2O4: C, 45.77; H, 4.07. Found: C, 45.60; H, 4.05
3.2.5. 1-Bromo-2-(2,5-dimethoxybenzyl)-4,5-dimethoxybenzene (16)
Yield: 75%, Rf: 0.56, cream solid. M.p. 97–99 °C. 1H NMR (400 MHz, CDCl3) δ: 7.03 (1H, s, Ar-H), 6.81 (1H, d, J = 8.0 Hz, Ar-H), 6.72 (1H, dd, J = 8.0 Hz, 3.0 Hz, Ar-H), 6.69 (1H, s, Ar-H), 6.57 (1H, d, J = 3.0 Hz, Ar-H), 3.99 (2H, s, C-H), 3.86 (3H, s, OCH3), 3.81 (3H, s, OCH3), 3.75 (3H, s, OCH3), 3.71 (3H, s, OCH3), 13C NMR (100 MHz, CDCl3) δ: 153.5 (OC), 151.6 (OC), 148.4 (OC), 147.9 (OC), 131.7 (C), 129.6 (C), 116.5 (CH), 115.4 (C-Br), 114.7 (CH), 113.7 (CH), 111.2 (CH), 111.1 (CH), 56.1 (OCH3), 55.9 (OCH3), 55.8 (OCH3), 55.6 (OCH3), 35.5 (CH2). IR (cm−1, CH2Cl2): 2997, 2937, 2834, 1602, 1574, 1500, 1463, 1436, 1379, 1257, 1217, 1163, 1112, 1030, 933, 860. Anal. Calcld for C17H19BrO4; C, 55.60; H,5.21. Found: C, 54.95; H, 5.22.
3.2.6. 1-Bromo-2-(2,5-dimethoxybenzyl)-4,5-dimethoxybenzene (17)
1-(2-bromo-4,5dimethoxybenzyl)-2,3,4-trimethoxybenzene (17) was synthesized by a different method than that of described previously [52].
Yield: 92%, Rf: 0.30, white solid. M.p. 79–80 °C, Lit. Mp:75–77 °C, 1H NMR (400 MHz, CDCl3) δ: 7.02 (1H, s, Ar-H), 6.68 (1H, d, J = 8.5 Hz, Ar-H), 6.63 (1H, s, Ar-H), 6.57 (1H, d, J = 8.6 Hz, Ar-H), 3.96 (2H, s, C-H), 3.87 (3H, s, OCH3), 3.84 (3H, s, OCH3), 3.83 (3H, s, OCH3), 3.82 (3H, s, OCH3), 3.73 (3H, s, OCH3). 13C NMR (100 MHz, CDCl3) δ: 152.6 (OC), 152.0 (OC), 148.5 (OC), 148.1 (OC), 142.5 (OC), 132.5 (C), 126.1 (C), 124.3 (CH), 115.5 (CH), 114.7 (C-Br), 113.8 (CH), 107.3 (CH), 56.4 (OCH3), 56.3 (OCH3), 56.2 (OCH3), 56.1 (OCH3), 56.0 (OCH3), 35.4 (CH2). Anal. Calcld for C18H21BrO5; C, 54.42; H, 5.33. Found: C, 54.10; H, 5.21.
3.2.7. General Procedure for the Synthesis of Bromophenols 18–21
Diaryl methane compounds (13–17) were dissolved in CH2Cl2. The solutions were cooled to 0 °C. To these solutions, for each methoxy group in the structure of these compounds, 3 equivalents of BBr3 were added dropwise under N2 atmosphere. Then, the mixtures were stirred at rt for 24 h. The reaction medium was cooled to 0 °C. Ice (20 g) and CH2Cl2 (50 mL) were added to the reaction medium and the organic phases were separated. Then, the water phase was extracted with ethyl acetate (2 × 50 mL). The organic layers were combined, dried over anhydrous Na2SO4 and the solvents were evaporated. The residue was crystallized from EtOAc/Hexane.
3.2.8. 4-Bromo-5-(2-bromo-4-hydroxybenzyl)benzene-1,2-diol (18)
Yield: 75%, white solid. M.p. 150–153 °C. 1H NMR (400 MHz, Acetone-d6) δ: 6.99 (1H, d, J = 2.5 Hz, Ar-H), 6.95–6.91 (1H, m, Ar-H), 6.80 (1H, d, J = 8.4 Hz, Ar-H), 6.68 (1H, dd, J = 8.4, 2.5 Hz, Ar-H), 6.36 (1H, s, Ar-H), 3.80 (2H, s, CH). 13C NMR (100 MHz, Acetone-d6) δ: 157.7 (OC), 145.4 (OC), 140.3 (OC), 132.3 (C), 130.8 (C), 127.2 (C-Br), 120.0 (CH), 119.8 (CH), 117.8 (C-Br), 117.2 (CH), 115.7 (CH), 113.3 (CH), 40.2 (CH2). IR (cm−1, CH2Cl2): 3324, 2392, 1686, 1605, 1490, 1429, 1350, 1274, 1226, 1185, 1143, 1031, 919, 872. Anal. Calcld for C13H10Br2O3; C41.75; H, 2.69. Found: C, 41.97; H, 2.63.
3.2.9. 4-Bromo-5-(2-bromo-4-hydroxybenzyl)benzene-1,2-diol (19)
Yield: 78%, cream solid. M.p.157–159 °C. 1H NMR (400 MHz, Acetone-d6) δ 6.91 (1H, s, Ar-H), 6.74 (1H, dd, J = 8.0, 2.0 Hz, Ar-H), 6.65 (1H, s, Ar-H), 6.64 (1H, d, J = 8.0 Hz, Ar-H), 6.49 (1H, s, Ar-H), 3.75 (2H, s, CH), 2.04 (3H, s, CH3). 13C NMR (100 MHz, Acetone-d6) δ 153.6 (OC), 145.5 (OC), 145.1 (OC), 132.2 (C), 131.6 (C), 128.5 (C), 127.0 (CH), 122.1 (CH), 119.5 (CH), 118.1 (CH), 115.6 (C-Br), 113.4 (CH), 35.4 (CH2), 20.3 (CH3). IR (cm−1, CH2Cl2): 3200, 2389, 1502, 1502, 1421, 1356, 1276, 1182, 1045, 919, 814. Anal. Calcld for C14H13BrO3; C 54.39; H, 4.24. Found: C, 54.10; H, 4.06.
3.2.10. 4-Bromo-5-(4-bromo-2,5-dihydroxybenzyl)benzene-1,2-diol (20)
Yield: 70%, cream solid. M.p.159–161 °C. 1H NMR (400 MHz, Acetone-d6) δ 8.11 (1H, s, OH), 8.08 (1H, s, OH), 8.04 (1H, s, OH), 7.99 (1H, s, OH), 6.91 (1H, s, Ar-H), 6.89 (1H, s, Ar-H), 6.54 (1H, s, Ar-H), 6.39 (1H, s, Ar-H), 3.69 (2H, s, CH). 13C NMR (100 MHz, Acetone-d6) δ 149.4 (OC), 147.9 (OC), 145.7 (OC), 145.5 (OC), 131.3 (C), 128.5 (C), 119.7 (CH), 119.4 (CH), 118.3 (C-Br and CH), 113.5 (CH), 107.2 (C-Br), 35.3 (CH2). IR (cm−1, CH2Cl2): 3215, 1685, 1602, 1500, 1420, 1355, 1274, 1192, 997, 935, 873. Anal. Calcld for C13H10Br2O4; C 40.03; H, 2.58. Found: C, 40.41; H, 2.50.
3.2.11. 4-Bromo-5-(2,5-dihydroxybenzyl)benzene-1,2-diol (21)
Yield: 82%, dark yellow. M.p.150–153 °C. 1H NMR (400 MHz, Acetone-d6) δ 6.91 (1H, s, Ar-H), 6.58 (1H, d, J = 8.5 Hz, Ar-H), 6.53 (1H, s, Ar-H), 6.40 (1H, dd, J = 8.5, 3.0 Hz, Ar-H), 6.27 (1H, d, J = 3.0 Hz, Ar-H), 3.76 (2H, s, CH). 13C NMR (100 MHz, Acetone-d6) δ 151.15 (OC), 148.65 (OC), 145.52 (OC), 145.20 (OC), 145.18 (OC), 132.06 (C), 128.15 (C), 119.61 (CH), 118.31 (CH), 117.44 (CH), 117.44 (CH), 116.28 (C-Br), 114.22 (CH), 35.59 (CH2). IR (cm−1, CH2Cl2): 3217, 2395, 2230, 1687, 1595, 1502, 1422, 1279, 1195, 1049, 876.Anal. Calcld for C13H11BrO4; C 50.18; H, 3.56. Found: C, 50.20; H, 3.45.
3.3. Biochemical Studies
3.3.1. Enzyme Activity Assays
In this work, the in vitro inhibition effects of bromophenols (13–21) on AChE activity were determined by Ellman’s method [76], as previously described [77]. The results were recorded spectrophotometrically at 412 nm. Acetylthiocholine iodide (AChI) was used as substrate, according to a prior study [78]. Both hCA isoforms were purified by using the Sepharose-4B-L-Tyrosine-sulfanilamide affinity technique [79]. Then, the purity of these CA isoenzymes was defined via the SDS-PAGE purity technique [80,81]. Furthermore, the hCA activity was determined using the esterase method at 348 nm, according to the method of Verpoorte et al. [82] and as given in prior studies [83,84].
3.3.2. Enzyme Inhibition Assays
In order to investigate the in vitro inhibitory mechanisms of bromophenols (13–21), kinetic studies were performed at different concentrations of bromophenols and various substrates [85,86]. From the Lineweaver–Burk graphs, the IC50 and Ki values of bromophenol (13–21) derivatives were calculated, and the inhibition type of bromophenols (13–21) against AChE and hCAs was determined, as given in prior studies [87,88,89].
3.3.3. Statistical Analyses
Statistical analyses were performed via an unpaired Student’s t-test with the use of the statistical program IBM SPSS Statistics 20. The results were recorded as means with their standard deviation (SD). p < 0.05 was the minimum significance level.
4. Conclusions
In the current study, new bromophenols were synthesized, and their hCAs and AChE inhibitory properties were investigated. The presence of different biologically functional groups (-OH, -OCH3, and -Br) in aromatic scaffolds of synthesized compounds influenced the activity of the studied enzymes. Our findings indicate that the investigated compounds 13–21 exhibited efficient hCA I, II, and AChE inhibition effects in the low nanomolar levels. These experimental findings confirm that substituted methoxy (-OCH3) and bromophenols may be used as leads for generating potent CAI and AChE inhibitors associated with some global disorders, including AD, epilepsy, and glaucoma.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217426/s1. 1H NMR and 13C NMR spectra of synthesized compounds.
Author Contributions
Methodology and investigation, N.O., Y.D. and A.M.; writing—original draft preparation and writing—review and editing, supervision, funding and acquisition, S.G. and İ.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are greatly indebted to Ataturk University Erzurum (Scientific Research Project, FBA-2017-6124) for the financial support and research conditions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are provided in a publicly accessible repository.
Acknowledgments
The authors are greatly indebted to Ataturk University Erzurum (Scientific Research Project, FBA-2017-6124) for the financial support and research conditions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Oztaşkın, N.; Çetinkaya, Y.; Taslimi, P.; Göksu, S.; Gulcin, I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015, 60, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Katsui, N.; Suzuki, Y.; Kitamura, S.; Irie, T. 5,6-dibromoprotocatechualdehyde and 2,3-dibromo-4,5-dihydroxybenzyl methyl ether: New dibromophenols from Rhodomela larix. Tetrahedron 1967, 23, 1185–1188. [Google Scholar] [CrossRef]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Liu, H.; Namikoshi, M.; Meguro, S.; Nagai, H.; Kobayashi, H.; Yao, X. Isolation and characterization of polybrominated diphenyl ethers as inhibitors of microtubule assembly from the marine sponge Phyllospongia dendyi collected at Palau. J. Nat. Prod. 2004, 67, 472–474. [Google Scholar] [CrossRef]
- Tian, L.W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.A.; Wellington, C.; Hooper, J.N.A.; Quinn, R.J. ApoE secretion modulating bromotyrosine derivative from the Australian marine sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 2014, 24, 3537–3540. [Google Scholar] [CrossRef]
- Lindsay, B.S.; Battershill, C.N.; Copp, B.R. Isolation of 2-(3′-bromo-4′-hydroxyphenol)ethanamine from the New Zealand ascidian Cnemidocarpa bicornuta. J. Nat. Prod. 1998, 61, 857–858. [Google Scholar] [CrossRef]
- Gribble, G.W. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-Integrin/FAK signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef]
- Xu, Q.; Luo, J.; Wu, N.; Zhang, R.S.; Shi, D.Y. BPN, a marine-derived PTP1B inhibitor, activates insulin signaling and improves insulin resistance in C2C12 myotubes. Int. J. Biol. Macromol. 2018, 106, 379–386. [Google Scholar] [CrossRef]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.; Hansen, E.; Isaksson, J.; Andersen, J. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar. Drugs 2013, 11, 2769–2784. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, T.H.; Lee, J.H.; Chae, C.S.; Chung, S.C.; Shin, D.S.; Shin, J.; Oh, K.B. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J. Agric. Food Chem. 2007, 55, 6923–6928. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef]
- Oh, K.B.; Lee, J.H.; Chung, S.C.; Shin, J.; Shin, H.J.; Kim, H.K.; Lee, H.S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Taniguchii, K.; Takashima, K.; Hayashi, I.; Suzuki, M. Feeding-deterrent bromophenols from Odonthalia corymbifera. Phytochemistry 1997, 45, 485–487. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Balaydın, H.T.; Gulcin, I.; Menzek, A.; Goksu, S.; Sahin, E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J. Enzyme Inhib. Med. Chem. 2010, 25, 685–695. [Google Scholar] [CrossRef]
- Akbaba, Y.; Balaydın, H.T.; Menzek, A.; Goksu, S.; Sahin, E.; Ekinci, D. Synthesis and biological evaluation of novel bromophenol derivatives as carbonic anhydrase inhibitors. Arch. Pharm. 2013, 346, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Balaydın, H.T.; Soyut, H.; Ekinci, D.; Göksu, S.; Beydemir, S.; Menzek, A.; Sahin, E. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J. Enzyme Inhib. Med. Chem. 2012, 27, 43–50. [Google Scholar] [CrossRef]
- Oztaşkın, N.; Taslimi, P.; Maras, A.; Goksu, S.; Gulcin, I. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg. Chem. 2017, 74, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Balaydin, H.T.; Akbaba, Y.; Menzek, A.; Sahin, E.; Goksu, S. First and short syntheses of biologically active, naturally occurring brominated mono-and dibenzyl phenols. Arkivoc 2009, XIV, 75–87. [Google Scholar]
- Balaydin, H.T.; Şentürk, M.; Goksu, S.; Menzek, A. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols and their derivatives including natural products: Vidalol B. Eur. J. Med. Chem. 2012, 54, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Scozzafava, A.; Supuran, C.T.; Akıncıoğlu, H.; Koksal, Z.; Turkan, F.; Alwasel, S. The effect of caffeic acid phenethyl ester (CAPE) metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione s-transferase, lactoperoxidase and carbonic anhydrase isoenzymes I, II, IX and XII. J. Enzyme Inhib. Med. Chem. 2016, 31, 1095–1101. [Google Scholar] [CrossRef]
- Küçük, M.; Gulcin, I. Purification and characterization of carbonic anhydrase enzyme from Black Sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on the enzyme activity. Environ. Toxicol. Pharmacol. 2016, 44, 134–139. [Google Scholar] [CrossRef]
- Akıncıoglu, A.; Topal, M.; Gulcin, I.; Goksu, S. Novel sulphamides and sulphonamides incorporating the tetralin scaffold as carbonic anhydrase and acetylcholine esterase inhibitors. Arch. Pharm. 2014, 347, 68–76. [Google Scholar] [CrossRef]
- Topal, A.; Atamanalp, M.; Oruc, E.; Demir, Y.; Beydemir, Ş.; Isik, A. In vivo changes in carbonic anhydrase activity and histopathology of gill and liver tissues after acute exposure to chlorpyrifos in rainbow trout. Arh. Hig. Rada. Toksikol. 2014, 65, 377–385. [Google Scholar] [CrossRef]
- Caglayan, C.; Taslimi, P.; Turk, C.; Gulcin, I.; Kandemir, F.M.; Demir, Y.; Beydemir, Ş. Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ. Sci. Pollut. Res. 2020, 27, 10607–10616. [Google Scholar] [CrossRef]
- Gümüş, M.; Babacan, S.N.; Demir, Y.; Sert, Y.; Koca, I.; Gulcin, I. Discovery of sulfadrug-pyrrole conjugates as carbonic anhydrase and acetylcholinesterase inhibitors. Arch. Pharm. 2022, 355, 2100242. [Google Scholar] [CrossRef]
- Hamide, M.; Gök, Y.; Demir, Y.; Yakalı, G.; Tok, T.T.; Aktas, A.; Sevinçek, R.; Güzel, B.; Gülcin, I. Pentafluorobenzyl-substituted benzimidazolium salts: Synthesis, characterization, crystal structures, computational studies and inhibitory properties of some metabolic enzymes. J. Mol. Struct. 2022, 1265, 133266. [Google Scholar] [CrossRef]
- Gulcin, I.; Taslimi, P. Sulfonamide inhibitors: A patent review 2013–present. Exp. Opin. Therap. Pat. 2018, 28, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, S.; Türkeş, C.; Arslan, M.; Demir, Y.; Beydemir, Ş. New isoindole-1,3-dione substituted sulfonamides as potent inhibitors of carbonic anhydrase and acetylcholinesterase: Design, synthesis, and biological evaluation. ChemistrySelect 2019, 4, 13347. [Google Scholar] [CrossRef]
- Tugrak, M.; Gul, H.I.; Demir, Y.; Gulcin, I. Synthesis of benzamide derivatives with thiourea-substituted benzenesulfonamides as carbonic anhydrase inhibitors. Arch. Pharm. 2021, 354, 2000230. [Google Scholar] [CrossRef] [PubMed]
- Tugrak, M.; Gul, H.I.; Demir, Y.; Levent, S.; Gulcin, I. Synthesis and in vitro carbonic anhydrases and acetylcholinesterase inhibitory activities of novel imidazolinone-based benzenesulfonamides. Arch. Pharm. 2021, 354, 2000375. [Google Scholar] [CrossRef]
- Mete, E.; Comez, B.; Gul, H.I.; Gulcin, I.; Supuran, C.T. Synthesis and carbonic anhydrase inhibitory activities of new thienyl-substituted pyrazoline benzenesulfonamides. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–5. [Google Scholar] [CrossRef]
- Ozmen Ozgün, D.; Gul, H.I.; Yamali, C.; Sakagami, H.; Gulcin, I.; Sukuroglu, M.; Supuran, C.T. Synthesis and bioactivities of pyrazoline benzensulfonamides as carbonic anhydrase and acetylcholinesterase inhibitors with low cytotoxicity. Bioorg. Chem. 2019, 84, 511–517. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, H.I.; Kazaz, C.; Levent, S.; Gulcin, I. Synthesis, structure elucidation, and in vitro pharmacological evaluation of novel polyfluoro substituted pyrazoline type sulfonamides as multi-target agents for inhibition of acetylcholinesterase and carbonic anhydrase I and II enzymes. Bioorg. Chem. 2020, 96, 103627. [Google Scholar] [CrossRef]
- Taslimi, P.; Sujayev, A.; Mamedova, S.; Kalın, P.; Gulcin, I.; Sadeghian, N.; Beydemir, S.; Küfrevioglu, Ö.İ.; Alwasel, S.H.; Farzaliyev, V.; et al. Synthesis and bioactivity of several new hetaryl sulfonamides. J. Enzyme Inhib. Med. Chem. 2017, 32, 137–145. [Google Scholar] [CrossRef]
- Gocer, H.; Akıncıoglu, A.; Goksu, S.; Gulcin, I. Carbonic anhydrase inhibitory properties of phenolic sulfonamides derived from dopamine related compounds. Arab. J. Chem. 2017, 10, 398–402. [Google Scholar] [CrossRef]
- Sepheri, N.; Mohammadi-Khanaposhtani, M.; Asemanipoor, N.; Hosseini, S.; Biglar, M.; Larijani, B.; Mahdavi, M.; Hamedifar, H.; Taslimi, P.; Sadeghian, N.; et al. Novel quinazolin-sulfonamid derivatives: Synthesis, characterization, biological evaluation, and molecular docking studies. J. Biomol. Struct. 2022, 40, 3359–3370. [Google Scholar]
- Aydin, B.O.; Anil, D.; Demir, Y. Synthesis of N-alkylated pyrazolo [3,4-d] pyrimidine analogs and evaluation of acetylcholinesterase and carbonic anhydrase inhibition properties. Arch. Pharm. 2021, 354, 2000330. [Google Scholar] [CrossRef] [PubMed]
- Burmaoğlu, S.; Yılmaz, A.O.; Polat, M.F.; Kaya, R.; Gulcin, I.; Algul, O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibitors. Bioorg. Chem. 2019, 85, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Garibov, E.; Taslimi, P.; Sujayev, A.; Bingöl, Z.; Çetinkaya, S.; Gulcin, I.; Beydemir, S.; Farzaliyev, V.; Alwasel, S.H.; Supuran, C.T. Synthesis of 4,5-disubstituted-2-thioxo-1,2,3,4-tetrahydropyrimidines and investigation of their acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase I/II inhibitory and antioxidant activities. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Erçag, A.; Zorlu, Y.; Demir, Y.; Gulcin, I. New Pd (II) complexes of the bisthiocarbohydrazones derived from isatin and disubstituted salicylaldehydes: Synthesis, characterization, crystal structures and inhibitory properties against some metabolic enzymes. J. Biol. Inorg. 2022, 27, 271–281. [Google Scholar] [CrossRef]
- Erdemir, F.; Barut Celepci, D.; Aktas, A.; Gok, Y.; Kaya, R.; Taslimi, P.; Demir, Y.; Gulcin, I. Novel 2-aminopyridine liganded Pd (II) N-heterocyclic carbene complexes: Synthesis, characterization, crystal structure and bioactivity properties. Bioorg. Chem. 2019, 91, 103134. [Google Scholar] [CrossRef]
- Türkan, F.; Huyut, Z.; Demir, Y.; Ertaş, F.; Beydemir, Ş. The effects of some cephalosporins on acetylcholinesterase and glutathione S-transferase: An in vivo and in vitro study. Arch. Physiol. Biochem. 2019, 125, 235–243. [Google Scholar] [CrossRef]
- Bayrak, Ç.; Taslimi, P.; Kahraman, H.S.; Gulcin, I.; Menzek, A. The first synthesis, carbonic anhydrase inhibition and anticholinergic activities of some bromophenol derivatives with S including natural products. Bioorg. Chem. 2019, 85, 128–139. [Google Scholar] [CrossRef]
- Bilginer, S.; Gul, H.I.; Anil, B.; Demir, Y.; Gulcin, I. Synthesis and in silico studies of triazene-substituted sulfamerazine derivatives as acetylcholinesterase and carbonic anhydrases inhibitors. Arch. Pharm. 2021, 354, 2000243. [Google Scholar] [CrossRef]
- Mahmudov, I.; Demir, Y.; Sert, Y.; Abdullayev, Y.; Sujayev, E.; Alwasel, S.H.; Gulcin, I. Synthesis and inhibition profiles of N-benzyl-and N-allyl aniline derivatives against carbonic anhydrase and acetylcholinesterase—A molecular docking study. Arab. J. Chem. 2022, 15, 103645. [Google Scholar] [CrossRef]
- Crombie, L.; Josephs, J.L. Rotenoid synthesis by Wadsworth-Emmons coupling and Mukaiyama cyclisation: Application to 5-thiorotenoids. J. Chem. Soc. 1993, 21, 2599–2604. [Google Scholar] [CrossRef]
- Jiang, B.; Shi, D.; Cui, Y.; Guo, S. Design, synthesis, and biological evaluation of bromophenol derivatives as protein tyrosine phosphatase 1B inhibitors. Arch. Pharm. 2012, 345, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl) methanone and its derivatives. Arch. Pharm. 2011, 345, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Sendil, K.; Sengul, E.; Gultekin, M.S.; Taslimi, P.; Gulcin, I.; Supuran, C.T. The synthesis of some β-lactams and investigation of their metal-chelating activity, carbonic anhydrase and acetylcholinesterase inhibition profiles. J. Enzyme Inhib. Med. Chem. 2016, 31, 79–88. [Google Scholar] [CrossRef]
- Taslimi, P.; Akıncıoğlu, H.; Gulcin, I. Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol. 2017, 31, e21973. [Google Scholar] [CrossRef]
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E.B.; et al. Synthesis, characterization, crystal structure, electrochemical studies and biological evaluation of metal complexes with thiosemicarbazone of glyoxylic acid. Polyhedron 2018, 155, 25–33. [Google Scholar] [CrossRef]
- Anil, D.; Ozturk Aydin, B.; Demir, Y.; Turkmenoglu, B. Design, synthesis, biological evaluation and molecular docking studies of novel 1H-1,2,3-triazole derivatives as potent inhibitors of carbonic anhydrase, acetylcholinesterase and aldose reductase. J. Mol. Struct. 2022, 1257, 132613. [Google Scholar] [CrossRef]
- Kocyigit, U.M.; Budak, Y.; Gurdere, M.B.; Erturk, F.; Yencilek, B.; Taslimi, P.; Gulcin, I.; Ceylan, M. Synthesis of chalcone-imide derivatives and investigation of their anticancer and antimicrobial activities, carbonic anhydrase and acetylcholinesterase enzymes inhibition profiles. Arch. Physiol. Biochem. 2018, 124, 61–68. [Google Scholar] [CrossRef]
- Taslimi, P.; Caglayan, C.; Gulcin, I. The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: An antidiabetic, anticholinergic, and antiepileptic study. J. Biochem. Mol. Toxicol. 2017, 31, e21995. [Google Scholar] [CrossRef]
- Caglayan, C.; Taslimi, P.; Demir, Y.; Kucukler, S.; Kandemir, F.M.; Gulcin, I. The effects of zingerone against vancomycin-induced lung, liver, kidney and testis toxicity in rats: The behavior of some metabolic enzymes. J. Biochem. Mol. Toxicol. 2019, 33, e22381. [Google Scholar] [CrossRef]
- Kucukoglu, K.; Gul, H.I.; Taslimi, P.; Gulcin, I.; Supuran, C.T. Investigation of inhibitory properties of some hydrazone compounds on hCA I, hCA II and AChE enzymes. Bioorg. Chem. 2019, 86, 316–321. [Google Scholar] [CrossRef]
- Ozensoy Guler, O.; Supuran, C.T.; Capasso, C. Carbonic anhydrase IX as a novel candidate in liquid biopsy. J. Enzyme Inhib. Med. Chem. 2020, 35, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Boztaş, M.; Çetinkaya, Y.; Topal, M.; Gulcin, I.; Menzek, A.; Sahin, E.; Tanc, M.; Supuran, C.T. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J. Enzyme Inhib. Med. Chem. 2015, 58, 640–650. [Google Scholar] [CrossRef]
- Gul, H.I.; Mete, E.; Taslimi, P.; Gulcin, I.; Supuran, C.T. Synthesis, carbonic anhydrase I and II inhibition studies of the 1,3,5-trisubstituted-pyrazolines. J. Enzyme Inhib. Med. Chem. 2017, 32, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yamali, C.; Gul, H.I.; Ece, A.; Taslimi, P.; Gulcin, I. Synthesis, molecular modeling, and biological evaluation of 4-[5-aryl-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl] benzenesulfonamides toward acetylcholinesterase, carbonic anhydrase I and II enzymes. Chem. Biol. Drug Des. 2018, 91, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L.; Erturk, A.; Akyüz, M.; Polat Kose, L.; Uc, E.M.; Bingol, Z.; Saglamtas, R.; Alwasel, S.; Gulcin, I. Screening of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzyme inhibition effects and antioxidant activity of coumestrol. Molecules 2022, 27, 3091. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Kalın, P.; Supuran, C.T.; Gulcin, I.; Alwasel, S.H. The impact of hydroquinone on acetylcholine esterase and certain human carbonic anhydrase isoenzymes (hCA I, II, IX, and XII). J. Enzyme Inhib. Med. Chem. 2015, 30, 941–946. [Google Scholar] [CrossRef]
- Saglik, B.N.; Çevik, U.A.; Osmaniye, D.; Levent, S.; Çavuşoğlu, B.K.; Demir, Y.; Ilgın, S.; Ozbay, Y.; Koparal, A.S.; Beydemir, S.; et al. Synthesis, molecular docking analysis and carbonic anhydrase I-II inhibitory evaluation of new sulfonamide derivatives Bioorg. Chem. 2019, 91, 103153. [Google Scholar]
- Alterio, V.; Di Fiore, A.; D’Ambrosio, K.; Supuran, C.T.; de Simone, G. Multiple binding modes of inhibitors to carbonic anhydrases: How to design specific drugs targeting 15 different isoforms? Chem. Rev. 2012, 112, 4421–4468. [Google Scholar] [CrossRef]
- Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef]
- Boztas, M.; Taslimi, P.; Yavari, M.A.; Gulcin, I.; Sahin, E.; Menzek, A. Synthesis and biological evaluation of bromophenol derivatives with cyclopropyl moiety: Ring opening of cyclopropane with monoester. Bioorg. Chem. 2019, 89, 103017. [Google Scholar] [CrossRef]
- Işık, M.; Demir, Y.; Durgun, M.; Türkeş, C.; Necip, A.; Beydemir, Ş. Molecular docking and investigation of 4-(benzylideneamino)-and 4-(benzylamino)-benzenesulfonamide derivatives as potent AChE inhibitors. Chem. Pap. 2020, 74, 1395. [Google Scholar] [CrossRef]
- Topal, F.; Gulcin, I.; Dastan, A.; Guney, M. Novel eugenol derivatives: Potent acetylcholinesterase and carbonic anhydrase inhibitors. Int. J. Biol. Macromol. 2017, 94, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, U.M.; Okten, S.; Cakmak, O.; Burhan, G.; Atas, M.; Taslimi, P.; Gulcin, I. Arylated quinoline and tetrahydroquinolines: Synthesis, characterization and their metabolic enzyme inhibitory and antimicrobial activities. ChemistrySelect 2022, 7, 202203469. [Google Scholar] [CrossRef]
- Yamali, C.; Gul, I.H.; Cakir, T.; Demir, Y.; Gulcin, I. Aminoalkylated phenolic chalcones: Investigation of biological effects on acetylcholinesterase and carbonic anhydrase I and II as potential lead enzyme inhibitors. Lett. Drug Des. Discov. 2020, 17, 1283–1292. [Google Scholar] [CrossRef]
- Ozaslan, M.S.; Saglamtas, R.; Demir, Y.; Genç, Y.; Saraçoglu, I.; Gulcin, I. Isolation of some phenolic compounds from Plantago subulata L. and determination of their antidiabetic, anticholinesterase, antiepileptic and antioxidant activity. Chem. Biodivers. 2022, 19, e202200280. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Aktaş, A.; Taslimi, P.; Gulcin, I.; Gok, Y. Novel NHC precursors: Synthesis, characterization, and carbonic anhydrase and acetylcholinesterase inhibitory properties. Arch. Pharm. 2017, 350, e1700045. [Google Scholar] [CrossRef]
- Lolak, N.; Akocak, S.; Turkes, C.; Taslimi, P.; Isik, M.; Beydemir, Ş.; Gulcin, I.; Durgun, M. Synthesis, characterization, inhibition effects, and molecular docking studies as acetylcholinesterase, α-glycosidase, and carbonic anhydrase inhibitors of novel benzenesulfonamides incorporating 1,3,5-triazine structural motifs. Bioorg. Chem. 2020, 100, 103897. [Google Scholar] [CrossRef]
- Cetin Cakmak, K.; Gulcin, I. Anticholinergic and antioxidant activities of usnic acid-an activity-structure insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Bytyqi-Damoni, A.; Kestane, A.; Taslimi, P.; Tüzün, B.; Zengin, M.; Genç Bilgiçli, H.; Gulçin, İ. Novel carvacrol based new oxypropanolamine derivatives: Design, synthesis, characterization, biological evaluation, and molecular docking studies. J. Mol. Struct. 2020, 1202, 127297. [Google Scholar] [CrossRef]
- Yigit, B.; Kaya, R.; Taslimi, P.; Isik, Y.; Karaman, M.; Yigit, M.; Ozdemir, I.; Gulcin, I. Midazolinium chloride salts bearing wingtip groups: Synthesis, molecular docking and metabolic enzymes inhibition. J. Mol. Struct. 2019, 1179, 709–718. [Google Scholar] [CrossRef]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 184221–184229. [Google Scholar] [CrossRef]
- Erdoğan, M.; Polat Köse, L.; Eşsiz, S.; Gulcin, I. Synthesis and biological evaluation of some 1-naphthol derivatives as antioxidants, acetylcholinesterase, and carbonic anhydrase inhibitors. Arch. Pharm. 2021, 354, e2100113. [Google Scholar] [CrossRef] [PubMed]
- Demir, Y.; Duran, H.E.; Durmaz, L.; Taslimi, P.; Beydemir, Ş.; Gulcin, I. The influence of some nonsteroidal anti-inflammatory drugs on metabolic enzymes of aldose reductase, sorbitol dehydrogenase, and α-glycosidase: A perspective for metabolic disorders. Appl. Biochem. Biotechnol. 2020, 190, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Burmaoglu, S.; Yilmaz, A.O.; Taslimi, P.; Algul, O.; Kılıç, D.; Gulcin, I. Synthesis and biological evaluation of phloroglucinol derivatives possessing α-glycosidase, acetylcholinesterase, butyrylcholinesterase, carbonic anhydrase inhibitory activity. Arch. Pharm. 2018, 351, e1700314. [Google Scholar] [CrossRef]
- Guney, M.; Coskun, A.; Topal, F.; Dastan, A.; Gulcin, I.; Supuran, C.T. Oxidation of cyanobenzocycloheptatrienes: Synthesis, photooxygenation reaction and carbonic anhydrase isoenzymes inhibition properties of some new benzotropone derivatives. Bioorg. Med. Chem. 2014, 22, 3537–3543. [Google Scholar] [CrossRef]
- Nar, M.; Cetinkaya, Y.; Gulcin, I.; Menzek, A. (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives as carbonic anhydrase isoenzymes inhibitors J. Enzyme Inhib. Med. Chem. 2013, 28, 402–406. [Google Scholar] [CrossRef]
- Sahin, I.; Bingol, Z.; Onur, S.; Gungor, S.A.; Kose, M.; Gulcin, I.; Tumer, F. Enzyme inhibition properties and molecular docking studies of 4-sulfonate containing aryl α-hydroxyphosphonates based hybrid molecules. Chem. Biodivers. 2022, 19, e202100787. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).