Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Ultrasonic Extraction and Liquid–Liquid Extraction
2.3. Selection of Antisolvent for Recrystallization
2.4. Single-Factor Experiment
2.5. RSM Experiment
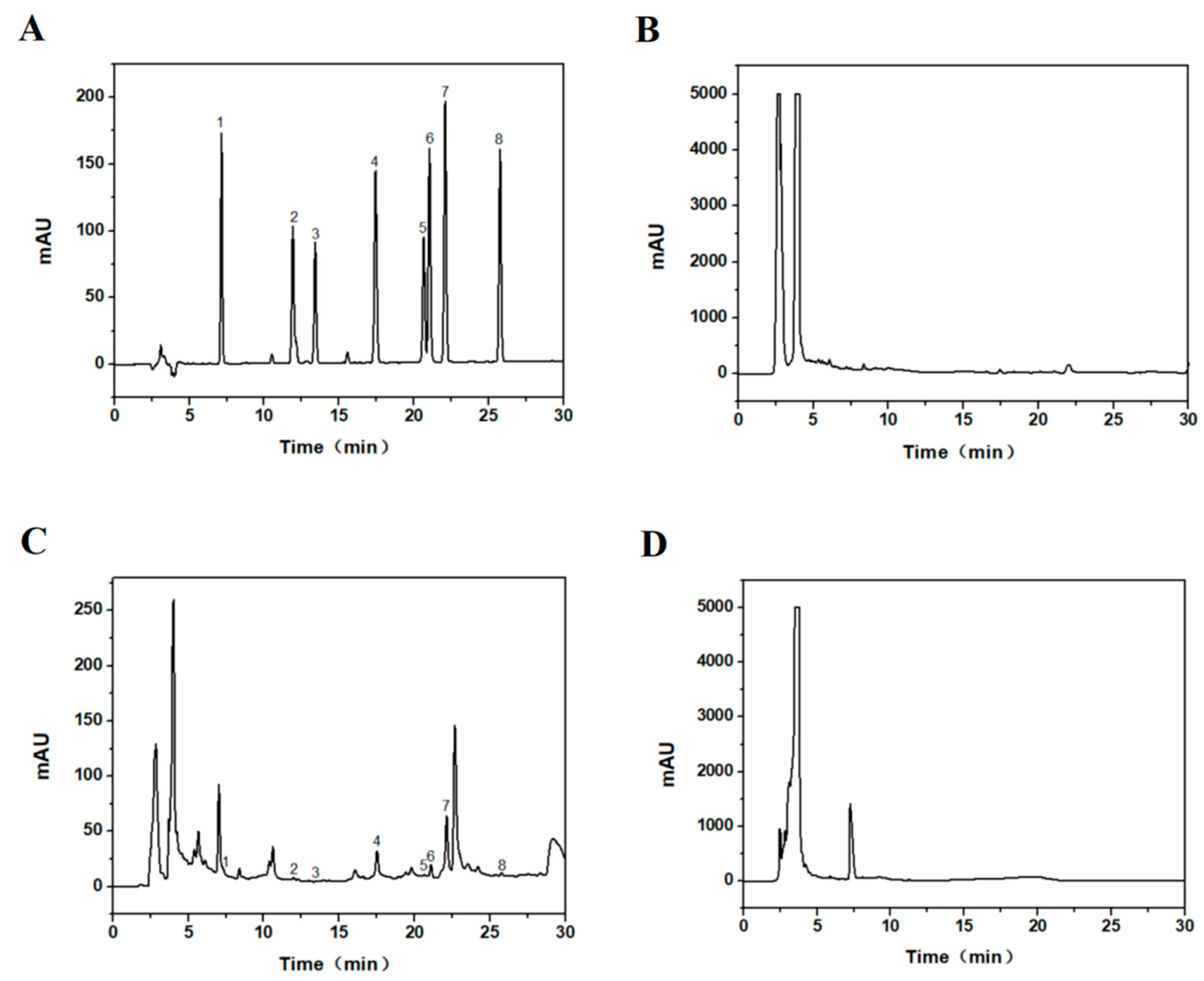
2.6. HPLC Detection of Taxane Yields
2.7. SEM
2.8. XRD
2.9. Raman Spectrum
3. Results
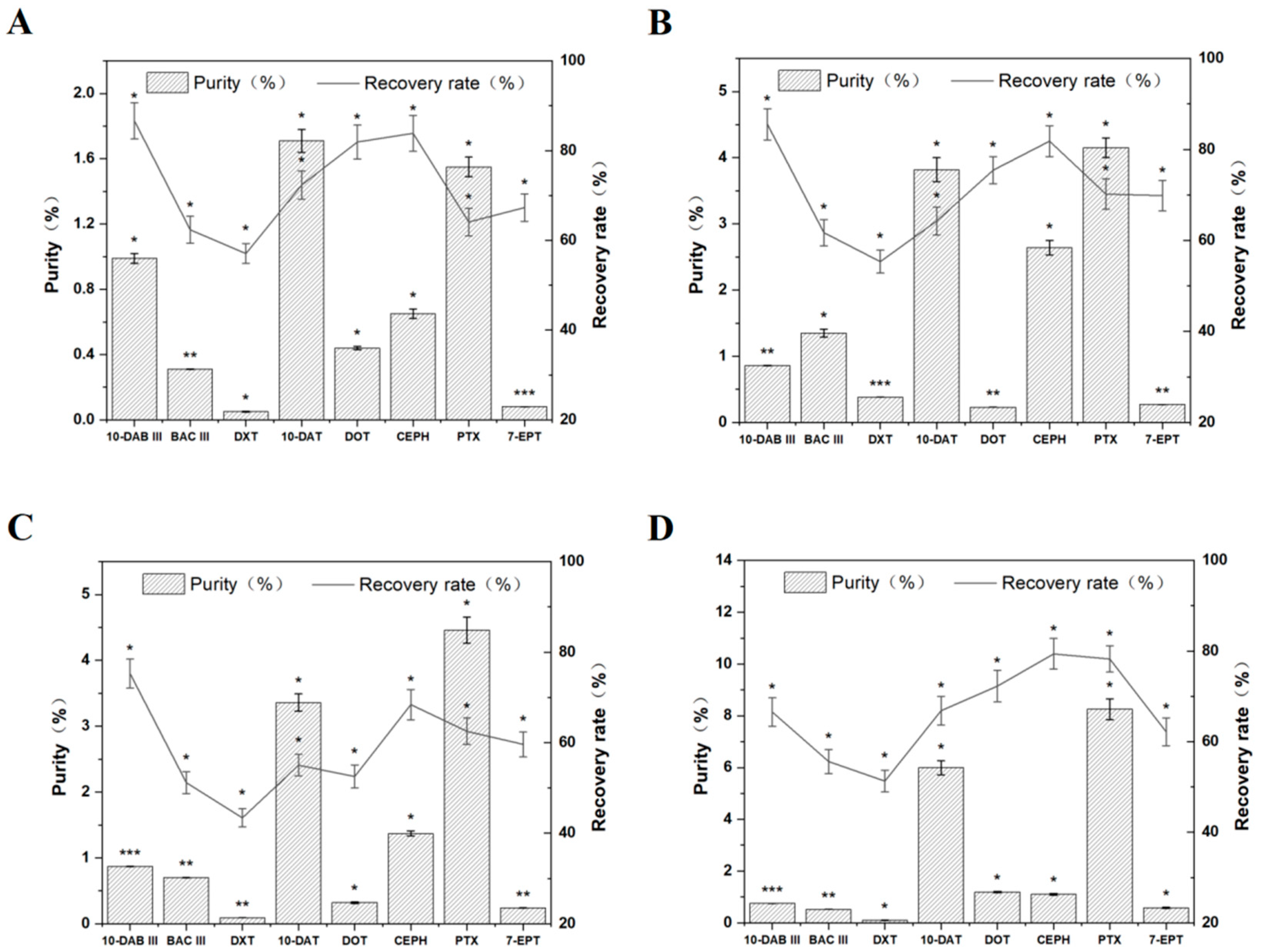
3.1. Selection of the Best Recrystallization Antisolvent
3.2. Analysis of Taxus cuspidata before and after Recrystallization by HPLC
3.3. Single-Factor Experiments
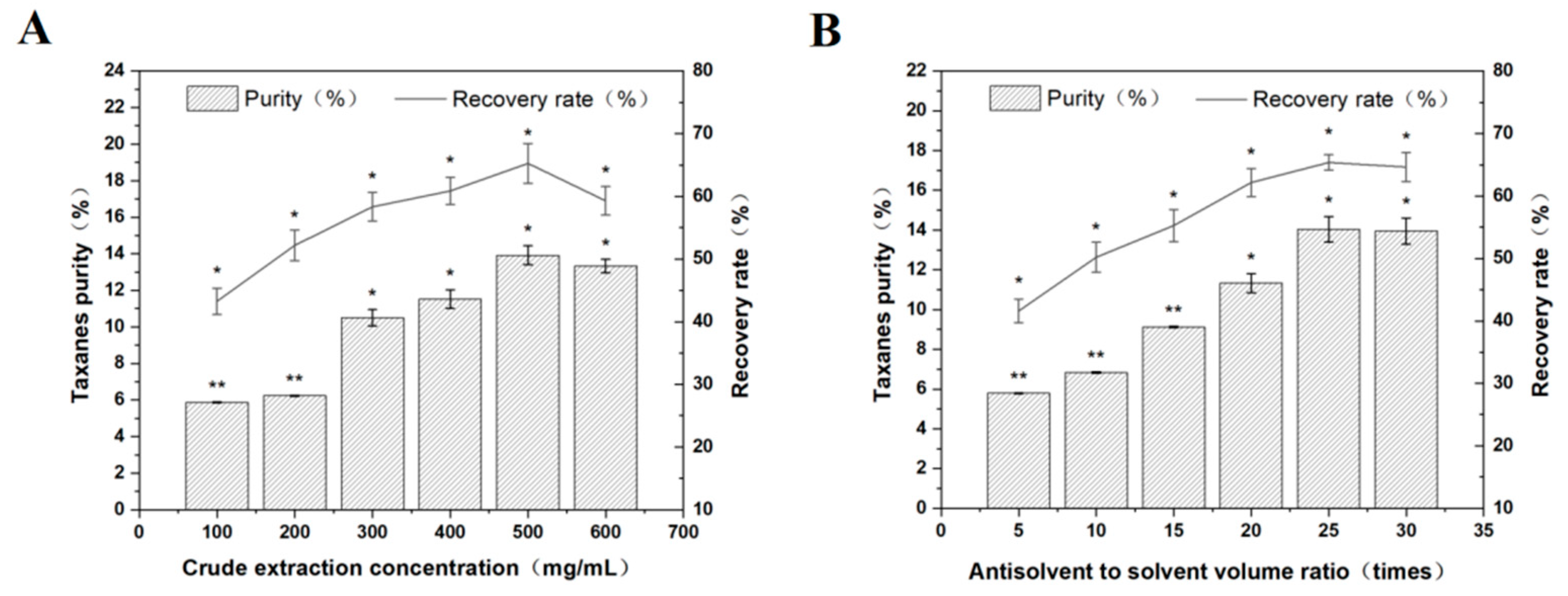
3.3.1. Crude Extraction Concentration
3.3.2. Antisolvent to Solvent Volume Ratio
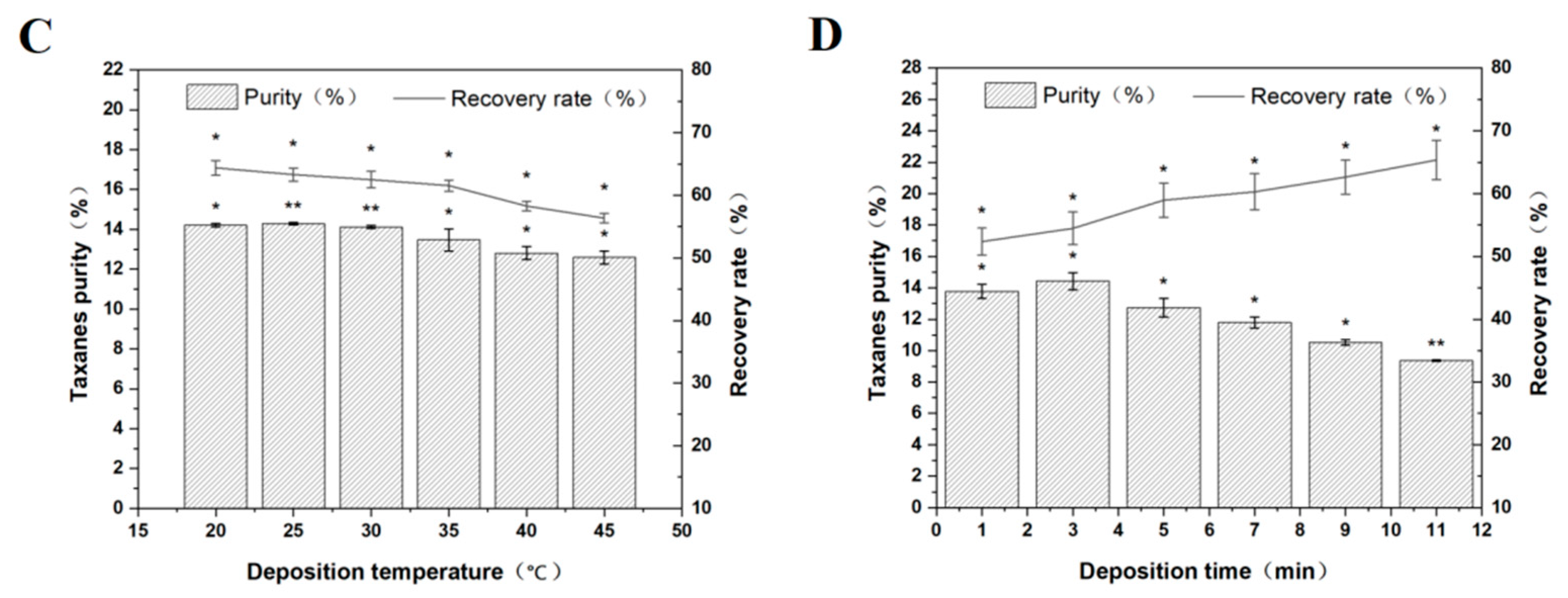
3.3.3. Deposition Temperature
3.3.4. Deposition Time
3.4. Construction of the RSM Model and the Optimization of Conditions
3.5. SEM Analysis of Taxus cuspidata before and after Recrystallization
3.6. XRD and Raman Spectroscopic Analysis of the Crude and Recrystallization Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, R.W.; Powell, R.G.; Smith, C.R.; Arnold, E.; Clardy, J. Antileukemic alkaloids from Taxus wallichiana zucc. J. Org. Chem. 1981, 46, 1469–1474. [Google Scholar] [CrossRef]
- Lange, B.M.; Conner, C.F. Taxanes and taxoids of the genus Taxus–A comprehensive inventory of chemical diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Zhuang, W.; Zhang, F.; Wang, Z. Recent research progress in taxol biosynthetic pathway and acylation reactions mediated by Taxus acyltransferases. Molecules 2021, 26, 2855. [Google Scholar] [CrossRef] [PubMed]
- Fett-Neto, A.G.; Dicosmo, F.; Reynolds, W.F.; Sakata, K. Cell culture of taxus as a source of the antineoplastic drug taxol and related taxanes. Bio/Technology 1992, 10, 1572–1575. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Butcher, M. Functional specialization in the forelimbs of two digging mammals: The american badger (taxidea taxus) and groundhog (marmota monax). FASEB J. 2011, 25, 3515–3524. [Google Scholar] [CrossRef]
- Liu, D.; Guo, Z.; Cui, X.; Fan, C. Comparison of five associations of Taxus cuspidata and their species diversity. Biodivers. Sci. 2020, 28, 94–105. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, J.; Liu, D.; Lu, A.; Wu, W. Identification of Taxus cuspidata Sieb.et Zucc endophytic fungi-new recorded-genus-species of China and the metabolite. J. For. Res. 2004, 15, 61–66. [Google Scholar] [CrossRef]
- Xiang, Y.; Lu, A.; Wu, W. Identification of Taxus cuspidata sieb. et Zucc. endophytic fungi-new species, species known and their metabolite. J. For. Res. 2003, 14, 290–294. [Google Scholar] [CrossRef]
- Cheng, Q.; Oritani, T.; Horiguchi, T. Four Novel Taxane Diterpenoids from the Needles of Japanese Yew, Taxus cuspidata. Biosci. Biotechnol. Biochem. 1999, 64, 894–898. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, Y.; Xiong, J.; Wang, F.; Yu, X. Extraction, Purification, and Biological Activities of Flavonoids from Branches and Leaves of Taxus cuspidata S. et Z. Bioresources 2021, 16, 2655–2682. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Zheng, T.; Guo, X.; Tang, Z. Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry. Open Life Sci. 2021, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Fang, W.; Zhou, J.; He, C.; Chen, W.; Fang, Q. Three New Taxane Diterpenoids from Needles and Stems of Taxus cuspidata. J. Nat. Prod. 2004, 58, 233–238. [Google Scholar] [CrossRef]
- Kawamura, F.; Kikuchi, Y.; Ohira, T.; Yastagai, M. Phenolic constituents of Taxus cuspidata I: Lignans from the roots. J. Wood Sci. 2000, 46, 167–171. [Google Scholar] [CrossRef]
- Ni, Z.; Wu, Y.; Dong, M.; Zhang, M.; Wang, Y.; Françoise, S.; Huo, C.; Shi, Q.; Gu, Y.; Hiromasa, K.; et al. Diabietane ether, a new dimeric abietane with an ether linkage from Taxus cuspidata needles. Z. Naturforsch. B. 2011, 66, 1083–1086. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Guo, W.; Li, Y.; Liu, X.; Wang, N.; Liu, B. Genomic diversity within Taxus cuspidata var. nana revealed by random amplified polymorphic DNA markers. Russ. J. Plant Physiol. 2006, 53, 684–688. [Google Scholar] [CrossRef]
- Lenka, S.K.; Nims, N.E.; Vongpaseuth, K.; Boshar, R.A.; Roberts, S.C.; Walker, E.L. Jasmonate-responsive expression of paclitaxel biosynthesis genes in Taxus cuspidata cultured cells is negatively regulated by the bHLH transcription factors TcJAMYC1, TcJAMYC2, and TcJAMYC4. Front. Plant Sci. 2015, 6, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Kishiko, O.; Sanro, T.; Masaya, S. Electron Microscopic Observation of Plastid Containing Taxol-like Substances in Callus Cells of Taxus cuspidata var. Nana. Pak. J. Biol. Sci. 2004, 7, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhou, Z. Characteristics of natural Japanese yew population in Muling Nature Reserve of Heilongjiang Province, China. J. For. Res. 2006, 17, 132–134. [Google Scholar] [CrossRef]
- Shi, Q.; Oritani, T.; Sugiyama, T. Three novel bicyclic taxane diterpenoids with verticillene skeleton from the needles of Chinese yew, Taxus chinensis var. Mairei. Planta Med. 1999, 62, 1114–1118. [Google Scholar] [CrossRef]
- Yao, X.; Da, B.; Chen, J.; Li, X.; Tang, B. Tissue culture and analysis on taxane diterpenoids in callus of Taxus chinensis var. Mairei, Chin. Tradit. Herb. Drugs. 2014, 45, 2696–2702. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L.; Chang, Y.; An, J.; Fu, Y. Application of green and recyclable menthol-based hydrophobic deep eutectic solvents aqueous for the extraction of main taxanes from Taxus chinensis needles. J. Mol. Liq. 2020, 326, 114–122. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, M.; Wang, Y.; Huo, C.; Shi, Q. A New Taxane from the Hard Wood of Taxus cuspidata. Z. Naturforsch. B. 2010, 65, 635–638. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, J.; Liang, Z.; Zhang, W.; Zhang, L.; Huo, Y.; Zhang, Y. Separation and identification of Taxol in the crude extracts of Taxus cuspidata and its callus culture with HPLC-ESI-MS/MS. Acta Pharmacol. Sin. 2008, 41, 863–866. [Google Scholar] [CrossRef]
- Shinjiro, Y.; Yuka, S.; Takato, K.; Shuhei, H.; Hitoshi, M. Enhanced productivity of paclitaxel and related taxanes in plant cell culture including aliphatic ionic liquids. Solvent Extr. Res. Dev. Jpn. 2018, 25, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Jerzy, K.; Atwal, A.S.; Subramaniam, M. Determination of Formaldehyde in Fresh and Retail Milk by Liquid Column Chromatography. J. AOAC Int. 1993, 76, 1010–1013. [Google Scholar] [CrossRef]
- Rokosik, E.; Dwiecki, K.; Rudzińska, M.; Siger, A.; Polewski, K. Column chromatography as a method for minor components removal from rapeseed oil. Grasas Aceites. 2019, 70, 9182. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Huang, X.; Jing, H.; Ye, X.; Jiang, C.; Shao, J.; Ma, C.; Wang, H. Separation of epigallocatechin gallate and epicatechin gallate from tea polyphenols by macroporous resin and crystallization. Anal. Methods. 2021, 13, 832–842. [Google Scholar] [CrossRef]
- Ke, C.; Ren, Y.; Gao, P.; Han, J.; Yang, X. Separation and purification of pyrroloquinoline quinone from fermentation broth by pretreatment coupled with macroporous resin adsorption. Sep. Purif. Technol. 2021, 257, 117962. [Google Scholar] [CrossRef]
- Lee, C.G.; Kim, J.H. Improved fractional precipitation method for purification of paclitaxel. Process Biochem. 2014, 49, 1370–1376. [Google Scholar] [CrossRef]
- Watchueng, J.; Kamnaing, P.; Gao, J.M.; Kiyota, T.; Yeboah, F.; Konishi, Y. Efficient purification of paclitaxel from yews using high-performance displacement chromatography technique. J. Chromatogr. A. 2011, 1218, 2929–2935. [Google Scholar] [CrossRef]
- Piletska, E.; Magumba, K.; Joseph, L.; Cruz, A.G.; Norman, R.; Singh, R.; Tabasso, A.F.S.; Jones, D.J.L.; Macip, S.; Piletsky, S. Molecular imprinting as a tool for determining molecular markers: A lung cancer case. RSC Adv. 2022, 12, 17747–17754. [Google Scholar] [CrossRef] [PubMed]
- Saeid, A.; Eun, J.B.; Sagor, M.S.A.; Rahman, A.; Akter, M.S.; Ahmed, M. Effects of Extraction and Purification Methods on Degradation Kinetics and Stability of Lycopene from Watermelon under Storage Conditions. J. Food Sci. 2016, 81, 2630–2638. [Google Scholar] [CrossRef] [PubMed]
- Lubaina, A.S.; Renjith, P.R.; Roshni, A.S.; Thompson, J. Identification and Quantification of Polyphenols from Pineapple Peel by High Performance Liquid Chromatography Analysis. Adv. Zool. Bot. 2020, 8, 431–438. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Luo, S. Preparative separation and enrichment of four taxoids from Taxus chinensis needles extracts by macroporous resin column chromatography. J. Sep. Sci. 2009, 32, 1284–1293. [Google Scholar] [CrossRef]
- Luebbert, C.; Sadowski, G. Moisture-induced phase separation and recrystallization in amorphous solid dispersions. Int. J. Pharm. 2017, 532, 635–646. [Google Scholar] [CrossRef]
- Karmwar, P.; Graeser, K.; Gordon, K.C.; Strachan, C.J.; Rades, T. Investigation of properties and recrystallisation behaviour of amorphous indomethacin samples prepared by different methods. Int. J. Pharm. 2011, 417, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Punochová, K.; Vukosavljevic, B.; Jaroslav, H.; Beránek, J.; Frantiek, T. Non-invasive insight into the release mechanisms of a poorly soluble drug from amorphous solid dispersions by confocal Raman microscopy. Eur. J. Pharm. Biopharm. 2016, 101, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Yadava, U.; Gupta, H.; Roychoudhury, M. Stabilization of Microtubules by Taxane Diterpenoids: Insight from Docking and MD simulations. J. Biol. Phys. 2015, 41, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wu, B.; Deng, W.; Liu, Y. Optimization of Ellagic Acid Purification from Pomegranate Husk by Antisolvent Recrystallization. Chem. Eng. Technol. 2018, 41, 1181–1198. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, M.; Feng, Z.; Wang, L.; Huang, Y. Purification of Ginkgo biloba Extract by Antisolvent Recrystallization. Chem. Eng. Technol. 2016, 39, 1301–1308. [Google Scholar] [CrossRef]
- Tarbe, M.; Pomyers, H.D.; Mugnier, L.; Bertin, D.; Mabrouk, K. Gram-scale purification of aconitine and identification of lappaconitine in Aconitum karacolicum. Fitoterapia 2017, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Alexander, W.; Samuel, A.O.; Felix, A.; Kwbena, D.; Salifu, S.L. Response surface methodology as a tool for optimization of the extraction of bioactive compounds from plant sources. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Xue, J.; Fan, E. Plant Phenolics Extraction from Flos Chrysanthemi: Response Surface Methodology Based Optimization and the Correlation Between Extracts and Free Radical Scavenging Activity. J. Food Sci. 2017, 82, 2726–2733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, G.; Yin, X.; Peng, X.; Li, C.; Wang, T.; Feng, J.; Ju, R. Response Surface Methodology Optimized Pilot Plant Extraction Process of 1-Deoxynojirimycin from Mulberry Leaves. J. Biobased Mater. Bioenergy 2019, 13, 207–213. [Google Scholar] [CrossRef]
- Kwak, H.J.; Park, S.J.; Kim, Y.N.; Yoo, G.; Jeong, E.J.; Kim, S.H. Optimization of extraction conditions for enhancing estrogenic activity of Rheum undulatum Linné using response surface methodology. Sep. Sci. Technol. 2020, 55, 2080–2089. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Meng, H.; Wang, S. Ultrasonic Extraction and Separation of Taxanes from Taxus cuspidata Optimized by Response Surface Methodology. Separations 2022, 9, 193. [Google Scholar] [CrossRef]
- Yazdani, F.; Edrissi, M. Effect of pressure on the size of magnetite nanoparticles in the coprecipitation synthesis. Mater. Sci. Eng. 2010, 171, 86–89. [Google Scholar] [CrossRef]
- Zhu, Y.; Demilie, P.; Davoine, P.; Paule, M. Application of density meter in the supersaturation determination of the two-component equilibrium systems. J. Cryst. Growth. 2004, 263, 459–465. [Google Scholar] [CrossRef]
- Fevotte, G.; Gherras, N.; Moutte, J. Batch cooling solution crystallization of ammonium oxalate in the presence of impurities: Study of solubility, supersaturation, and steady-state inhibition. Cryst. Growth Des. 2013, 13, 2737–2748. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, J.M. Ultrasound-Assisted Micellar Extraction for Paclitaxel Purification from Taxus chinensis. Korean Inst. Chem. Eng. 2021, 59, 106–111. [Google Scholar] [CrossRef]
- Min, H.S.; Kim, J.H. Negative pressure cavitation fractional precipitation for the purification of paclitaxel from Taxus chinensis. Korean J. Chem. Eng. 2022, 39, 58–62. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.H. Development of an improved acetone-water fractional precipitation process for purification of paclitaxel from Taxus chinensis and its kinetic and thermodynamic analysis. Korean Chem. Eng. Res. 2021, 59, 379–392. [Google Scholar] [CrossRef]
- Park, G.Y.; Kim, G.J.; Kim, J.H. Effect of tar compounds on the purification efficiency of paclitaxel from Taxus chinensis. J. Ind. Eng. Chem. 2015, 21, 151–154. [Google Scholar] [CrossRef]
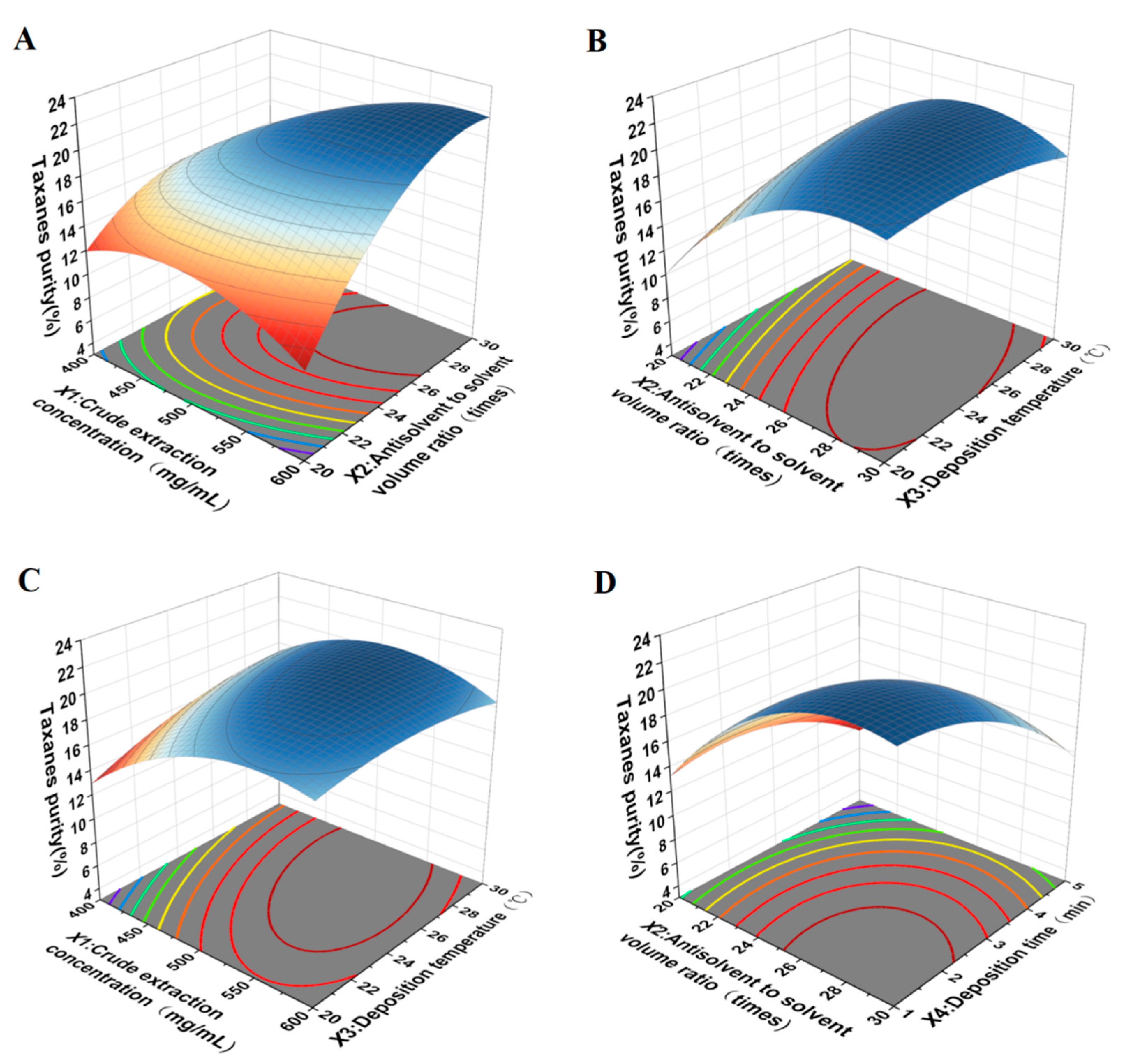
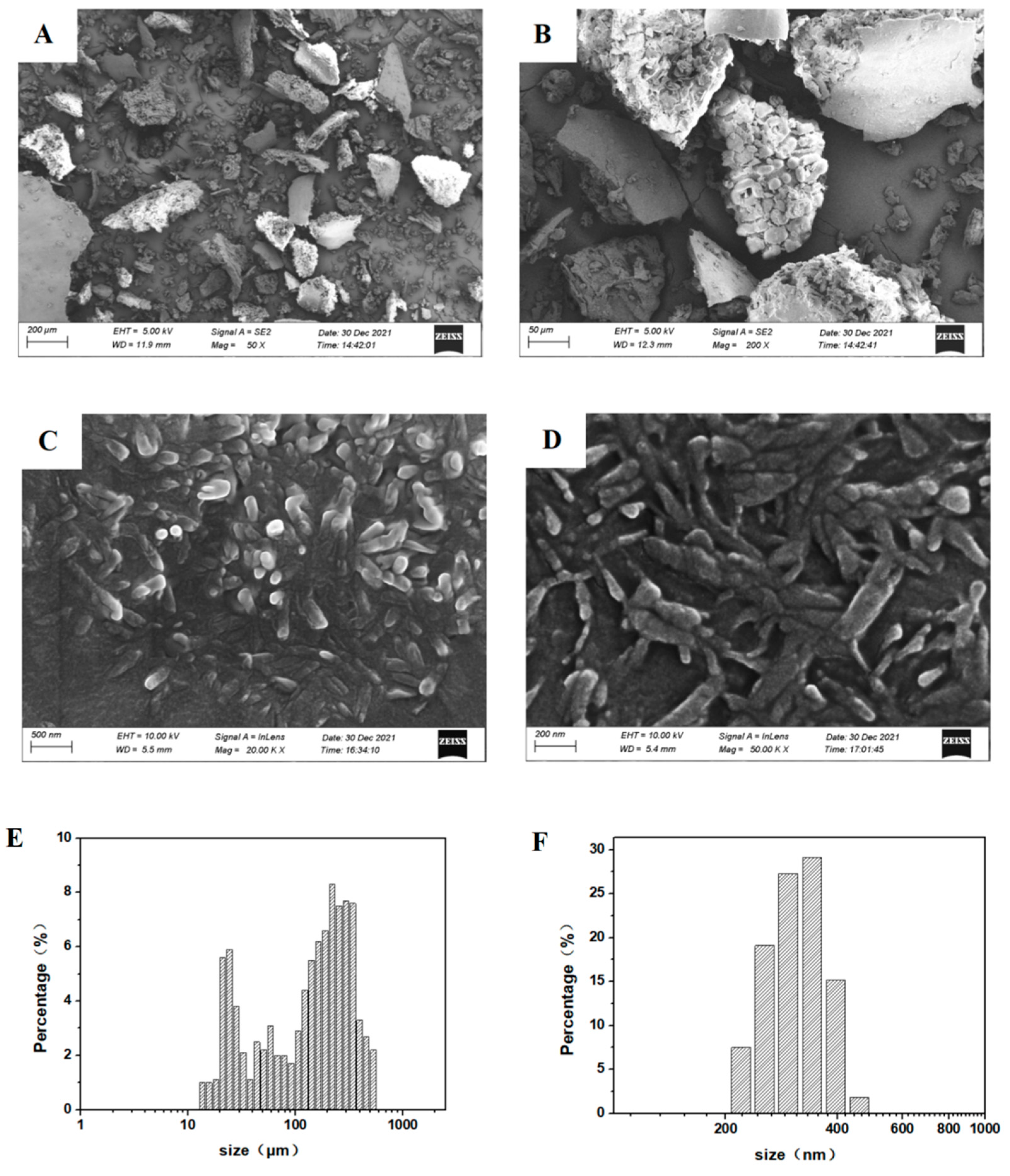
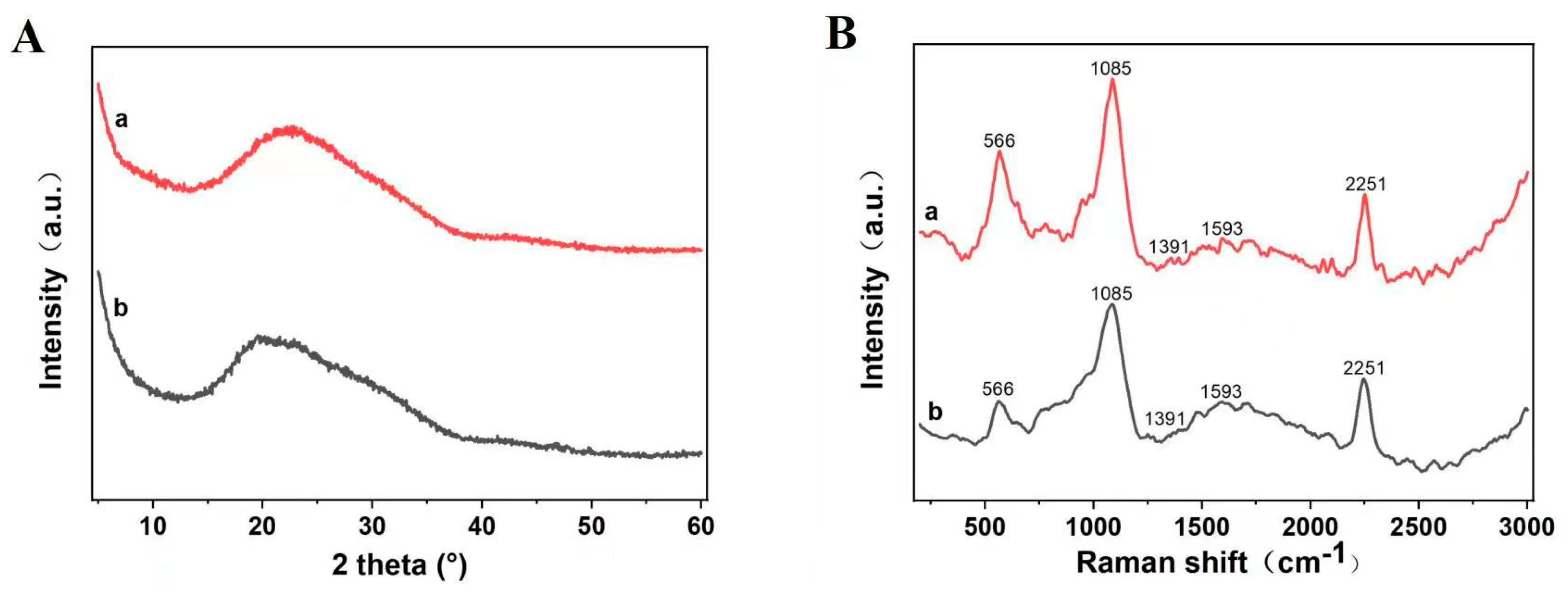
| Run | X1 | X2 | X3 | X4 | Taxanes Purity (%) |
|---|---|---|---|---|---|
| 1 | 400.00 | 25.00 | 25.00 | 5.00 | 10.6358 |
| 2 | 400.00 | 20.00 | 25.00 | 3.00 | 12.5983 |
| 3 | 400.00 | 25.00 | 25.00 | 1.00 | 17.3129 |
| 4 | 500.00 | 30.00 | 30.00 | 3.00 | 19.1577 |
| 5 | 600.00 | 25.00 | 30.00 | 3.00 | 18.3369 |
| 6 | 500.00 | 30.00 | 25.00 | 5.00 | 13.1014 |
| 7 | 500.00 | 25.00 | 25.00 | 3.00 | 20.3471 |
| 8 | 500.00 | 25.00 | 30.00 | 1.00 | 21.2453 |
| 9 | 500.00 | 20.00 | 25.00 | 1.00 | 13.3078 |
| 10 | 500.00 | 25.00 | 25.00 | 3.00 | 19.1931 |
| 11 | 600.00 | 20.00 | 25.00 | 3.00 | 10.4839 |
| 12 | 500.00 | 25.00 | 20.00 | 5.00 | 12.5033 |
| 13 | 500.00 | 25.00 | 25.00 | 3.00 | 20.6922 |
| 14 | 500.00 | 25.00 | 20.00 | 1.00 | 18.7964 |
| 15 | 500.00 | 20.00 | 25.00 | 5.00 | 10.1201 |
| 16 | 600.00 | 25.00 | 25.00 | 5.00 | 13.985 |
| 17 | 500.00 | 20.00 | 30.00 | 3.00 | 14.0391 |
| 18 | 500.00 | 30.00 | 20.00 | 3.00 | 21.1741 |
| 19 | 400.00 | 25.00 | 30.00 | 3.00 | 16.7901 |
| 20 | 500.00 | 20.00 | 20.00 | 3.00 | 9.5607 |
| 21 | 400.00 | 25.00 | 20.00 | 3.00 | 12.4958 |
| 22 | 500.00 | 25.00 | 25.00 | 3.00 | 19.6704 |
| 23 | 500.00 | 25.00 | 25.00 | 3.00 | 21.2981 |
| 24 | 600.00 | 30.00 | 25.00 | 3.00 | 20.9644 |
| 25 | 600.00 | 25.00 | 25.00 | 1.00 | 20.3397 |
| 26 | 500.00 | 25.00 | 30.00 | 5.00 | 16.0481 |
| 27 | 400.00 | 30.00 | 25.00 | 3.00 | 12.7904 |
| 28 | 600.00 | 25.00 | 20.00 | 3.00 | 18.5439 |
| 29 | 500.00 | 30.00 | 25.00 | 1.00 | 21.0477 |
| Source of Variation | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 436.06 | 14 | 31.15 | 37.52 | <0.0001 | *** |
| X1 | 33.44 | 1 | 33.44 | 40.28 | <0.0001 | *** |
| X2 | 121.13 | 1 | 121.13 | 145.91 | <0.0001 | *** |
| X3 | 13.11 | 1 | 13.11 | 15.79 | 0.0014 | ** |
| X4 | 105.95 | 1 | 105.95 | 127.62 | <0.0001 | *** |
| X1X2 | 26.46 | 1 | 26.46 | 31.88 | <0.0001 | *** |
| X1X3 | 5.07 | 1 | 5.07 | 6.10 | 0.0270 | * |
| X1X4 | 0.026 | 1 | 0.026 | 0.031 | 0.8621 | |
| X2X3 | 10.55 | 1 | 10.55 | 12.70 | 0.0031 | ** |
| X2X4 | 5.66 | 1 | 5.66 | 6.82 | 0.0205 | * |
| X3X4 | 0.30 | 1 | 0.30 | 0.36 | 0.5572 | |
| X12 | 43.89 | 1 | 43.89 | 52.87 | <0.0001 | *** |
| X22 | 78.00 | 1 | 78.00 | 93.96 | <0.0001 | *** |
| X32 | 5.54 | 1 | 5.54 | 6.68 | 0.0216 | * |
| X42 | 31.55 | 1 | 31.55 | 38.00 | <0.0001 | *** |
| Residual | 11.62 | 14 | 0.83 | |||
| Lack of fit | 8.87 | 10 | 0.89 | 1.29 | 0.4353 | |
| Pure error | 2.76 | 4 | 0.69 | |||
| Total | 447.68 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations 2022, 9, 304. https://doi.org/10.3390/separations9100304
Zhang Y, Zhao Z, Li W, Tang Y, Meng H, Wang S. Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations. 2022; 9(10):304. https://doi.org/10.3390/separations9100304
Chicago/Turabian StyleZhang, Yajing, Zirui Zhao, Wenlong Li, Yuanhu Tang, Huiwen Meng, and Shujie Wang. 2022. "Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method" Separations 9, no. 10: 304. https://doi.org/10.3390/separations9100304
APA StyleZhang, Y., Zhao, Z., Li, W., Tang, Y., Meng, H., & Wang, S. (2022). Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations, 9(10), 304. https://doi.org/10.3390/separations9100304





