Abstract
A novel, sensitive, and low-cost HPLC method for the rapid determination of favipiravir (FVR) in rat plasma was developed and validated, and the effect of vitamin C on FVR pharmacokinetic parameters was investigated. FVR and oxcarbazepine (IS) were separated using a mobile phase of 50% acetonitrile and 50% water (with 0.25% trifluoroacetic acid) at 1.0 mL/min flow rate and detected at λmax 289 nm. The intra- and interday values for FVR in plasma were less than 15%, with low, medium, and high QC levels for the relative recovery rate, according to ICH guidelines. Cmax values in the control and experimental groups were 558 ± 124.42 and 979.13 ± 138.10 ng/mL, respectively; t1/2 values were 7.15 ± 1.60 and 9.09 ± 1.14 h, AUC(0-t) values were 5697.70 ± 536.58 and 7381.62 ± 1577.58 ng.h/mL, and AUC(0-∞) values were 5697.70 ± 536.58 and 8192.36 ± 1721.67, respectively. According to the results, the experimental group’s Cmax of FVR was 75.17% higher than the control group’s, the Vz/F was lower, and the t1/2 was 1.86 h longer. The technique developed for determining FVR in plasma was useful for FVR pharmacokinetics and food–drug interaction investigations.
1. Introduction
The global coronavirus disease 2019 (COVID-19) pandemic has encouraged researchers to work on developing and validating medications or vaccinations to prevent or slow the spread of this fatal disease [1,2]. The potentially lethal illness is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. Vitamin C (Vit C) was identified as important in the earlier coronavirus epidemic caused by severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) [4]. Interleukin-6 and endothelin-1 levels are elevated in many COVID-19 patients [5]. These mediators might explain why COVID-19 pneumonia is age-dependent and the predominance of males, obese or hypertensive individuals, people of color, and cigarette smoking [6]. There is substantial proof that large doses of Vit C can inhibit these mediators [7].
Furthermore, Vit C is inexpensive and non-hazardous. As a result, as a prophylactic, a relatively small dosage of Vit C is recommended, and in severe cases of COVID-19, an intravenous regimen of a high dose might be helpful [7,8]. Clinical studies are now underway that are expected to give more conclusive data. Similar to the causing of SARS-linked acute respiratory distress syndrome (ARDS), in SARS-CoV-2, inherent immunity is activated by contagion, resulting in a “cytokine storm” and the response of inflammation and failure of many organs [9,10,11]. A SARS retrospective review revealed that the worsening after two weeks was due to immunopathological damage rather than uncontrolled viral multiplication [12].
Favipiravir (FVR) is a synthetic prodrug found in Toyoma’s chemical library and was used to investigate the antiviral action of chemical compounds active against the virus of influenza. A/PR/8/34, a lead compound, was subsequently identified as T-1105, and its derivatives were shown to have antivirus properties [1,13,14]. FVR is prepared by chemical modification of the the pyrazine moiety of T-1105 (Figure 1). In 2014, it was licensed in Japan to treat influenza infections [14]. FVR is given as a prodrug. It has a high bioavailability (94%), protein binding (54%), and a limited volume of distribution (10–20 L). After a single dosage, it achieves Cmax in two hours. After several doses, both Tmax and half-life increase [15]. The hydroxylated version of FVR has a short half-life (2.5–5 h), resulting in fast renal clearance. Aldehyde oxidases and, to a lesser extent, xanthine oxidases are responsible for drug elimination. FVR pharmacokinetics are dose-dependent as well as time-dependent. The cytochrome P450 system does not metabolize FVR, but CYP2C8 does block one of its components. As a result, it must be taken with care when combined with medications metabolized by the system of CYP2C8 [14]. FVR (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is a powerful and specific inhibitor of RNA-dependent RNA polymerase (RdRp), a key enzyme in replicating RNA viruses. Toyama Chemical Co., Ltd., discovered FVR by screening a chemical library for antiviral activity against the influenza virus [16]. FUJIFILM and MediVector developed FVR on a global scale (FAV, FUJIFILM; Toyama Chemical, AdisInsight, n.d.). FVR (prodrug) is a purine rule analog that undergoes inside-cell phosphoribosylation to form active FAV ribofuranosyl-5B-triphosphate (FAV-RTP). It is a very specific and effective inhibitor of RNA virus RNA-dependent RNA polymerase (RdRp) [17].
Figure 1.
Chemical structure of (a) FAV, (b) OCBZ (IS), and (c) vitamin C.
Figure 1 depicts Vit C (ascorbic acid), a simple low-molecular-weight carbohydrate with an ene-diol structure making it a widespread and necessary water-soluble electron donor [18]. The discovery of Vit C is related to a long history of diligent search into the origin of the ancient hemorrhagic disease scurvy [19]. Vit C is required for the figuration and maintenance of connective tissues. It is found in reduced (ascorbate) and oxidized forms such as dehydro-ascorbic acid, both easily interconvertible and physiologically active. As a result, it serves as a significant antioxidant. Vit C is an oxidized acid that may be damaged by oxygen, alkali, and heat [19]. Vit C is a free radical scavenger that protects DNA, proteins, and lipids from oxidative damage. Vit C is an antioxidant used in the body but may have some roles in organs. Although vitamin C is known to have antioxidant effects, the clinical implication of its use in the setting of ARDS from viral pneumonia is not established [20,21]. There are reports of the use of high-dose intravenous vitamin C as part of treatment for ARDS associated with SARS-CoV2 infection, but no randomized controlled trials or concrete evidence supports its use [22]. The production of adverse effects from food–drug and drug–drug interactions can be used in the study of the efficacy of drug therapy, safety, and the patient’s nutritional status [23].
Antiviral medicines are among these pharmaceuticals, including therapeutic agents such as FVR and supporting agents such as ascorbic acid [24]. A method for evaluating the concentration of FVR in human plasma by HPLC was established [1]. In addition, another method for evaluating FVR in the presence of co-administered medication has been developed [25]. In the literature, no previous studies were reported concerning the combination pharmacokinetics of FAV and vitamin C. So, our aim of this study is to explore the pharmacokinetic parameters of FAV after single dosing and evaluate the possible effect that occurs if vitamin C changes the pharmacokinetics of FAV.
2. Materials and Methods
2.1. Materials
FAV (purity > 98%, CAS: 1971436-31-2) and oxcarbazepine (OCBZ, purity > 98%, CAS: 312172–10979-03) were procured from Sigma-USA (St. Louis, MO, USA). Triliptal tablets were procured from Novartis. FAV was obtained as Sancovir tablets (200 mg); Sana Pharma’s Sancovir (FAV) drug was recently approved by Jordan Food and Drug Administration. Vitamin C effervescent Redoxon tablet (Bayer) was purchased. Methanol (R-OH) and acetonitrile (ACN) were of HPLC grade and were purchased from Merck (Darmstadt, Germany).
2.2. Chromatographic Conditions and Instrumentation
After experimenting with numerous permutations of flow rate, column temperature, mobile phase composition, wavelength, and chromatography column, the ideal conditions were determined. The analysis method was completed using an Agilent 1100-12 liquid chromatographic system equipped with a G-1480-9A vacuum degasser, G1481-1A quaternary pump, G1486-1A column oven, G1818-1A auto-sampler, and G1813-BL DAD detector. A ZORBAX Eclipse XDB-C18 (4.6 × 150 mm, 5.0 μm, Agilent, Santa Clara, CA, USA) chromatographic column was used for separation; the separation temperature at 38 °C. A mixture of 50% ACN and 50% water (with 0.25% trifluoroacetic acid) was used as a chromatographic mobile phase with a 1.0 mL/min flow rate. The optimum λ-max for the detection was set at 289 nm.
2.3. Standard and Quality Preparation Control (QC) Samples
After being accurately weighed, 25 mg of FVR was dissolved in 25 mL of methanol, which yielded a stock solution of 1.0 mg/mL. Methanol was used to dilute the stock solution to prepare concentrations of 100, 10, and 1 µg/mL. Separately, OCBZ (1 mg) was dissolved in 1 mL of methanol and then diluted to 4.0 µg/mL standard solution. The solutions were all kept in a fridge at 5.0 °C. Standard calibration curves were formed by incorporating suitable quantities of the working solutions into rat plasma. FVR samples were prepared in concentrations of 10.0, 25.0, 50.0, 100.0, 250.0, 500.0, 1000.0, and 2000.0 ng/mL, whereas IS concentration was 400.0 ng/mL. QC samples were prepared the same way, with three levels of plasma concentration (25.0, 500.0, and 1500.0 ng/mL).
2.4. Preparation of Sample
The samples were thawed at room temperature before analysis. An aliquot of 20.0 µL of the IS working solution (4 µg/mL) and 200 µL of NaOH (1M) were added to 200 µL of a collected sample of plasma in a 2 mL Eppendorf tube. After that, 1 mL of ethyl acetate was added. The tubes were vortexed for 1 min before being centrifuged at 4000 rpm for 8 min at 5 °C. An aliquot of 900 µL supernatant organic layer was then transferred into an Eppendorf tube and dried at 40 °C under a stream of nitrogen. After redissolving the dried residue in the mobile phase volume of 100 µL, a 10 µL aliquot was injected into the HPLC for analysis.
2.5. Method of Validation
The method’s selectivity was examined, and FVR and OCBZ were identified in spiked and blank plasma. By examining the spiked calibration of samples in a row for three days, calibration curves were developed and confirmed [26]. The ratio between the analyte and standard internal areas was plotted on the y-axis, the analyte (FVR) concentrations were plotted on the x-axis, and the standard curve was fitted using weighted (1/χ2) least-squares linear regression (range of 10–2000 ng/mL) of concentration. In six replicates, accuracy and precision were assessed by analyzing QC samples at three concentration points (25, 500, and 1500 ng/mL). Intraday precision was estimated on the same day, and interday precision was measured continuously for 72 h. FVR recovery was evaluated at 3 QC levels (25.0, 500.0, and 1500.0 ng/mL, n = 8) by comparing peak analytics in the sample with the analyte added before extraction with the equivalent solution after extraction. Every concentration recovery should have an RSD of less than 15%. The stabilization of FVR in plasma was tested by examining six plasma replicate samples at three levels of concentration (25, 500, and 1500 ng/mL) under various conditions. In the beginning, the short-term stability of the spiked samples was assessed after 24 h of exposure at room temperature. The freeze–thaw stability was assessed following three complete freeze–thaw cycles (20 °C) on sequential days. Finally, the samples’ long-range standard serrated plasma durability was evaluated after 20 days of storage at 20 °C.
2.6. Animals
Male Sprague–Dawley rats weighing 225 ± 25 g were procured from the Applied Science University (ASU) in Amman. For one week in the laboratory, the rats were acclimatized to their new surroundings. The tests were approved by Petra University’s Institutional Animal Ethics Committee (date: 1 April 2021, No. 2020-04/13RR).
2.7. System Suitability Criteria
The method relies on being capable of distinguishing the FVR and OCBZ peaks apart from one another. Well retained (k’ values >> 1) and very well separated, the FVR and OCBZ peaks are not critical to performance. There was no discernable asymmetry among the peaks. Selectivity values (α) for the critical distances are satisfactory. Indicative of a system well-suited for high-performance quantitative analysis, the resolution factors for the corresponding critical separations are around 0.5, corresponding to the baseline resolution (i.e., Rs ≈ 1.0).
2.8. Planning of the Study
Sixteen male Sprague–Dawley rats were randomly divided into two groups: Group A (experimental group receiving 5 mL/kg of Vit C juice for seven days) and Group B (control group receiving normal saline for seven days). A single dose of FVR (5 mg/kg) was administered to all rats. At time points 0.25, 0.50, 0.75, 1.25, 1.50, 2, 3, 6, 9, 12, 15, 18, 21, 24, and 48 h following FVR oral treatment, blood samples (0.3 mL) were withdrawn from the tail vein into heparinized 1.5 mL polythene tubes. The samples were directly centrifuged for 10 min at 10,000 rpm. The collected plasma (200 µL) was kept at 20 °C until analysis.
2.9. Statistical Analysis
The mean and standard deviation (S.D.) were used for the results. The compartmental analysis used DAS 2.0 (Drug and Statistics) software to calculate the pharmacokinetic parameters. The statistical analyses were evaluated by unpaired t-test (SPSS 19.0, Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Sensitivity
The LLOQ of FVR in rat plasma was found at 10 ng/mL. Significant results with high linearity and low variability are evidenced in a sensitive method with low LLOQ (10 ng/mL). The absence of interference at the retention time of blank plasma, FVR, and OCBZ (IS) confirmed the method’s sensitivity.
3.2. Linearity and Selectivity
Over the 10–2500 ng/mL concentration range, the peak area vs. concentration ratio was fitted with linear regression. The result showed the calibration curve’s usual equations: y = 0.01239x + 0.06381, r2 = 0.999. FVR and OCBZ were well isolated from endogenous materials from the experimental conditions. Figure 2 demonstrates an example of chromatograms from a blank plasma sample, a plasma sample spiked with FVR and OCBZ, and a sample from rats taken 1 h following oral administration of FVR. FVR and OCBZ had retention times of 6.464 and 5.441 min, respectively.
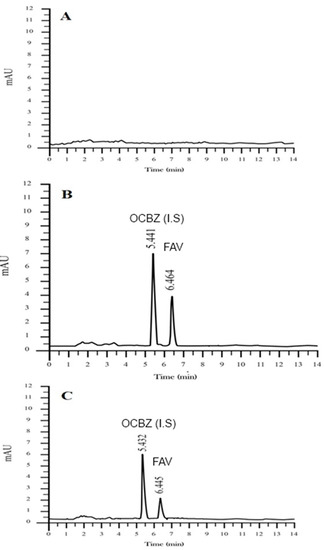
Figure 2.
HPLC chromatograms for FVR and OCBZ (I.S) in rat plasma: (A)—chromatogram of blank; (B)—chromatogram of blank spiked with FVR (30 µg/mL) and OCBZ and (C)—chromatogram of rat plasma sample after oral administration of FVR and OCBZ.
3.3. Precision and Accuracy
The accuracy and precision data evidenced that the assay’s repeatability, intermediate precision, and bias values were within the acceptable limits of ±20% at the LOQ and ±15% at other concentration levels [27]. The method’s precision was evaluated by estimating RSD values for QCs at three concentration levels (25, 500, and 1500 ng/mL) for three days. The intraday and interday precision for FVR at concentration levels 25, 500, and 1500 ng/mL in rat plasma were found in the ranges of 95.39–103.56% and 89.23–104.60%, respectively. At three concentrations, the intraday RSDs were measured as 4.68%, 4.72%, and 3.96%, while the interday RSDs were 6.38%, 4.84%, and 3.74%, respectively. Table 1 shows the results of the assays. The findings showed that values were within a reasonable range, and the approach was precise and accurate.

Table 1.
Accuracy and precision for FVR of QC rat plasma samples.
3.4. Recovery
The mean extraction recoveries for FVR and OCBZ (IS) from plasma at three QC concentrations were found in the ranges of 60.76–64.18% and 62.68–63.55%, respectively, while RSD values were in the ranges of 2.16 to 2.48% and 2.19 to 2.91%, respectively (Table 2).

Table 2.
Recovery after extraction of FAV and OCBZ (IS).
3.5. Stability
The precision (RSDs) and accuracy (RE%, relative error, ((actual concentration observed − spiked concentration)/spiked concentration) × 100%) of three QC samples of plasma spiked with FVR (25, 500, and 1500 ng/mL) were found to be lower than 10%, and FVR demonstrated high plasma stability at room temperature for 24 h, after three freeze–thaw cycles, and after storage at −75 °C for four weeks (Table 3). The results of these studies indicated that rat plasma samples containing FVR could be handled in standard laboratory conditions without losing significant drug content.

Table 3.
Stability tests for determination of precision (RSDs) and accuracy (relative error, RE%) for FVR in stock solution.
3.6. Studies of the Impact of Vit C Juice on the Pharmacokinetics of FVR
Figure 3 depicts the mean plasma concentration–time profiles of FVR in the two treatment groups, namely Group A (experimental group receiving 5 mL/kg of Vit C juice for seven days) and Group B (control group receiving normal saline for seven days), following an oral dose of FVR (5 mg/kg). Table 4 shows the suitable pharmacokinetic parameters. FVR pharmacokinetic properties were considerably influenced by Vit C juice, as shown in Figure 3 and Table 4. Vitamin C juice enhanced the Cmax of FVR by 44.20% compared to the control. Furthermore, FVR AUC(0-∞) was elevated by 22.75%.
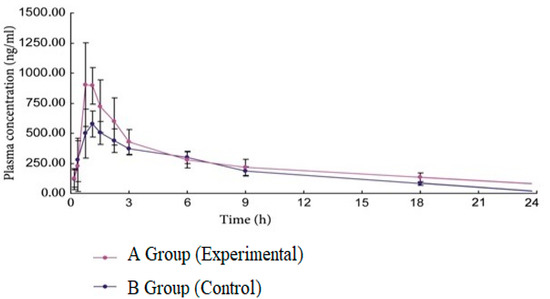
Figure 3.
The mean plasma concentration–time profiles of FVR after oral administration of FVR (5 mg/kg) in the different treatment groups.

Table 4.
Pharmacokinetic parameters of FAV and vitamin C after oral administration.
Furthermore, the Vz/F was lowered, and t1/2 was increased by 1.86 h. As the results show, the Vit C juice considerably impacts the pharmacokinetics of FVR in rats. Food–drug interactions can have a negative impact on the safety and efficacy of medication therapy, as well as the patient’s nutritional state [28]. The exact amount of adverse drug responses caused by food–drug interactions is unknown, and regrettably, the issue receives significant attention only when a severe adverse medication response occurs due to a food–drug interaction [29].
FVR is mainly metabolized by aldehyde oxidase and xanthine oxidase and excreted into urine [30]. Vit C is a frequent inhibitor of these metabolic enzymes, which retard drug metabolism via liver metabolism, resulting in higher drug concentrations in the bloodstream and more unpleasant effects [31,32]. Their potential to interact with vitamin C, like pharmaceuticals, must not be overlooked.
Despite considerable metabolism changes between types, animal investigations’ outcomes might give therapeutic medicine guidelines. As a result, while combining vitamin C juice with FVR, the medicine’s dosage should be modified to guarantee effectiveness and avoid unwanted effects. Specific pharmaceutical medications or supplements taken with food can significantly impact the therapy’s efficacy and safety. There is a lack of understanding of some interactions, particularly the implications of food–drug interactions [32].
4. Conclusions
FAV was detected in rat plasma using the HPLC-DAD technique in this investigation. HPLC-DAD is a simple, accurate, reproducible, and sensitive method with a short time of detection. It was appropriate for FAV pharmacokinetics and food–drug reaction investigations. Vit C juice may enhance FAV plasma exposure in the body, implying that vitamin C juice may decrease FAV metabolism. Patients taking FAV should be cautious of food–drug interactions when consuming vitamin C juice.
Author Contributions
Conceptualization, M.H. (Mohammad Hailat) and Z.Z.; methodology, R.A.-S.; software, W.A.D.; validation, O.A.-M., R.A.-S. and I.A.-A.; formal analysis, M.H. (Mohammed Hamad); investigation, R.A.; resources, M.H. (Mohammad Hailat) and W.A.D.; data curation, M.H. (Mohammad Hailat); writing—original draft preparation, M.H. (Mohammad Hailat); writing—review and editing, M.K.A.; visualization, R.A.; supervision, M.H. (Mohammad Hailat); project administration, W.A.R. and M.H. (Mohammad Hailat). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Petra University’s Institutional Animal Ethics Committee (date: 1 April, 2021, No. 2020-04/13RR).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hailat, M.; Al-Ani, I.; Hamad, M.; Zakareia, Z.; Abu Dayyih, W. Development and Validation of a Method for Quantification of Favipiravir as COVID-19 Management in Spiked Human Plasma. Molecules 2021, 26, 3789. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H. An Updated Review on Betacoronavirus Viral Entry Inhibitors: Learning from Past Discoveries to Advance COVID-19 Drug Discovery. Curr. Top. Med. Chem. 2021, 21, 571–596. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Bardaweel, S.K.; Zhong, H.A. An Updated Review on SARS-CoV-2 Main Proteinase (MPro): Protein Structure and Small-Molecule Inhibitors. Curr. Top. Med. Chem. 2021, 21, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; de Man, A.M.E. Vitamin C and COVID-19. Front. Med. 2021, 7, 1013. [Google Scholar] [CrossRef]
- Feyaerts, A.F.; Luyten, W. Vitamin C as prophylaxis and adjunctive medical treatment for COVID-19? Nutrition 2020, 79, 110948. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.Q. Cytokine Release Syndrome in Severe COVID-19: Interleukin-6 Receptor Antagonist Tocilizumab may be the Key to Reduce Mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Hoang, X.; Shaw, G.; Fang, W.; Han, B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J. Glob. Antimicrob. Resist. 2020, 23, 256–262. [Google Scholar] [CrossRef]
- Hamad, M.; Dayyih, W.A.; Raad, R.; Dayyih, A.; Al Ani, I.; Mallah, E.; Salih, H.; Zakarya, Z.; Arafat, T. The Effect of Some Fruit Juices on Glimepiride Pharmacokinetic in Rat Plasma by Using High Performance Liquid Chromatography-Mass Spectrometry. Biomed. Pharmacol. J. 2017, 10, 1665–1675. [Google Scholar] [CrossRef]
- Contini, C.; Caselli, E.; Martini, F.; Maritati, M.; Torreggiani, E.; Seraceni, S.; Vesce, F.; Perri, P.; Rizzo, L.; Tognon, M. COVID-19 Is a Multifaceted Challenging Pandemic Which Needs Urgent Public Health Interventions. Microorganisms 2020, 8, 1228. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; on behalf of the HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Rayyan, W.A. Seroprevalence of SARS-CoV-2 antibodies among Jordanian citizens: A cross-sectional study of the demographic and clinical factors that ameliorate serum IgG concentration. J. Appl. Pharm. Sci. 2022, 0, 1–6. [Google Scholar] [CrossRef]
- Peiris, J.; Chu, C.; Cheng, V.; Chan, K.; Hung, I.; Poon, L.; Law, K.; Tang, B.; Hon, T.; Chan, C.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef]
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-De-Hoyo, R. The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials. Sci. Rep. 2021, 11, 11022. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A new and emerging antiviral option in COVID-19. Med. J. Armed Forces India 2020, 76, 370–376. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Guedj, J.; Anglaret, X.; Laouénan, C.; Madelain, V.; Taburet, A.-M.; Baize, S.; Sissoko, D.; Pastorino, B.; Rodallec, A.; et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLOS Negl. Trop. Dis. 2017, 11, e0005389. [Google Scholar] [CrossRef]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B 2017, 93, 449–463. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18. [Google Scholar] [CrossRef]
- Carpenter, K.J. The Discovery of Vitamin, C. Ann. Nutr. Metab. 2012, 61, 259–264. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Tamimi, L.N.; Takruri, H.R.; Zakaria, Z.Z.; Abu Dayyih, W. Hepatic uptake, storage and release of vitamin A in humans. IJBPAS 2020, 9, 862–876. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020, 12, 100190. [Google Scholar] [CrossRef]
- Ayo, J.; Agu, H.; Madaki, I. Food and drug interactions: Its side effects. Nutr. Food Sci. 2005, 35, 243–252. [Google Scholar] [CrossRef]
- Wu, R.; Wang, L.; Kuo, H.-C.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 6, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Eldin, G.M.; Ismail, S.M.; Zine, N.; Elaissari, A.; Jaffrezic-Renault, N.; Errachid, A. Innovative electrochemical sensor for the precise determination of the new antiviral COVID-19 treatment Favipiravir in the presence of coadministered drugs. J. Electroanal. Chem. 2021, 895, 115422. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Ezzeldin, E.; Anwer, K.; Imam, F. Eco-Friendly UPLC-MS/MS Quantitation of Delafloxacin in Plasma and Its Application in a Pharmacokinetic Study in Rats. Separations 2021, 8, 146. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N.; Khan, A.Y. Food Drug Interactions. Oman Med. J. 2011, 26, 77–83. [Google Scholar] [CrossRef]
- Williams, L.; Davis, J.A.; Lowenthal, D.T. The influence of food on the absorption and metabolism of drugs. Med. Clin. North Am. 1993, 77, 815–829. [Google Scholar] [CrossRef]
- Mishima, E.; Anzai, N.; Miyazaki, M.; Abe, T. Uric Acid Elevation by Favipiravir, an Antiviral Drug. Tohoku J. Exp. Med. 2020, 251, 87–90. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Zhang, B.; Sanchez, R.; Golde, D.W. Vitamin C Is a Kinase Inhibitor: Dehydroascorbic Acid Inhibits IκBα Kinase β. Mol. Cell Biol. 2004, 24, 6645–6652. [Google Scholar] [CrossRef]
- Donovan, M. Pediatric and Adult Nutrition in Chronic Diseases, Developmental Disabilities, and Hereditary Metabolic Disorders. J. Nutr. Educ. Behav. 2018, 50, 323. [Google Scholar] [CrossRef]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).