Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
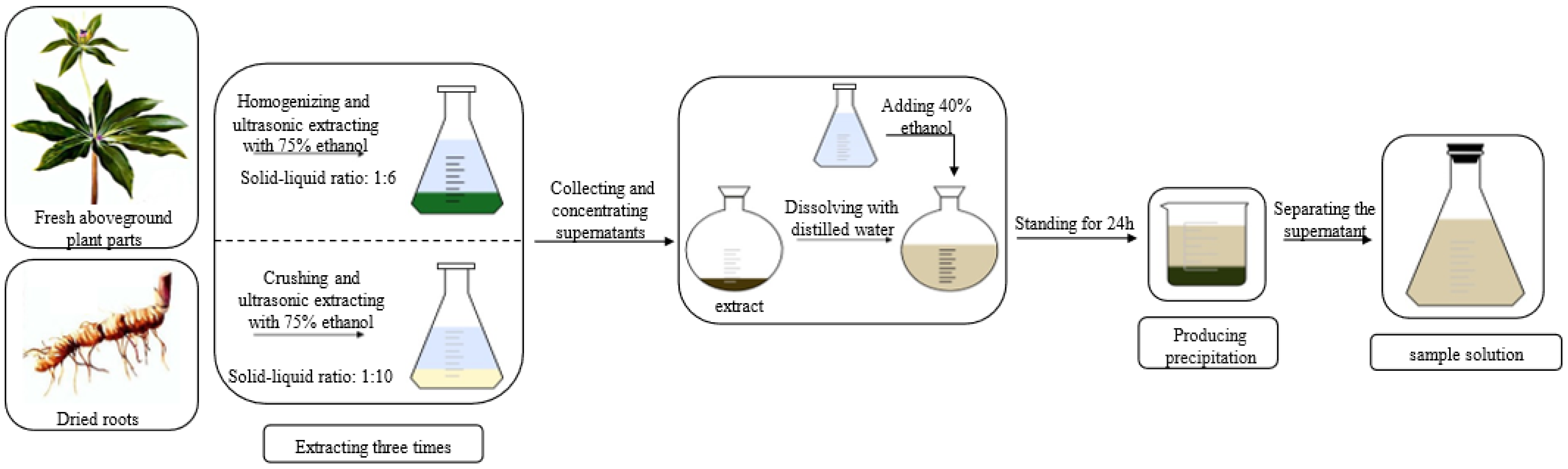
2.2. Preparation of Sample Solution
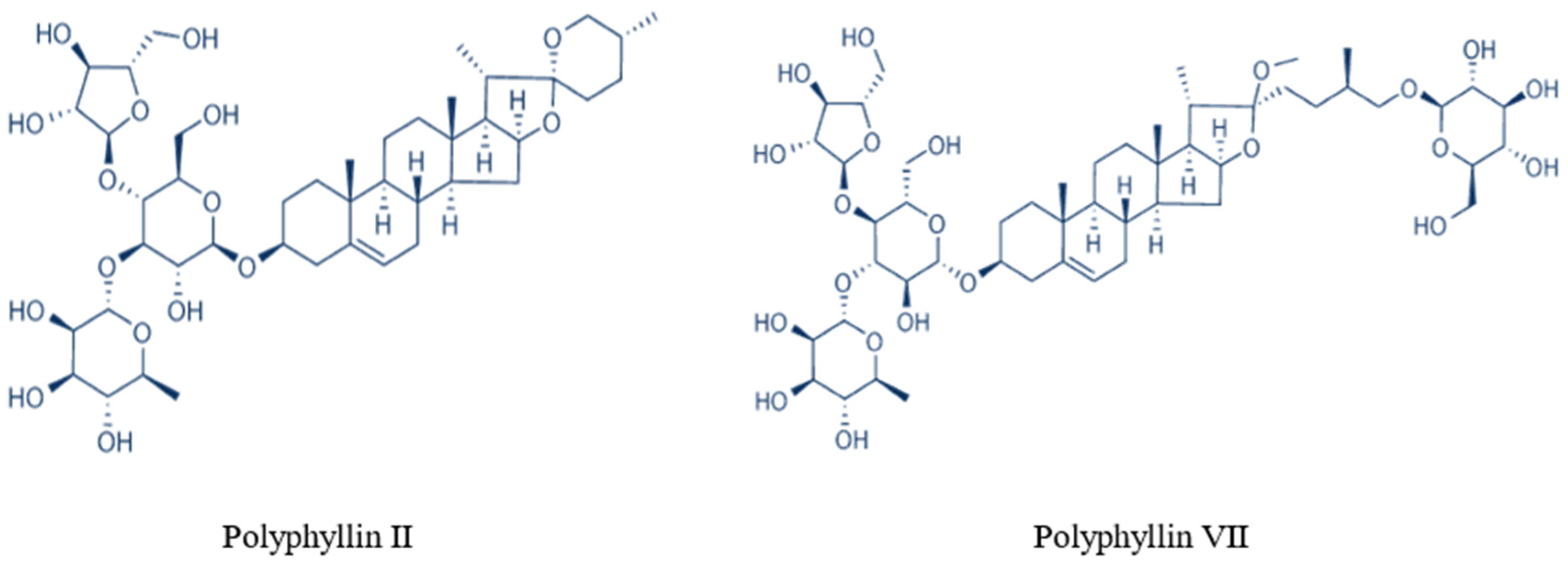
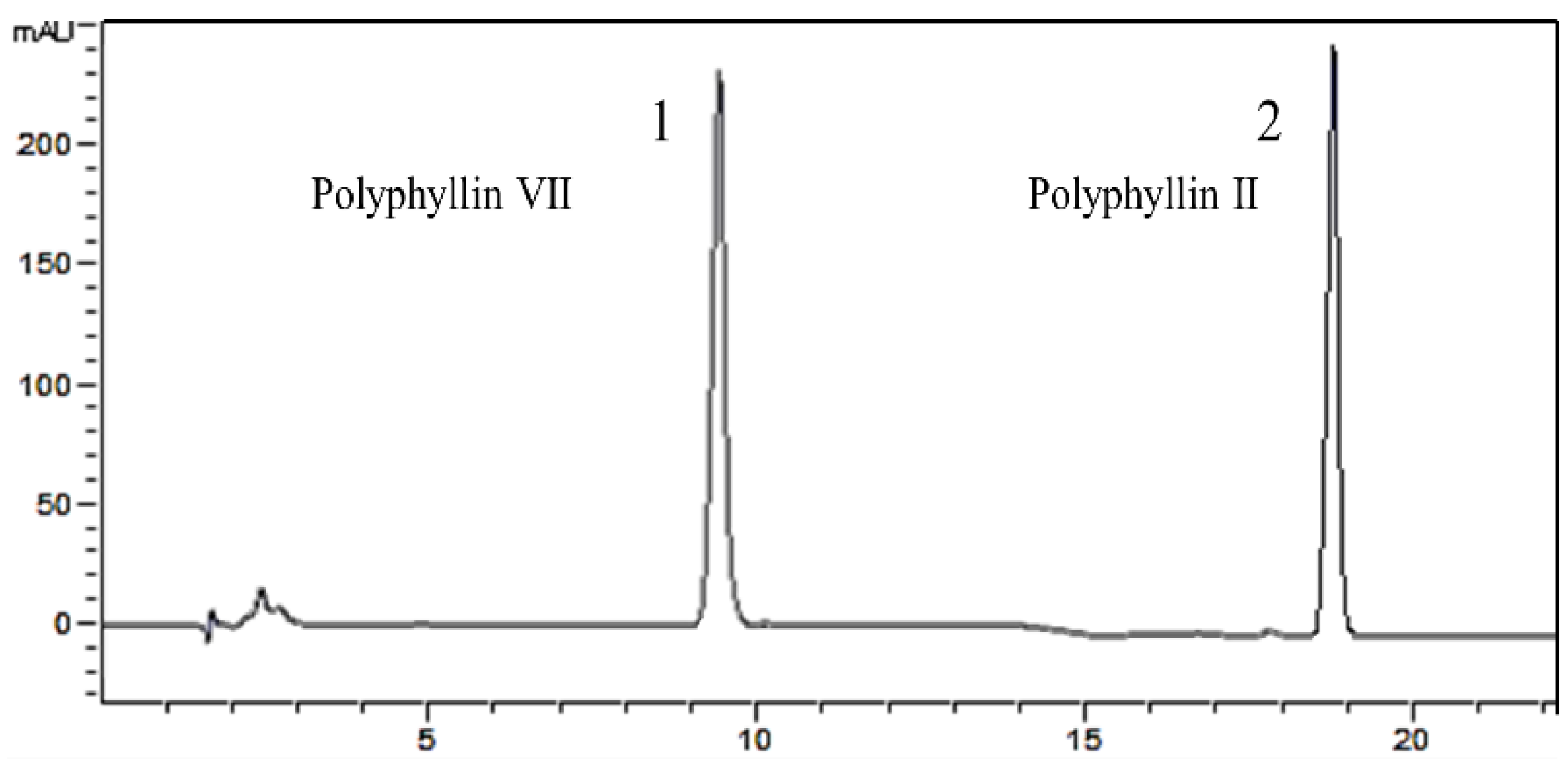
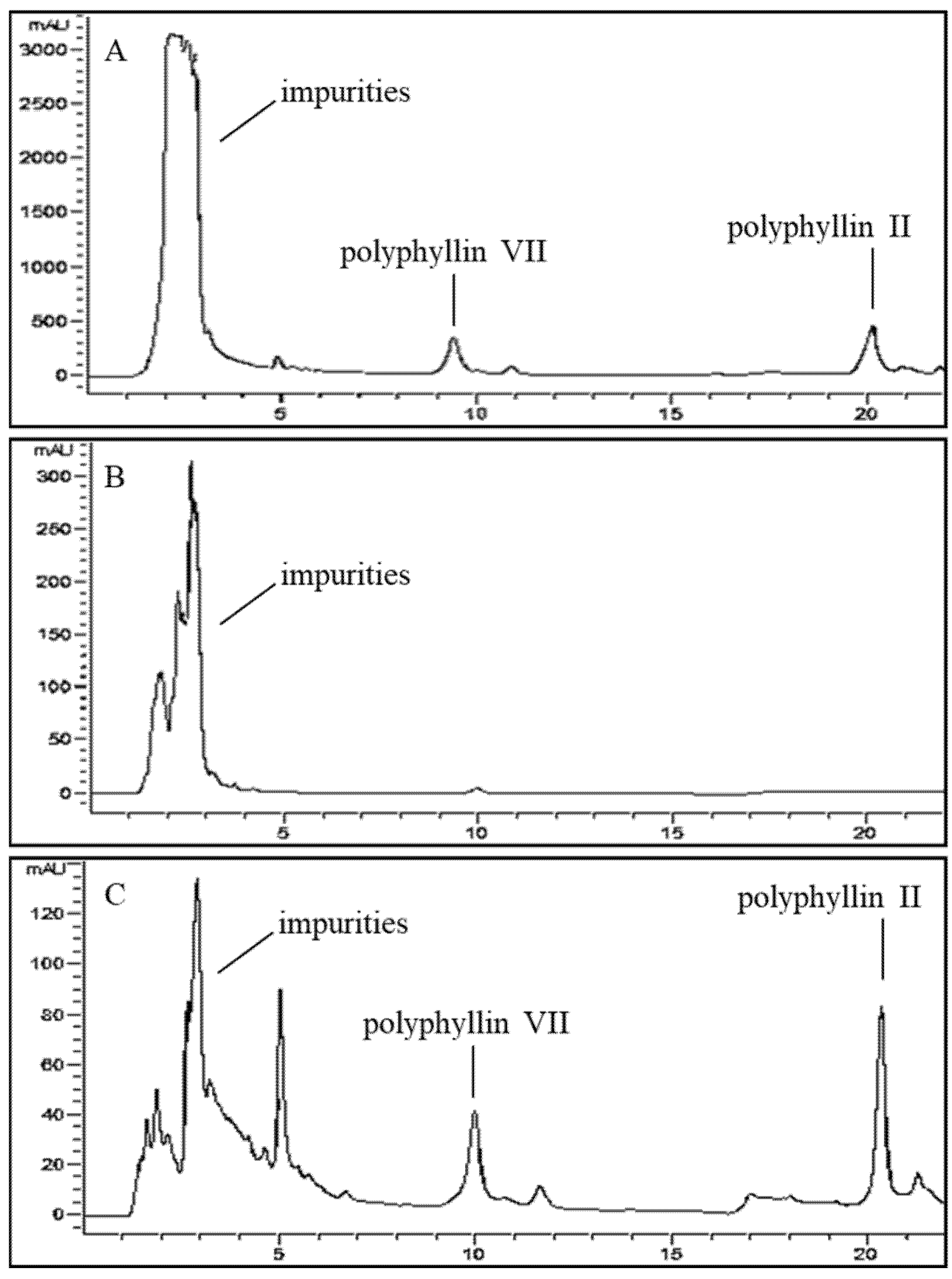
2.3. HPLC Analysis of Polyphyllin II and Polyphyllin VII
2.4. Optimization of Resins
2.5. Static Adsorption Kinetic and Fitting Equations
2.6. Isothermal Curve and Adsorption Thermodynamics Equation
2.7. Dynamic Adsorption-Desorption Behavior on Resin Column
2.8. Dynamic Data Fitting Adsorption Kinetic Equation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Static Adsorption and Desorption
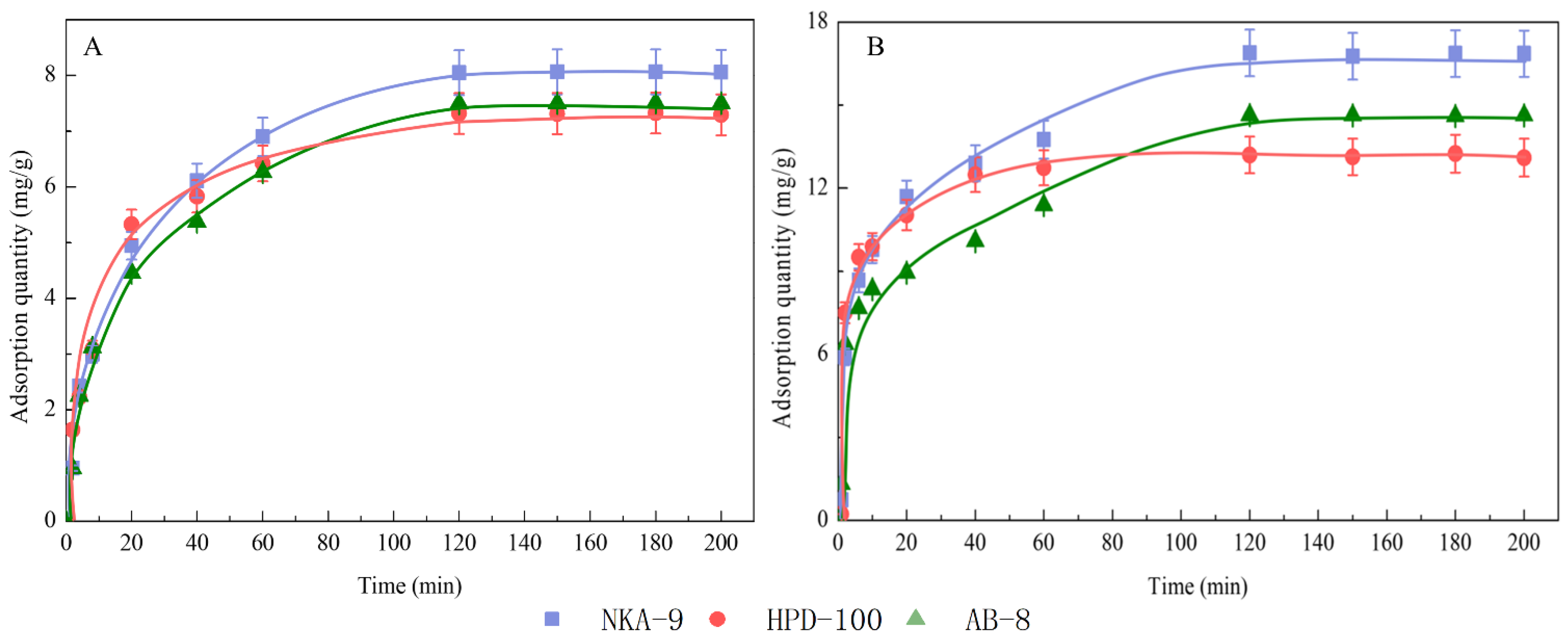
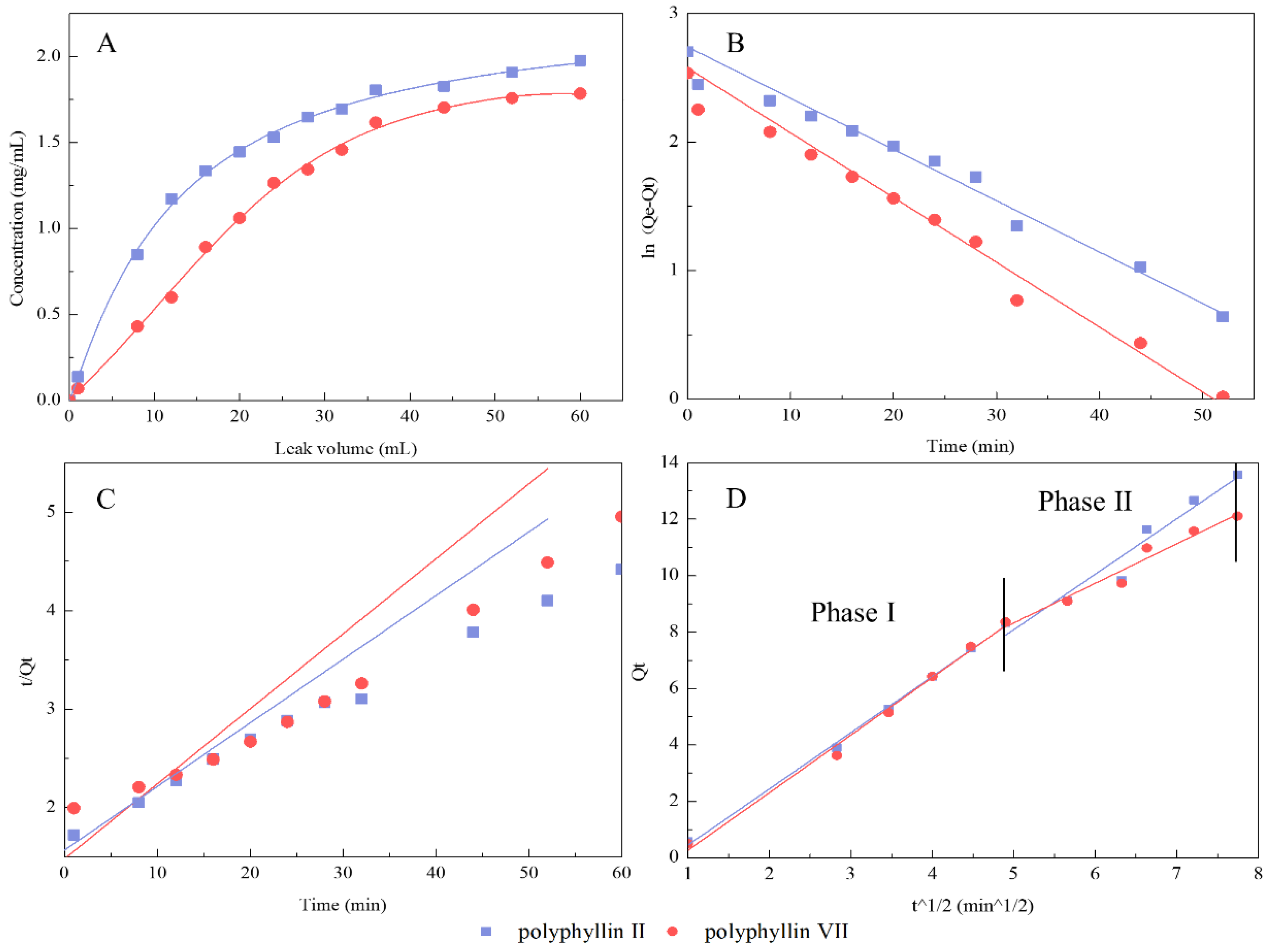
3.2. Adsorption Kinetic Curves and Equations
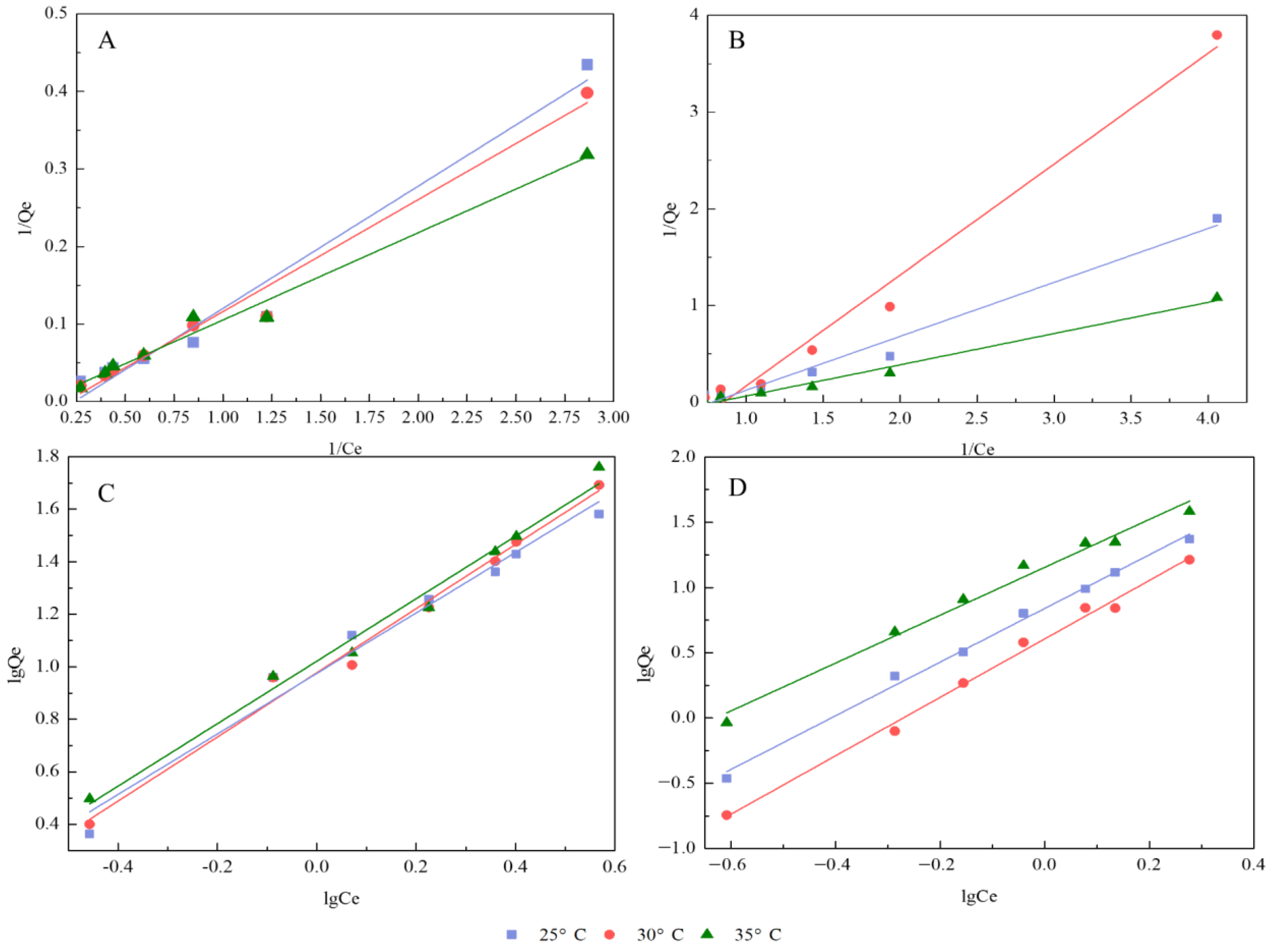
3.3. Adsorption Isotherms and Thermodynamics
3.4. Dynamic Leakage Curve and Kinetic Fitting Equations
3.5. Optimization of Dynamic Desorption Elution on NKA-9 Resin Column
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Balkhi, M.H.; Mohammad, M.A.; Tisserant, L.P.; Boitel-Conti, M. Development of a liquid-liquid extraction method of resveratrol from cell culture media using solubility parameters. Sep. Purif. Technol. 2016, 170, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.J.; Wei, Y.; Yuan, Q.P. Preparative separation of gallic acid from Chinese traditional medicine by high-speed counter-current chromatography and followed by preparative liquid chromatography. Sep. Purif. Technol. 2006, 55, 40–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Ouyang, X.; Chen, J.; Zhao, L.; Qiu, X. Separation of aromatic monomers from oxidatively depolymerized products of lignin by combining Sephadex and silica gel column chromatography. Sep. Purif. Technol. 2018, 191, 250–256. [Google Scholar] [CrossRef]
- Du, Z.; Wang, K.; Tao, Y.; Chen, L.; Qiu, F. Purification of baicalin and wogonoside from Scutellaria baicalensis extracts by macroporous resin adsorption chromatography. J. Chromatogr. B 2012, 908, 143–149. [Google Scholar] [CrossRef]
- Yang, F.; Yang, L.; Wang, W.; Liu, Y.; Zhao, C.; Zu, Y. Enrichment and Purification of Syringin, Eleutheroside E and Isofraxidin from Acanthopanax senticosus by Macroporous Resin. Int. J. Mol. Sci. 2012, 13, 8970–8986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, M.; Zhang, L. Adsorption/desorption characteristics and chromatographic purification of polyphenols from Vernonia patula (Dryand.) Merr. using macroporous adsorption resin. Ind. Crops Prod. 2021, 170, 113729. [Google Scholar] [CrossRef]
- Gao, M.; Huang, W.; Liu, C. Separation of scutellarin from crude extracts of Erigeron breviscapus (vant.) Hand. Mazz. by macroporous resins. J. Chromatogr. B 2007, 858, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, T.; Xiao, W.; Wang, Z.; Ding, G.; Zhao, L. Enrichment and Purification of Total Ginkgo Flavonoid O-Glycosides from Ginkgo Biloba Extract with Macroporous Resin and Evaluation of Anti-Inflammation Activities In Vitro. Molecules 2018, 23, 1167. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.H.; Silveira, D.; Ferreira-Dias, S. Selective adsorption of limonin and naringin from orange juice to natural and synthetic adsorbents. Eur. Food Res. Technol. 2002, 215, 462–471. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, R.; Li, J.; Yang, X.; He, J.; Wang, H.; Chang, Y. Separation and Enrichment of Three Coumarins from Angelicae pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity. Molecules 2019, 24, 2664. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, Y.; Li, M.; Yan, H. Selective adsorption of tea polyphenols from aqueous solution of the mixture with caffeine on macroporous crosslinked poly(N-vinyl-2-pyrrolidinone). React. Funct. Polym. 2007, 68, 768–774. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Zhang, L.; Zhou, J.; Yu, Y.; Zhang, S.; Zhou, Y. Green and efficient extraction of total glucosides from Paeonia lactiflora Pall. ‘Zhongjiang’ by subcritical water extraction combined with macroporous resin enrichment. Ind. Crops Prod. 2019, 141, 111699. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part 1; China Medical Science Press: Beijing, China, 2020; p. 260. [Google Scholar]
- Wang, W.; Liu, Y.; Sun, M.; Sai, N.; You, L.; Dong, X.; Yin, X.; Ni, J. Hepatocellular Toxicity of Paris Saponins I, II, VI and VII on Two Kinds of Hepatocytes-HL-7702 and HepaRG Cells, and the Underlying Mechanisms. Cells 2019, 8, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Meng, P.; Zhang, P.; Zheng, T.; Huang, L.; Ye, F.; Lei, P. The Pharmacokinetics and Tissue Distributions of Nine Steroidal Saponins from Paris polyphyllain Rats. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 665–673. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Chen, C.; Li, Z.; Chen, Y.; Zhang, X.; Wang, L.; Zhou, J. Polyphyllin III-Induced Ferroptosis in MDA-MB-231 Triple-Negative Breast Cancer Cells Can Be Protected against by KLF4-Mediated Upregulation of xCT. Front. Pharmacol. 2021, 12, 670224. [Google Scholar] [CrossRef] [PubMed]
- Thapa, C.B.; Paudel, M.R.; Bhattarai, H.D.; Pant, K.K.; Devkota, H.P.; Adhikari, Y.P.; Pant, B. Bioactive secondary metabolites in Paris polyphylla Sm. and their biological activities: A review. Heliyon 2022, 8, e08982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.; Liu, K.; Lu, T.; He, C. In Vitro and In Vivo Anti-Inflammatory Effects of Polyphyllin VII through Downregulating MAPK and NF-κB Pathways. Molecules 2019, 24, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, D.; Yang, C.; Li, C.; Zou, Y.; Feng, B.; Li, L.; Liu, W.; Luo, Q.; Chen, Z.; Huang, C. Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofilin activity and the AKT/NF-κB pathway. Biol. Open 2020, 9, bio046854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Dong, X.; You, L.; Sai, N.; Leng, X.; Yang, C.; Yin, X.; Ni, J. Apoptosis in HepaRG and HL-7702 cells inducted by polyphyllin II through caspases activation and cell-cycle arrest. J. Cell. Physiol. 2019, 234, 7078–7089. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, B.; Huang, J.; Bai, X.; Liang, Z.; Yi, X.; Xu, N.; Huang, Y.; Jiao, A. Network Pharmacology Reveals Polyphyllin II as One Hit of Nano Chinese Medicine Monomers against Nasopharyngeal Carcinoma. Bioinorg. Chem. Appl. 2021, 2021, 9959634. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hsieh, M.; Chen, C.; Lin, J.; Lo, Y.; Chuang, Y.; Chien, S.; Chen, M. Polyphyllin G induce apoptosis and autophagy in human nasopharyngeal cancer cells by modulation of AKT and mitogen-activated protein kinase pathways in vitro and in vivo. Oncotarget 2016, 7, 70276–70289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.; Chien, S.; Lin, J.; Yang, S.; Chen, M. Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomed. Int. J. Phytother. Phytopharmacol. 2016, 23, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Ren, P.; Pi, G.; Shi, R.; Yuan, Z.; Wang, C. High selective purification of flavonoids from natural plants based on polymeric adsorbent with hydrogen-bonding interaction. J. Chromatogr. A 2009, 1216, 8331–8338. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, W.; Xiu, Z.; Jiang, A.; Men, L.; Hao, K.; Sun, X.; Cao, D. Preparative Purification of Total Flavonoids from Sophora tonkinensis Gagnep. by Macroporous Resin Column Chromatography and Comparative Analysis of Flavonoid Profiles by HPLC-PAD. Molecules 2019, 24, 3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Li, Y.; Li, X.; Zhao, X.; Ma, X.; Zhou, X.; Cui, Y.; Ma, S.; Xu, W.; Ren, C. Preparation of Macroporous High Adsorbent Resin and Its Application for Heavy Metal Ion Removal. ChemistrySelect 2021, 6, 9038–9045. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J. Effective extraction with deep eutectic solvents and enrichment by macroporous adsorption resin of flavonoids from Carthamus tinctorius L. J. Pharm. Biomed. Anal. 2019, 176, 112804. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Zhang, R. Purification and antioxidant properties of triterpenic acids from blackened jujube (Ziziphus jujuba Mill.) by macroporous resins. Food Sci. Nutr. 2021, 9, 5070–5082. [Google Scholar] [CrossRef]
- Wang, F.; Ma, X.; Qu, L. Combined Application of Macroporous Resins and Preparative High-Performance Liquid Chromatography for the Separation of Steroidal Saponins from Stems and Leaves of Paris polyphylla. Chromatographia 2021, 84, 917–925. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chu, X.; Zhang, X.; Kan, Q.; Liu, R.; Fu, X. Adsorption and Desorption Characteristics of Total Flavonoids from Acanthopanax senticosus on Macroporous Adsorption Resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Y.; Qiao, X.; Lu, Y.; Chen, M.; Li, P.; Wen, X.; Yang, J. Structural characterisation and discrimination of the aerial parts of Paris polyphylla var. yunnanensis and Paris polyphylla var. chinensis by UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem. Anal. 2019, 30, 437–446. [Google Scholar] [CrossRef]
- Qin, X.; Ni, W.; Chen, C.; Liu, H. Seeing the light: Shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J. Ethnopharm. 2018, 224, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Takabi, A.S.; Shirani, M.; Semnani, A. Apple stem as a high performance cellulose based biosorbent for low cost and eco-friendly adsorption of crystal violet from aqueous solutions using experimental design: Mechanism, kinetic and thermodynamics. Environ. Technol. Innov. 2021, 24, 101947. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Ma, X.; Zhang, K.; Li, S.; Wang, X.; Liu, X.; Liu, J.; Fan, W.; Li, Y.; et al. Study on the kinetic model, thermodynamic and physicochemical properties of Glycyrrhiza polysaccharide by ultrasonic assisted extraction. Ultrason.—Sonochem. 2019, 51, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Intriago, L.A.; Gorozabel-Mendoza, M.L.; Mosquera, A.C.; Delgado-Demera, M.H.; Duarte, M.M.M.B.; Rodríguez-Díaz, J.M. Kinetics, equilibrium, and thermodynamics of the blue 19 dye adsorption process using residual biomass attained from rice cultivation. Biomass Convers. Biorefin. 2020, 12, 3843–3855. [Google Scholar] [CrossRef]
- Wasilewska, M.; Derylo-Marczewska, A. Adsorption of Non-Steroidal Anti-Inflammatory Drugs on Alginate-Carbon Composites-Equilibrium and Kinetics. Materials 2022, 15, 6049. [Google Scholar] [CrossRef] [PubMed]
- Davidescu, C.M.; Ardelean, R.; Popa, A. New polymeric adsorbent materials used for removal of phenolic derivatives from wastewaters. Pure Appl. Chem. 2019, 91, 443–458. [Google Scholar] [CrossRef]
- Sheshdeh, R.K.; Abbasizadeh, S.; Nikou, M.R.K.; Badii, K.; Sharafi, M.S. Liquid Phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J. Environ. Health Sci. Eng. 2014, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasilewska, M.; Marczewski, A.W.; Deryło-Marczewska, A.; Sternik, D. Nitrophenols removal from aqueous solutions by activated carbon—Temperature effect of adsorption kinetics and equilibrium. J. Environ. Chem. Eng. 2021, 9, 105459. [Google Scholar] [CrossRef]
- Bell, J.P.; Tsezos, M. Removal of Hazardous Organic Pollutants by Biomass Adsorption. Water Pollut. Control Fed. 1987, 59, 191–198. [Google Scholar] [CrossRef]
- Ho, Y.S.; Ofomaja, A.E. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005, 40, 3455–3461. [Google Scholar] [CrossRef]
- Cao, S.; Pan, S.; Yao, X.; Fu, H. Isolation and Purification of Anthocyanins from Blood Oranges by Column Chromatography. Agric. Sci. China 2010, 9, 207–215. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, H.; Shao, Q.; Bilia, A.R.; Wang, Y.; Zhao, X. Enrichment and Purification of the Bioactive Flavonoids from Flower of Abelmoschus manihot (L.) Medic Using Macroporous Resins. Molecules 2018, 23, 2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Rai, B.N.; Rai, L.C. Ni (II) and Cr (VI) sorption kinetics by Microcystis in single and multimetallic system. Process Biochem. 2001, 36, 1205–1213. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Li, Y.; Wang, C.; Hou, G.; Cong, W.; Zhao, F. Purification of spinosin from Ziziphi Spinosae Semen using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2019, 42, 3134–3140. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.; Zhang, R.; Yao, H.; Ge, K.; Zhang, M.; Ma, L. Enrichment of chelidonine from Chelidonium majus L. using macroporous resin and its antifungal activity. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1070, 7–14. [Google Scholar] [CrossRef]
- Sun, J.; Su, X.; Zhang, Z.; Hu, D.; Hou, G.; Zhao, F.; Sun, J.; Cong, W.; Wang, C.; Li, H. Separation of three chromones from Saposhnikovia divaricata using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2021, 44, 3287–3294. [Google Scholar] [CrossRef]
- Hou, M.; Hu, W.; Xiu, Z.; Shi, Y.; Hao, K.; Cao, D.; Guan, Y.; Yin, H. Efficient enrichment of total flavonoids from Pteris ensiformis Burm. extracts by macroporous adsorption resins and in vitro evaluation of antioxidant and antiproliferative activities. J. Chromatogr. B 2020, 1138, 121960. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Chen, L.; Liu, T.; Sha, R.; Mao, J. Enrichment and separation of steroidal saponins from the fibrous roots of Ophiopogon japonicus using macroporous adsorption resins. RSC Adv. 2019, 9, 6689–6698. [Google Scholar] [CrossRef] [Green Version]
- Luk, C.H.J.; Yip, J.; Yuen, C.W.M.; Pang, S.K.; Lam, K.H.; Kan, C.W. Biosorption Performance of Encapsulated Candida krusei for the removal of Copper(II). Sci. Rep. 2017, 7, 2159. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, L.; Mao, J.; Liu, S.; Huang, J.; You, Y.; Mei, L. Kinetic and thermodynamic studies of sulforaphane adsorption on macroporous resin. J. Chromatogr. B 2016, 1028, 231–236. [Google Scholar] [CrossRef]
- Han, Z.; Kong, S.; Sui, H.; Li, X.; Zhang, Z. Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs. Materials 2019, 12, 2438. [Google Scholar] [CrossRef] [Green Version]
- Ling, M.; Xia, G.; Zhi, Y. Static and dynamic studies of adsorption by four macroporous resins to enrich oridonin from Rabdosia rubescens. Chin. J. Chem. Eng. 2020, 32, 151–158. [Google Scholar] [CrossRef]




| Resin | Surface Area (m2/g) | Average Pore Diameter (nm) | Moisture Content (%) | Polarity |
|---|---|---|---|---|
| XAD7HP | ≥380 | 4.5 | 70.65 | Weak polar |
| HPD-100 | 650–700 | 8.5–9.0 | 69.00 | Nonpolar |
| HPD-500 | 500–550 | 5.5–7.5 | 67.66 | Polar |
| NKA-9 | 500–550 | 10.0–12.0 | 72.50 | Polar |
| D101 | 480–520 | 25.0–28.0 | 67.50 | Nonpolar |
| AB-8 | 480–520 | 13.0–14.0 | 71.64 | Weak polarity |
| HP-20 | 500–600 | 29.0–30.0 | 67.00 | Nonpolar |
| Time (min) | A (%) | B (%) |
|---|---|---|
| 0.0 | 57.0 | 43.0 |
| 13.0 | 57.0 | 43.0 |
| 14.0 | 45.0 | 55.0 |
| 22.0 | 45.0 | 55.0 |
| Polyphyllin II | Polyphyllin VII | ||||||
|---|---|---|---|---|---|---|---|
| NKA-9 | HPD-100 | AB-8 | NKA-9 | HPD-100 | AB-8 | ||
| Pseudo-first-order model | |||||||
| Qe | 7.925 | 6.618 | 6.640 | 14.068 | 12.190 | 11.169 | |
| K1 | 0.083 | 0.108 | 0.135 | 0.145 | 0.184 | 0.133 | |
| R2 | 0.951 | 0.967 | 0.964 | 0.911 | 0.855 | 0.841 | |
| Pseudo-second-order model | |||||||
| K2 | 0.015 | 0.051 | 0.032 | 0.039 | 0.026 | 0.010 | |
| R2 | 0.974 | 0.943 | 0.967 | 0.994 | 0.946 | 0.943 | |
| Weder-Morris intragranular diffusion model | |||||||
| Kp * | 1.220 | 1.082 | 1.212 | 1.858 | 0.835 | 1.107 | |
| Bangham model | |||||||
| K | 11.264 | 17.365 | 12.164 | 63.368 | 96.376 | 83.323 | |
| a | 0.801 | 0.655 | 0.707 | 0.742 | 0.548 | 0.356 | |
| Resin | Material | Coefficient of Determination (R2) | Reference | |
|---|---|---|---|---|
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||
| HPD-300 | three chromones (Prim-O-glucosylcimifugin, cimifugin, and 5-O-methylvisamminoside) | 0.983, 0.935, 0.967 | >0.990 | [48] |
| NKA-II | total flavonoids (Pteris ensiformis Burm.) | 0.974 | 0.998 | [49] |
| XAD-7HP | total steroidal saponins (Ophiopogon japonicus (L. f.) Ker-Gawl) | 0.973 | 0.990 | [50] |
| NKA-9 | two saponins (polyphyllin II and polyphyllin VII) | 0.951, 0.911 | 0.974, 0.994 | this study |
| Temperature (°C) | Langmuir Equation | Freundlich Equation | |||||
|---|---|---|---|---|---|---|---|
| Qm | KL | R2 | KF | 1/n | R2 | ||
| Polyphyllin II | |||||||
| 25 | 26.323 | 0.240 | 0.965 | 9.434 | 1.153 | 0.972 | |
| 30 | 34.879 | 0.198 | 0.979 | 9.488 | 1.222 | 0.986 | |
| 35 | 57.111 | 0.067 | 0.982 | 10.504 | 1.899 | 0.984 | |
| Polyphyllin VII | |||||||
| 25 | 23.064 | 0.777 | 0.973 | 6.925 | 2.240 | 0.994 | |
| 30 | 10.209 | 0.584 | 0.981 | 4.047 | 2.058 | 0.992 | |
| 35 | 38.722 | 0.799 | 0.985 | 14.305 | 1.835 | 0.982 | |
| Polyphyllin II | Polyphyllin VII | ||||||
|---|---|---|---|---|---|---|---|
| T (K) | ΔG° (kJ/mol) | ΔS° (kJ/mol) | ΔH° (kJ/mol) | ΔG° (kJ/mol) | ΔS° (kJ/mol) | ΔH° (kJ/mol) | |
| 298 | −2148.80 | 46.17 | 8198.35 | −1106.06 | 201.86 | 55360.13 | |
| 308 | −1348.45 | −1395.48 | |||||
| Resin | Material | Optimal Temperature | Reference |
|---|---|---|---|
| NKA-II (Polar) | total flavonoids (Pteris ensiformis Burm.) | 25 °C | [49] |
| HPD-300 (nonpolar) | three chromones (Prim-O-glucosylcimifugin, cimifugin, and 5-O-methylvisamminoside) | 25 °C | [48] |
| NKA-9 (Polar) | two saponins (polyphyllin II and polyphyllin VII) | 35 °C | this study |
| Material | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| Qe | k1 | R2 | k2 | R2 | ||
| Polyphyllin II | 15.479 | 0.092 | 0.972 | 0.00391 | 0.861 | |
| Polyphyllin VII | 13.132 | 0.116 | 0.977 | 0.00266 | 0.902 | |
| Concentration of Ethanol (%) | Polyphyllin II | Polyphyllin VII | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P (%) | R (%) | Y (%) | P (%) | R (%) | Y (%) | P (%) | R (%) | Y (%) | |||
| 20 | 0.24 | 0.24 | ─ | 5.36 | 4.56 | ─ | 5.6 | 2.54 | ─ | ||
| 30–50 | 35.28 | 68.30 | 68.46 | 49.69 | 84.61 | 88.65 | 84.97 | 76.98 | 93.16 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wu, J.; Qin, L.; Wang, G.; Li, P.; Yu, A.; Liu, A.; Sun, R. Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin. Molecules 2022, 27, 6626. https://doi.org/10.3390/molecules27196626
Zhang X, Wu J, Qin L, Wang G, Li P, Yu A, Liu A, Sun R. Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin. Molecules. 2022; 27(19):6626. https://doi.org/10.3390/molecules27196626
Chicago/Turabian StyleZhang, Xiaoya, Junli Wu, Long Qin, Guangxi Wang, Ping Li, Anmin Yu, Aizhong Liu, and Rui Sun. 2022. "Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin" Molecules 27, no. 19: 6626. https://doi.org/10.3390/molecules27196626
APA StyleZhang, X., Wu, J., Qin, L., Wang, G., Li, P., Yu, A., Liu, A., & Sun, R. (2022). Separation and Purification of Two Saponins from Paris polyphylla var. yunnanensis by a Macroporous Resin. Molecules, 27(19), 6626. https://doi.org/10.3390/molecules27196626







