A Simple High-Throughput Field Sample Preparation Method Based on Matrix-Induced Sugaring-Out for the Simultaneous Determination of 5-Hydroxymethylfurfural and Phenolic Compounds in Honey
Abstract
1. Introduction
2. Results and Discussion
2.1. High-Throughput Field Matrix-Induced Sugaring-Out
2.2. High-Throughput Optimization of Method Parameters
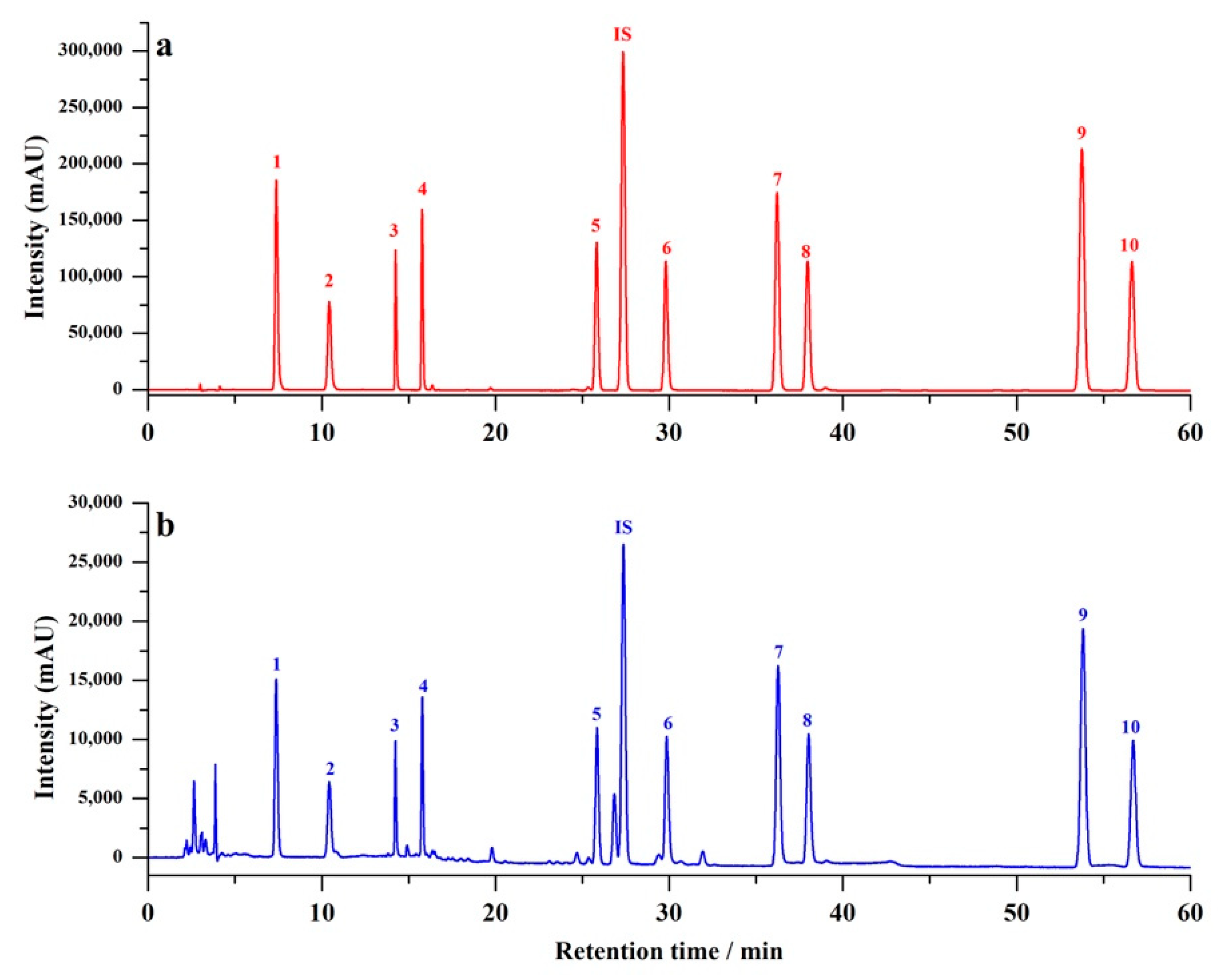
2.3. Analytical Performance
2.4. Real Samples Analysis
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Optimization of High-Throughput Field Matrix-Induced Sugaring-Out
3.3. Optimal Protocol of High-Throughput Field Matrix-Induced Sugaring-Out
3.4. HPLC Analysis
3.5. Method Validation
3.6. Real Samples Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical methods used in the quality control of honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Valverde, S.; Ares, A.M.; Stephen Elmore, J.; Bernal, J. Recent trends in the analysis of honey constituents. Food Chem. 2022, 387, 132920. [Google Scholar] [CrossRef] [PubMed]
- Serra Bonvehí, J.; Ventura Coll, F. Flavour index and aroma profiles of fresh and processed honeys: Flavour index and aroma profiles of honeys. J. Sci. Food Agric. 2003, 83, 275–282. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, J.; Zhao, H.; Wu, F.; Xue, X.; Wu, L.; Cao, W. Volatile compounds of five types of unifloral honey in northwest China: Correlation with aroma and floral origin based on HS-SPME/GC–MS combined with chemometrics. Food Chem. 2022, 384, 132461. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.; Sanna, G. Recent advances in the analysis of phenolic compounds in unifloral honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef]
- Zhao, L.; Ren, C.; Xue, X.; Lu, H.; Wang, K.; Wu, L. Safflomin A: A novel chemical marker for Carthamus tinctorius L. (safflower) monofloral honey. Food Chem. 2022, 366, 130584. [Google Scholar] [CrossRef]
- Zhao, T.; Zhao, L.; Wang, M.; Qi, S.; Xue, X.; Wu, L.; Li, Q. Identification of characteristic markers for monofloral honey of Astragalus membranaceus var. mongholicus Hsiao: A combined untargeted and targeted MS-based study. Food Chem. 2023, 404, 134312. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, M.W.; Namieśnik, J. Challenges in preparing honey samples for chromatographic determination of contaminants and trace residues. Trends Anal. Chem. 2008, 27, 785–793. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Mansour, F.R. Homogeneous liquid–liquid extraction as an alternative sample preparation technique for biomedical analysis. J. Sep. Sci. 2022, 45, 185–209. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Apyari, V.V.; Gorbunova, M.V.; Tolmacheva, V.V.; Zolotov, Y.A. Homogeneous Liquid–Liquid Microextraction of Organic Compounds. J. Anal. Chem. 2020, 75, 1371–1383. [Google Scholar] [CrossRef]
- Valente, I.M.; Moreira, M.M.; Neves, P.; da Fé, T.; Gonçalves, L.M.; Almeida, P.J.; Rodrigues, J.A. An insight on salting-out assisted liquid-liquid extraction for phytoanalysis. Phytochem. Anal. 2017, 28, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tu, X.; Wu, D.; Gao, Z.; Wu, S.; Huang, S. Comparison of the partition efficiencies of multiple phenolic compounds contained in propolis in different modes of acetonitrile–water-based homogenous liquid–liquid extraction. Molecules 2019, 24, 442. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.A.; Hammad, S.F.; Bedair, A.; Mansour, F.R. Sugaring-out induced homogeneous liquid-liquid microextraction as an alternative mode for biological sample preparation: A comparative study. J. Sep. Sci. 2021, 44, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.A.; Hammad, S.F.; Bedair, A.; Elshafeey, A.H.; Mansour, F.R. Determination of favipiravir in human plasma using homogeneous liquid-liquid microextraction followed by HPLC/UV. Bioanalysis 2022, 14, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Sun, F.; Wu, S.; Liu, W.; Gao, Z.; Huang, S.; Chen, W. Comparison of salting-out and sugaring-out liquid–liquid extraction methods for the partition of 10-hydroxy-2-decenoic acid in royal jelly and their co-extracted protein content. J. Chromatogr. B 2018, 1073, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, S.; Zhang, J.; Yu, F.; Miao, X.; Tu, X. Salting-out-assisted liquid–liquid extraction of 5-hydroxymethylfurfural from honey and the determination of 5-hydroxymethylfurfural by high-performance liquid chromatography. Anal. Methods 2019, 11, 4835–4841. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; González-Sálamo, J.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Evolution and applications of the QuEChERS method. Trends Anal. Chem. 2015, 71, 169–185. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. Miniaturized salting-out assisted liquid-liquid extraction combined with disposable pipette extraction for fast sample preparation of neonicotinoid pesticides in bee pollen. Molecules 2020, 25, 5703. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, F.; Tao, Y.; Du, C.; Yang, W.; Chen, W.; Tu, X. Micro salting-out assisted matrix solid-phase dispersion: A simple and fast sample preparation method for the analysis of bisphenol contaminants in bee pollen. Molecules 2021, 26, 2350. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Feriduni, B.; Afshar Mogaddam, M.R. Development of a new extraction method based on counter current salting-out homogenous liquid–liquid extraction followed by dispersive liquid–liquid microextraction: Application for the extraction and preconcentration of widely used pesticides from fruit juices. Talanta 2016, 146, 772–779. [Google Scholar] [PubMed]
- Fan, Y.; Hu, S.; Liu, S. Salting-out assisted liquid-liquid extraction coupled to dispersive liquid-liquid microextraction for the determination of chlorophenols in wine by high-performance liquid chromatography: Sample preparation. J. Sep. Sci. 2014, 37, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, X.; Hu, Q.; Zhang, C.; Li, F.; Wang, F.; Feng, Q. Salting-out assisted liquid-liquid extraction coupled to dispersive liquid-liquid microextraction for the determination of bisphenol a and six analogs (B, E, F, S, BADGE, BFDGE) in canned coffee drinks by ultra-performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2021, 14, 441–452. [Google Scholar]
- Tabaraki, R.; Heidarizadi, E. Spectrophotometric determination of phenol and chlorophenols by salting out assisted liquid-liquid extraction combined with dispersive liquid-liquid microextraction. Spectrochim. Acta Part A 2019, 215, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.M.; Ahmadi, R.; Yamini, Y. Homogeneous liquid-liquid microextraction based on deep eutectic solvents. Trends Anal. Chem. 2022, 149, 116566. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Homogenous liquid-liquid micro-extraction of pollutants in complex matrices. Microchem. J. 2021, 170, 106750. [Google Scholar] [CrossRef]
- Chen, W.; Wu, S.; Zhang, J.; Yu, F.; Hou, J.; Miao, X.; Tu, X. Matrix-Induced Sugaring-Out: A simple and rapid sample preparation method for the determination of neonicotinoid pesticides in honey. Molecules 2019, 24, 2761. [Google Scholar] [CrossRef]
- Alkan, C.; Çabuk, H. Matrix-induced sugaring-out liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of organophosphorus pesticides in fruit jams. Sep. Sci. Plus 2022, 5, 416–423. [Google Scholar] [CrossRef]
- Bogdanov, S.; Ruoff, K.; Persano Oddo, L. Physico-chemical methods for the characterisation of unifloral honeys: A review. Apidologie 2004, 35, S4–S17. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.d.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, J.; Tao, Y.; Du, C.; Yang, W.; Chen, W.; Tu, X. High-throughput subzero-temperature assisted homogenous liquid-liquid extraction for the fast sample preparation of multiple phenolic compounds in propolis. J. Chromatogr. B 2021, 1179, 122823. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A review focussing on its manufacture. Starch 1990, 42, 314–321. [Google Scholar] [CrossRef]
- Subramanian, R.; Umesh Hebbar, H.; Rastogi, N.K. Processing of honey: A review. Int. J. Food Prop. 2007, 10, 127–143. [Google Scholar] [CrossRef]
- Turkut, G.M.; Degirmenci, A.; Yildiz, O.; Can, Z.; Cavrar, S.; Yaylaci Karahalil, F.; Kolayli, S. Investigating 5-hydroxymethylfurfural formation kinetic and antioxidant activity in heat treated honey from different floral sources. J. Food Meas. Charact. 2018, 12, 2358–2365. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. High 5-hydroxymethylfurfural concentrations are found in malaysian honey samples stored for more than one year. Food Chem. Toxicol. 2010, 48, 2388–2392. [Google Scholar] [CrossRef]
- ICH Q2(R1). Validation of Analytical Procedures: Text and Methodology. 2005. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 14 November 2022).


| Analytes | Linear Equation | Linearity Range (μg/mL) | r2 | LOD (μg/g) | LOQ (μg/g) |
|---|---|---|---|---|---|
| 5-Hydroxymethylfurfural | y = 2.1198x | 1.44–40 | 0.9998 | 0.18 | 0.54 |
| Protocatechuic acid | y = 0.3618x | 0.76–21 | 0.9997 | 0.90 | 4.04 |
| Pinocembrin | y = 0.9226x | 0.09–2.5 | 0.9997 | 0.17 | 0.51 |
| Caffeic acid | y = 0.8331x | 0.18–5 | 0.9996 | 0.51 | 1.02 |
| 3,4-Dimethoxycinnamic acid | y = 0.7344x | 0.22–6 | 0.9994 | 0.39 | 1.17 |
| Quercetin | y = 0.2954x | 0.72–20 | 0.9992 | 0.69 | 4.14 |
| Apigenin | y = 0.6312x | 0.54–15 | 0.9996 | 0.83 | 2.50 |
| Kaempferol | y = 0.3666x | 0.72–20 | 0.9989 | 1.35 | 4.04 |
| Chrysin | y = 0.9792x | 0.36–10 | 0.9997 | 0.35 | 1.57 |
| Galangin | y = 0.4508x | 0.58–16 | 0.9993 | 1.01 | 3.03 |
| Analytes | Spiked Levels (μg/g) | Intra-Day | Inter-Day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | |||||||
| Recovery (%) ± SD (n = 6) | RSD (%) | Recovery (%) ± SD (n = 6) | RSD (%) | Recovery (%) ± SD (n = 6) | RSD (%) | Recovery (%) ± SD (n = 6) | RSD (%) | ||
| 5-Hydroxymethylfurfural | 5.7 | 98.63 ± 2.40 | 2.44 | 101.99 ± 3.99 | 3.91 | 97.85 ± 2.06 | 2.11 | 99.49 ± 3.32 | 3.34 |
| 11.4 | 104.96 ± 4.14 | 3.94 | 107.97 ± 3.63 | 3.36 | 102.07 ± 4.25 | 4.16 | 105.00 ± 4.51 | 4.30 | |
| 28.6 | 102.07 ± 3.28 | 3.22 | 96.33 ± 1.67 | 1.73 | 97.93 ± 3.50 | 3.57 | 98.78 ± 3.71 | 3.76 | |
| Protocatechuic acid | 4.2 | 88.13 ± 1.75 | 1.98 | 88.83 ± 1.70 | 1.91 | 89.78 ± 1.58 | 1.77 | 88.91 ± 1.72 | 1.94 |
| 8.3 | 83.98 ± 1.68 | 2.00 | 89.06 ± 1.97 | 2.21 | 85.86 ± 2.42 | 2.82 | 86.30 ± 2.89 | 3.35 | |
| 20.8 | 86.98 ± 1.72 | 1.98 | 85.22 ± 3.41 | 4.00 | 84.14 ± 3.49 | 4.15 | 85.45 ± 3.05 | 3.57 | |
| Pinocembrin | 0.5 | 92.58 ± 2.19 | 2.37 | 95.22 ± 1.71 | 1.79 | 96.56 ± 1.53 | 1.59 | 94.79 ± 2.42 | 2.55 |
| 1.0 | 94.74 ± 2.00 | 2.12 | 97.04 ± 2.92 | 3.00 | 95.20 ± 3.37 | 3.54 | 95.66 ± 2.84 | 2.97 | |
| 2.4 | 100.45 ± 1.90 | 1.89 | 96.54 ± 3.38 | 3.50 | 97.67 ± 2.66 | 2.72 | 98.22 ± 3.06 | 3.11 | |
| Caffeic acid | 1.1 | 95.61 ± 2.80 | 2.92 | 96.02 ± 2.65 | 2.76 | 96.16 ± 2.80 | 2.92 | 95.93 ± 2.59 | 2.70 |
| 2.2 | 95.77 ± 2.73 | 2.85 | 96.40 ± 3.76 | 3.90 | 95.58 ± 2.76 | 2.89 | 95.92 ± 2.95 | 3.08 | |
| 5.5 | 92.87 ± 1.29 | 1.39 | 92.78 ± 3.65 | 3.93 | 93.37 ± 3.20 | 3.43 | 93.00 ± 2.74 | 2.94 | |
| 3,4-Dimethoxycinnamic acid | 1.2 | 105.17 ± 3.84 | 3.65 | 106.44 ± 2.96 | 2.78 | 105.75 ± 1.82 | 1.72 | 105.79 ± 2.86 | 2.70 |
| 2.3 | 105.43 ± 3.09 | 2.93 | 110.38 ± 4.06 | 3.68 | 106.99 ± 2.99 | 2.79 | 107.60 ± 3.85 | 3.58 | |
| 5.8 | 106.51 ± 0.96 | 0.90 | 102.60 ± 2.23 | 2.17 | 103.66 ± 2.14 | 2.07 | 104.25 ± 2.44 | 2.34 | |
| Quercetin | 4.0 | 113.50 ± 4.51 | 3.98 | 117.11 ± 3.66 | 3.13 | 111.77 ± 3.44 | 3.08 | 114.13 ± 4.32 | 3.79 |
| 8.0 | 104.75 ± 3.64 | 3.48 | 100.29 ± 4.18 | 4.17 | 107.06 ± 1.18 | 1.10 | 104.03 ± 4.22 | 4.06 | |
| 20.2 | 96.07 ± 3.69 | 3.84 | 93.60 ± 3.34 | 3.57 | 98.28 ± 3.39 | 3.45 | 95.99 ± 3.81 | 3.97 | |
| Apigenin | 3.1 | 102.87 ± 3.92 | 3.81 | 102.36 ± 1.88 | 1.84 | 96.35 ± 2.60 | 2.69 | 100.53 ± 4.10 | 4.08 |
| 6.1 | 101.77 ± 4.86 | 4.78 | 95.01 ± 1.54 | 1.62 | 94.19 ± 3.23 | 3.43 | 96.99 ± 4.79 | 4.94 | |
| 15.4 | 99.52 ± 4.37 | 4.39 | 95.21 ± 2.32 | 2.44 | 94.71 ± 3.03 | 3.20 | 96.48 ± 3.85 | 4.00 | |
| Kaempferol | 3.8 | 103.70 ± 3.45 | 3.33 | 101.69 ± 3.67 | 3.61 | 99.74 ± 3.86 | 3.87 | 101.71 ± 3.82 | 3.76 |
| 7.7 | 103.59 ± 3.10 | 2.99 | 99.96 ± 2.65 | 2.65 | 100.95 ± 3.15 | 3.12 | 101.50 ± 3.21 | 3.16 | |
| 19.2 | 102.36 ± 3.96 | 3.87 | 98.02 ± 2.06 | 2.10 | 100.65 ± 3.02 | 3.00 | 100.34 ± 3.45 | 3.44 | |
| Chrysin | 2.0 | 105.11 ± 2.98 | 2.84 | 101.20 ± 3.18 | 3.14 | 100.36 ± 2.99 | 2.98 | 102.23 ± 3.57 | 3.49 |
| 4.0 | 104.70 ± 3.18 | 3.03 | 105.44 ± 2.32 | 2.32 | 104.57 ± 2.22 | 2.12 | 104.91 ± 2.51 | 2.40 | |
| 10.2 | 101.48 ± 1.99 | 1.96 | 106.82 ± 4.42 | 4.14 | 103.67 ± 2.30 | 2.22 | 103.99 ± 3.68 | 3.54 | |
| Galangin | 2.9 | 104.95 ± 2.48 | 2.36 | 107.77 ± 2.75 | 2.55 | 104.59 ± 2.61 | 2.50 | 105.77 ± 2.86 | 2.71 |
| 5.9 | 106.67 ± 2.84 | 2.66 | 108.00 ± 2.47 | 2.28 | 103.81 ± 3.49 | 3.36 | 106.16 ± 3.32 | 3.12 | |
| 14.7 | 109.74 ± 2.72 | 2.48 | 104.54 ± 1.63 | 1.56 | 105.68 ± 2.91 | 2.76 | 106.65 ± 3.28 | 3.07 | |
| Sample ID | Detected Compounds (μg/g, Mean ± SD, n = 3) | ||||
|---|---|---|---|---|---|
| 5-Hydroxymethylfurfural | Protocatechuic Acid | Caffeic Acid | 3,4-Dimethoxycinnamic Acid | Kaempferol | |
| C1 | 34.96 ± 3.25 | - | - | - | - |
| C2 | 9.67 ± 1.11 | - | - | - | - |
| C3 | 6.14 ± 0.17 | - | - | - | - |
| C4 | 20.14 ± 0.15 | - | - | - | - |
| C5 | 8.21 ± 0.47 | - | - | - | - |
| C6 | 85.88 ± 1.07 | - | 1.30 ± 0.02 | - | |
| C7 | 43.23 ± 1.65 | <LOQ | - | - | - |
| C8 | 14.16 ± 1.04 | - | - | - | - |
| C9 | 1.85 ± 0.12 | - | - | - | - |
| C10 | 72.07 ± 0.54 | - | - | - | - |
| C11 | 18.16 ± 1.35 | - | - | - | - |
| C12 | 27.97 ± 0.54 | - | - | - | <LOQ |
| R1 | <LOQ | - | - | - | - |
| R2 | <LOQ | - | - | <LOQ | - |
| R3 | <LOQ | - | - | <LOQ | - |
| R4 | <LOQ | - | - | 2.02 ± 0.03 | - |
| R5 | <LOQ | - | - | <LOQ | - |
| R6 | <LOQ | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, X.; Yu, F.; Jin, Q.; Du, C.; Chen, J.; Yang, J.; He, Y.; Huang, S.; Chen, W. A Simple High-Throughput Field Sample Preparation Method Based on Matrix-Induced Sugaring-Out for the Simultaneous Determination of 5-Hydroxymethylfurfural and Phenolic Compounds in Honey. Molecules 2022, 27, 8373. https://doi.org/10.3390/molecules27238373
Tu X, Yu F, Jin Q, Du C, Chen J, Yang J, He Y, Huang S, Chen W. A Simple High-Throughput Field Sample Preparation Method Based on Matrix-Induced Sugaring-Out for the Simultaneous Determination of 5-Hydroxymethylfurfural and Phenolic Compounds in Honey. Molecules. 2022; 27(23):8373. https://doi.org/10.3390/molecules27238373
Chicago/Turabian StyleTu, Xijuan, Fengjie Yu, Qian Jin, Chunping Du, Jiaxu Chen, Ji Yang, Yuchang He, Shaokang Huang, and Wenbin Chen. 2022. "A Simple High-Throughput Field Sample Preparation Method Based on Matrix-Induced Sugaring-Out for the Simultaneous Determination of 5-Hydroxymethylfurfural and Phenolic Compounds in Honey" Molecules 27, no. 23: 8373. https://doi.org/10.3390/molecules27238373
APA StyleTu, X., Yu, F., Jin, Q., Du, C., Chen, J., Yang, J., He, Y., Huang, S., & Chen, W. (2022). A Simple High-Throughput Field Sample Preparation Method Based on Matrix-Induced Sugaring-Out for the Simultaneous Determination of 5-Hydroxymethylfurfural and Phenolic Compounds in Honey. Molecules, 27(23), 8373. https://doi.org/10.3390/molecules27238373








