Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei
Abstract
1. Introduction
2. Results and Discussions
2.1. The Flavonoid Compositions of FEAK
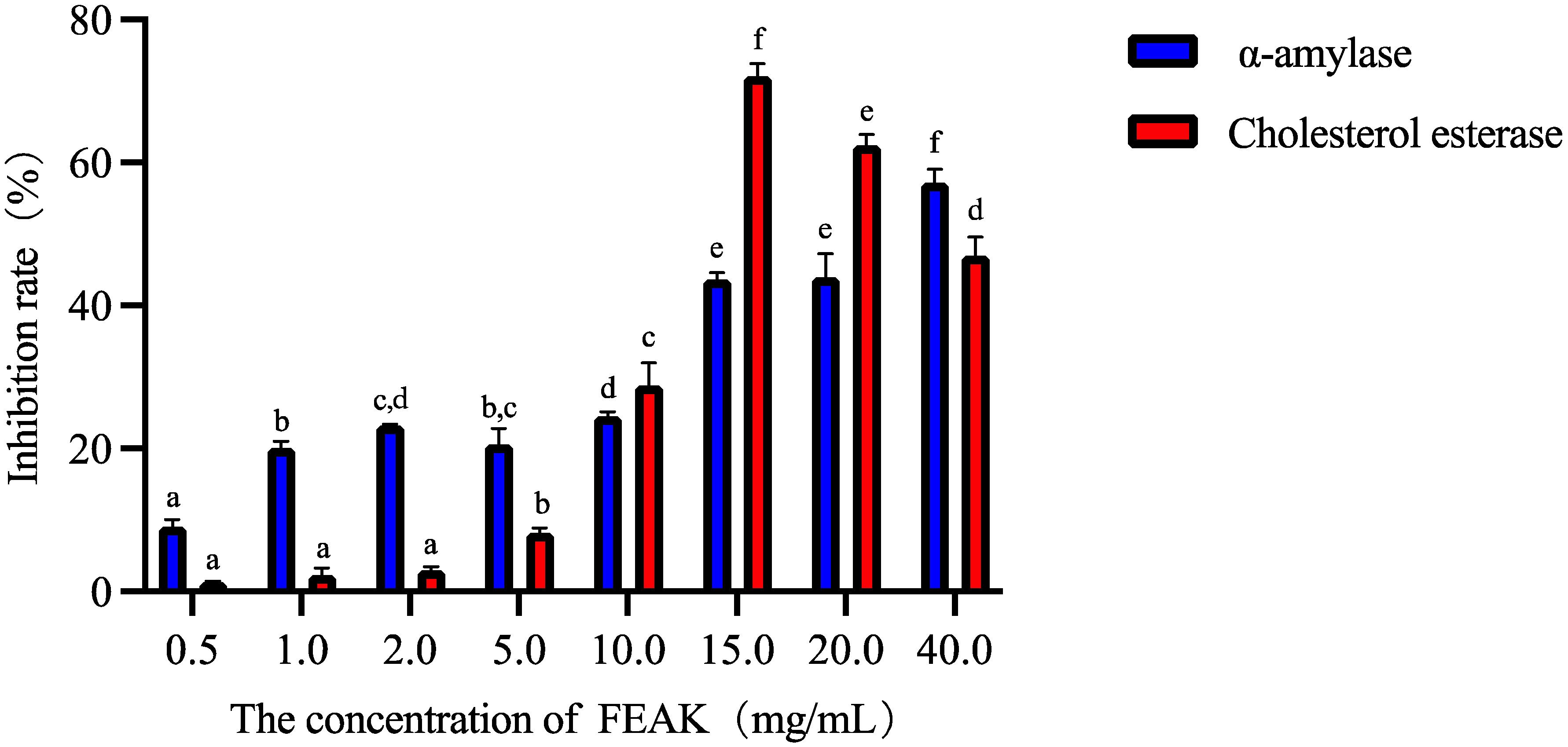
2.2. The Inhibition Activities on the α-Amylase and Cholesterol Esterase
2.3. Effect of FEAK on the Intracellular Levels of TC and TG in HepG2 Cells
2.4. Effect of FEAK on Glucose Uptake in HepG2 Cells
2.5. Evaluation of Hypoglycemic Efficacy of FEAK In Vivo in a Zebrafish Model
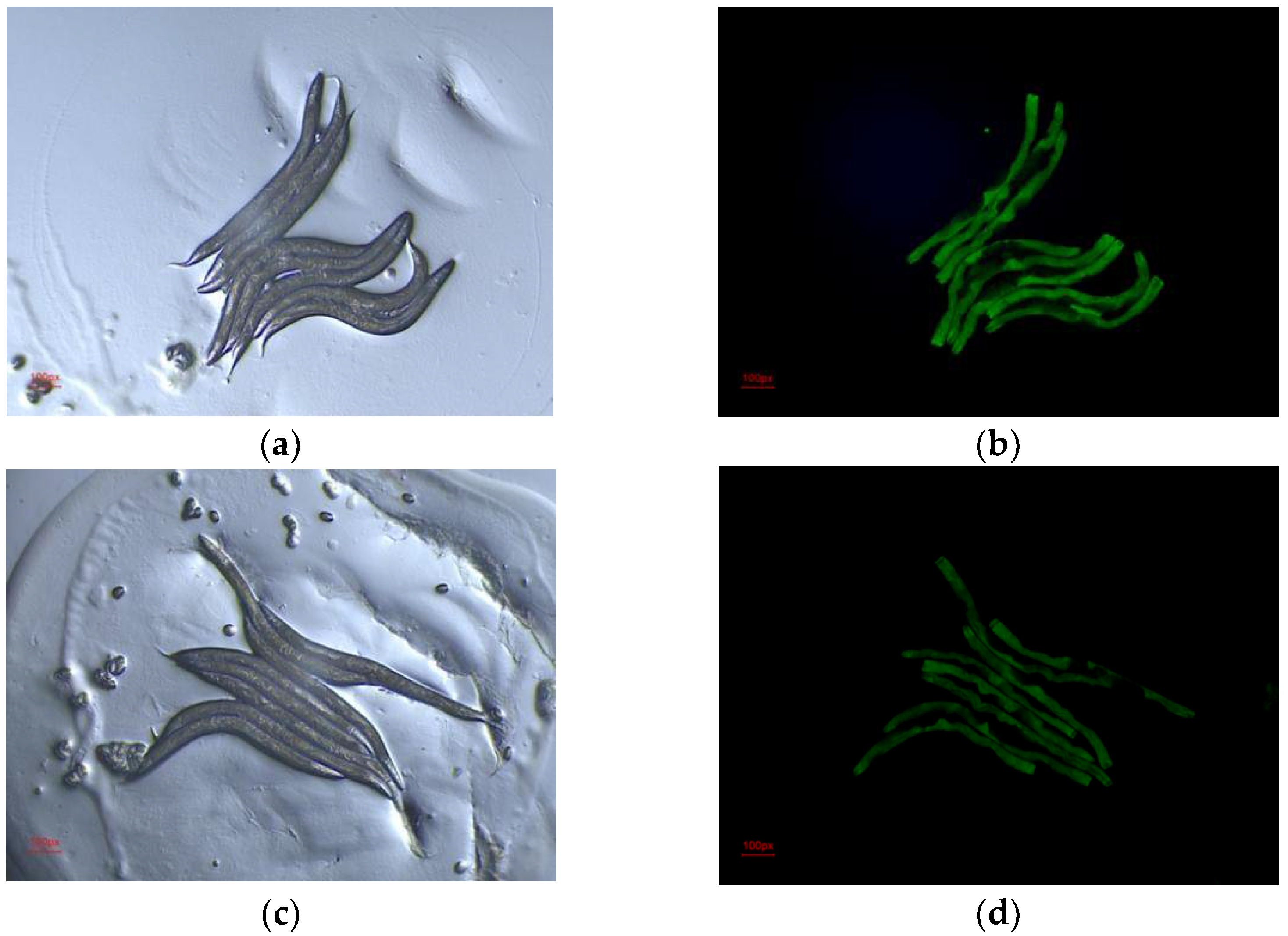
2.6. Evaluation of Hypolipidemic Efficacy of FEAK In Vivo in a C. elegans Model
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Preparation of Flavonoid-Rich Extract from Angelica keiskei
3.3. Analysis of the Flavonoid Composition of FEAK
3.4. Evaluation of the Hypoglycemic and Hypolipidemic Activity of FEAK In Vitro
3.4.1. Measurement of α-Amylase Inhibitory Activity
3.4.2. Measurement of Cholesterol Esterase Inhibitory Activity in Porcine Pancreas
3.4.3. Glucose Consumption Assay in HepG-2 Cells
3.4.4. Total Cholesterol (TC) and Triglyceride (TG) Assay in HepG-2 Cells
3.5. Experimental Analysis of In Vivo Hypoglycemic Effect in a Zebrafish Model
3.5.1. Sample Preparation
3.5.2. Evaluation of the Hypoglycemic Effect of FEAK in a Diabetic Zebrafish Model
3.6. Evaluation of Hypolipidemic Effect of FEAK in C. elegans Model
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lu, E.; Wu, H.; Zhou, C. Study on diabetes complicated with hyperlipidemia. Chin. J. Neuroanat. 2013, 29, 346–350. [Google Scholar]
- Diabetes Branch of Chinese Medical Association. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chin. J. Pract. Intern. Med. 2018, 38, 292–344. [Google Scholar] [CrossRef]
- Ma, L. Research progress of related adverse reactions and non-cardiovascular effects of statins. Guide China Med. 2018, 16, 8–9. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Y.X.; Chen, J.B.; Zhang, Y.Q.; Yu, X.W.; Tang, X.N. Research progress of hypoglycemic effect of traditional Chinese Medicine. J. Pract. Gynecol. Endocrinol. 2018, 5, 198. [Google Scholar] [CrossRef]
- Rong, Y.Z.; Gu, X.Z.; Li, D.N.; Chen, L.H.; Zhang, Y.H.; Wang, Z.W. Characterization of aroma, sensory and taste properties of Angelica keiskei tea. Eur. Food Res. Technol. 2021, 247, 1665–1677. [Google Scholar] [CrossRef]
- Gao, X.; Jiang, Y.; Xu, Q.; Liu, F.; Pang, X.; Wang, M.; Li, Q.; Li, Z. 4-Hydroxyderricin Promotes Apoptosis and Cell Cycle Arrest through Regulating PI3K/AKT/MTOR Pathway in Hepatocellular Cells. Foods 2021, 10, 2036. [Google Scholar] [CrossRef]
- Amalia, R.; Aulifa, D.L.; Zain, D.N.; Pebiansyah, A.; Levita, J. The Cytotoxicity and Nephroprotective Activity of the Ethanol Extracts of Angelica keiskei Koidzumi Stems and Leaves against the NAPQI-Induced Human Embryonic Kidney (HEK293) Cell Line. Evid. Based Complementary Altern. Med. 2021, 2021, e6458265. [Google Scholar] [CrossRef]
- Zhang, J. Effect of Ashitaba Chalcone on Lipid Metabolism of Rats with Type II Diabetes. Food Sci. 2015, 36, 250–254. [Google Scholar]
- Zhang, W.; Jin, Q.; Luo, J.; Wu, J.H.; Wang, Z.W. Phytonutrient and anti-diabetic functional properties of flavonoid-rich ethanol extract from Angelica keiskei leaves. J. Food Sci. Technol. 2018, 55, 4406–4412. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.P.; Zhao, Y.; Li, L.; Zhong, J.Y. Effect of ashitabe chalcones on the mRNA expression of PI3K and Akt in hepatocytes of rats with diabetes. J. Hyg. Res. 2013, 42, 466–469. [Google Scholar]
- Bu, S.; Xue, Q.; Yuan, C.; Chen, Y.; Cao, F. Effects of Three Flavonoids Extracted from Ginkgo biloba on Lipid Metabolism in 3T3-L1 Cells. J. Shandong Agric. Univ. Nat. Sci. Ed. 2020, 51, 598–604. [Google Scholar]
- Yuan, W.; Yan, M.X.; Wang, Y.T.; Liu, X.; Gong, Y.L. Optimized preparation of eugenol microcapsules and its effect on hepatic steatosis in HepG2 cells. Drug Dev. Ind. Pharm. 2020, 47, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wu, Q.X.; Wei, X.; Qin, X.M. Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Biosci. 2020, 37, 100682. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.M.; Chen, Z.; Zhao, Z.; Li, Y.C.; Deng, Q.; Huang, Z.J.; Zhang, X.; Li, C.; Zhou, M.G.; et al. Multilevel logistic regression analysis on hypercholesterolemia related risk factors among adults in China. Chin. J. Prev. Med. 2018, 52, 151–157. [Google Scholar]
- Amanguli, A.; Ma, H.; Lan, W. Study on improvement effect of total flavonoids from Matricaria recutita on lipid deposition in HepG2 cells. China Pharm. 2022, 33, 1306–1312. [Google Scholar]
- Matsumoto, K.; Yokoyama, S.; Gato, N. Bile Acid-Binding Activity of Young Persimmon (Diospyros kaki) Fruit and Its Hypo lipidemic Effect in Mice. Phytother. Res. 2010, 24, 205–210. [Google Scholar] [CrossRef]
- Liu, C. Effects of Red Raspberry and Its Extracts on Fat Accumulation in HepG2 Cells. Master’s Thesis, Northeast Forestry University, Harbin, China, 2019. [Google Scholar]
- Lu, Y.C.; Liu, Y.X.; Wu, W.; Zhou, F.; Ji, B.P.; Su, C.Y. Preventive Effect of Blueberry Polyphenols on Oleic Acid-induced Fat Accumulation in HepG2 Cells. Food Sci. 2011, 32, 308–312. [Google Scholar]
- Malle, E.K.; Zammit, N.W.; Walters, S.N.; Koay, Y.C.; Wu, J.; Tan, B.M.; Villanueva, J.E.; Brink, R.; Loudovaris, T.; Cantley, J.; et al. Nuclear Factor ΚB–Inducing Kinase Activation as a Mechanism of Pancreatic β Cell Failure in Obesity. J. Experiment. Med. 2015, 212, 1239–1254. [Google Scholar] [CrossRef]
- Zong, H.; Bao, B. Construction of rict-1/daf-2&Pdhs-3::dhs-3::gfp in C. elegans. J. HeFei Univ. Technol. 2018, 41, 1695–1699. [Google Scholar]
- Arneson, L.; Gleeson, M.; Connaughton, V. Induction of Hyperglycemia and Microvascular Retinal Complications in Zebrafish, Danio Rerio. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1739. [Google Scholar]
- Capiotti, K.M.; Antonioli, R.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent Impaired Glucose Metabolism in a Zebrafish Hyperglycemia Model. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Zou, Y.; Shan, H.; Zhao, Y.; Lv, H. Study on Optimum of Extraction and Determination of Total Flavonoids in Angelica keiskei Koidzumi. Food Ind. 2012, 33, 44–47. [Google Scholar]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Yuca, H.; Özbek, H.; Demirezer, L.Ö.; Güvenalp, Z. Assessment of the α-glucosidase and α-amylase inhibitory potential of Paliurus spina-christi Mill. and its terpenic compounds. Med. Chem. Res. 2022, 31, 1393–1399. [Google Scholar] [CrossRef]
- Su, J.; Ma, C.; Yang, L.; Wang, H.; Gao, C.; Nie, R. Inhibition of pancreatic cholesterol esterase activities and cholesterol micelle of EGCG and quercetin. Sci. Technol. Food Ind. 2015, 36, 346–349. [Google Scholar]
- Shi, H. Effect of Purple Sweet Potato Anthocyanins on Lipid Metabolism in HepG2 Cells Induced by Oleic Acid. Port Health Control 2019, 24, 27–31, 35. [Google Scholar]



| Compounds | Class | Relative Quantification (%) (Mean ± SE) |
|---|---|---|
| Aureusidin-4-O-glucoside | Aurones | 5.395 ± 0.082 |
| Xanthoangelol | Chalcones | 3.995 ± 0.041 |
| 4-Hydroxyderricin | Chalcones | 3.767 ± 0.015 |
| Kaempferol-3-O-(6″-malonyl)glucoside | Flavonols | 3.622 ± 0.116 |
| Kaempferol-3-O-(6″-malonyl)galactoside | Flavonols | 3.482 ± 0.065 |
| Luteolin-7-O-rutinoside | Flavones | 3.311 ± 0.212 |
| Kaempferol-3-O-glucoside-7-O-rhamnoside | Flavonols | 3.251 ± 0.007 |
| Luteolin-7-O-neohesperidoside (Lonicerin) | Flavones | 3.172 ± 0.003 |
| Kaempferol-3-O-neohesperidoside | Flavonols | 3.170 ± 0.072 |
| Kaempferol-3-O-glucorhamnoside | Flavonols | 3.125 ± 0.058 |
| Diosmetin-7-O-galactoside | Flavones | 2.888 ± 0.034 |
| Quercetin-5-O-β-d-glucoside | Flavonols | 2.805 ± 0.095 |
| 6-C-MethylKaempferol-3-glucoside | Flavones | 2.755 ± 0.009 |
| Diosmetin-7-O-glucoside | Flavones | 2.750 ± 0.028 |
| Luteolin-7-O-glucoside (Cynaroside) | Flavones | 2.739 ± 0.406 |
| Hispidulin-7-O-Glucoside | Flavones | 2.678 ± 0.027 |
| Chrysoeriol-7-O-(6″-malonyl)glucoside | Flavones | 1.997 ± 0.326 |
| Diosmetin-7-O-rutinoside (Diosmin) | Flavones | 1.995 ± 0.015 |
| Luteolin-4′-O-glucoside | Flavones | 1.851 ± 0.171 |
| Quercetin-3-O-galactoside (Hyperin) | Flavonols | 1.785 ± 0.062 |
| Cyanidin-3-O-glucoside (Kuromanin) | Anthocyanidins | 1.688 ± 0.191 |
| Quercetin-3-O-glucoside (Isoquercitrin) | Flavonols | 1.683 ± 0.038 |
| Hispidulin-7-O-(6″-O-p-Coumaroyl)Glucoside | Flavones | 1.650 ± 0.055 |
| Kaempferol-3-O-glucoside (Astragalin) | Flavonols | 1.644 ± 0.036 |
| Dihydrokaempferide | Flavanonols | 1.527 ± 0.080 |
| Luteolin-3′-O-glucoside | Flavones | 1.506 ± 0.018 |
| Luteolin-7,3′-di-O-glucoside | Flavones | 1.394 ± 0.053 |
| Isobavachalcone | Chalcones | 1.36 ± 0.028 |
| Kaempferol-3-O-galactoside-4′-O-glucoside | Flavonols | 1.297 ± 0.041 |
| Xanthoangelol F | Chalcones | 1.277 ± 0.044 |
| Yuanhuanin | Flavones | 1.216 ± 0.147 |
| Quercetin-7-O-glucoside | Flavonols | 1.172 ± 0.014 |
| Quercetin-4′-O-glucoside (Spiraeoside) | Flavonols | 1.165 ± 0.027 |
| Hesperetin-5-O-glucoside | Flavanones | 1.145 ± 0.028 |
| Sample | Fluorescence Intensity | δ (Contrast Value) |
|---|---|---|
| 0 mg/mL | 0.1208 ± 0.0211 a | |
| 10 mg/mL | 0.1237 ± 0.0272 a | 2.40% |
| 25 mg/mL | 0.1573 ± 0.0108 b | 26.66% |
| 50 mg/mL | 0.1711 ± 0.0082 b | 41.64% |
| 100 mg/mL | 0.2031 ± 0.0008 c | 68.12% |
| Group | Concentration (μg/mL) | Mortality (%) | Phenotype |
|---|---|---|---|
| Normal control group | - | 0 | No obvious abnormality |
| Model control group | - | 0 | No obvious abnormality |
| FEAK group | 31.2 | 0 | The state is similar to that of the model control group |
| 62.5 | 0 | ||
| 125 | 0 | ||
| 250 | 0 | ||
| 500 | 0 |
| Group | Concentration (μg/mL) | Blood Glucose Value (mmol/L, Mean ± SE) |
|---|---|---|
| Normal control group | - | 0.92 ± 0.04 *** |
| Model control group | - | 2.31 ± 0.13 |
| Positive control group | 20 | 1.07 ± 0.04 *** |
| Sample group | 125 | 2.00 ± 0.13 |
| 250 | 1.65 ± 0.10 * | |
| 500 | 1.31 ± 0.05 *** |
| Tube | α-Amylase Solution (μL) | Sample (μL) | Starch Solution (μL) | PBS (μL) |
|---|---|---|---|---|
| Blank 1 | 300 | - | 300 | 150 |
| Blank control 2 | - | - | 300 | 450 |
| Sample 3 | 300 | 150 | 300 | - |
| Sample control 4 | - | 150 | 300 | 300 |
| Tube | Cholesterol Esterase Solution (μL) | Sample (μL) | PNPB (μL) | Buffer (mL) |
|---|---|---|---|---|
| Blank 1 | 50 | - | 10 | 1 |
| Blank control 2 | - | - | 10 | 1 |
| Sample 3 | 50 | 25 | 10 | 1 |
| Sample control 4 | - | 25 | 10 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, L.; Wang, R.; Fang, Z.; Sun, M.; Sun, X.; Wu, J.; Dang, Y.; Liu, J. Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei. Molecules 2022, 27, 6625. https://doi.org/10.3390/molecules27196625
Tu L, Wang R, Fang Z, Sun M, Sun X, Wu J, Dang Y, Liu J. Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei. Molecules. 2022; 27(19):6625. https://doi.org/10.3390/molecules27196625
Chicago/Turabian StyleTu, Lanlan, Rui Wang, Zheng Fang, Mengge Sun, Xiaohui Sun, Jinhong Wu, Yali Dang, and Jianhua Liu. 2022. "Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei" Molecules 27, no. 19: 6625. https://doi.org/10.3390/molecules27196625
APA StyleTu, L., Wang, R., Fang, Z., Sun, M., Sun, X., Wu, J., Dang, Y., & Liu, J. (2022). Assessment of the Hypoglycemic and Hypolipidemic Activity of Flavonoid-Rich Extract from Angelica keiskei. Molecules, 27(19), 6625. https://doi.org/10.3390/molecules27196625







