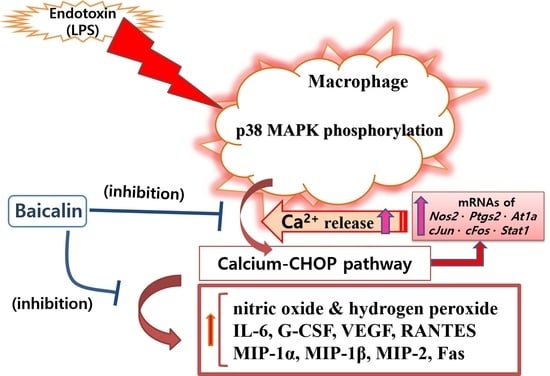
Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Cell Viability
2.3. NO Production
2.4. Intracellular Calcium Release
2.5. Hydrogen Peroxide Production
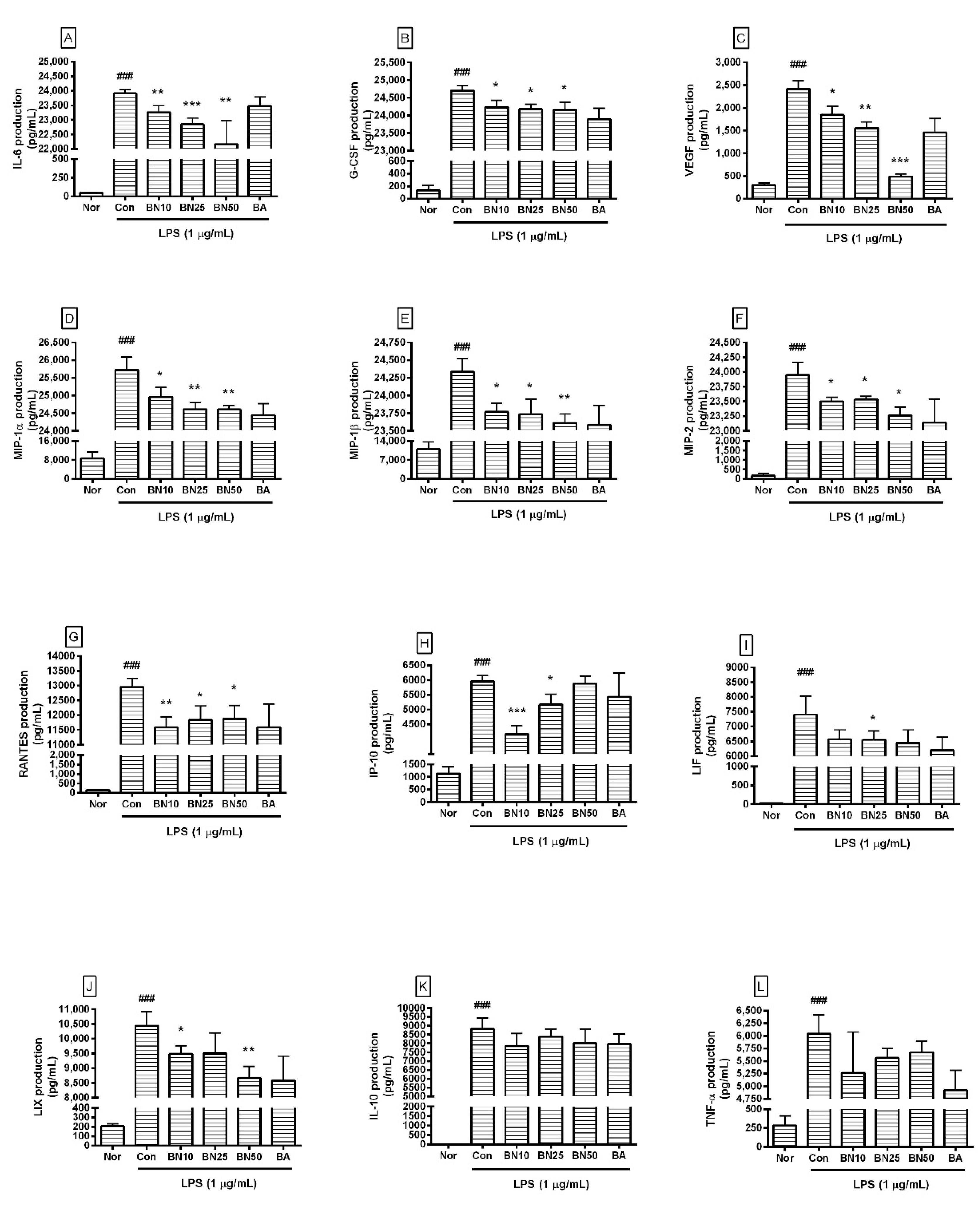
2.6. Multiplex Cytokine Assay
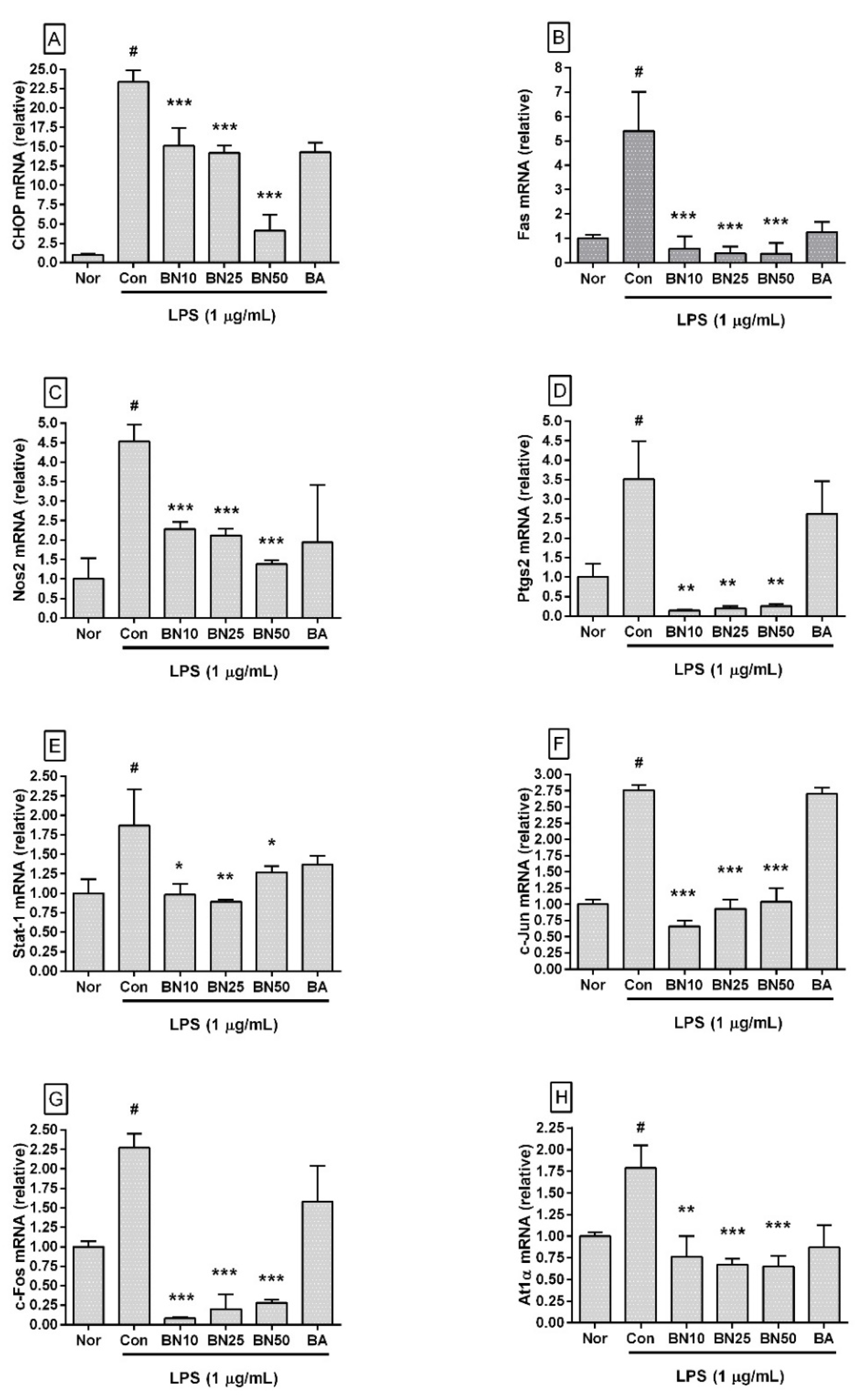
2.7. Quantitative RT-PCR
2.8. Flow Cytometric Analysis of Phosphorylated P38 MAPK and Fas
2.9. Statistical Analyses
3. Results
3.1. Effect of Baicalin on Cell Viability
3.2. Effect of Baicalin on NO Production
3.3. Effect of Baicalin on Intracellular Calcium Release
3.4. Effect of Baicalin on Hydrogen Peroxide Production
3.5. Effect of Baicalin on Cytokine Production
3.6. Effect of Baicalin on mRNA Expression Levels of Inflammatory Genes in RAW 264.7
3.7. Effect of Baicalin on Levels of Phosphorylated P38 MAPK and Fas in RAW 264.7 Macrophages
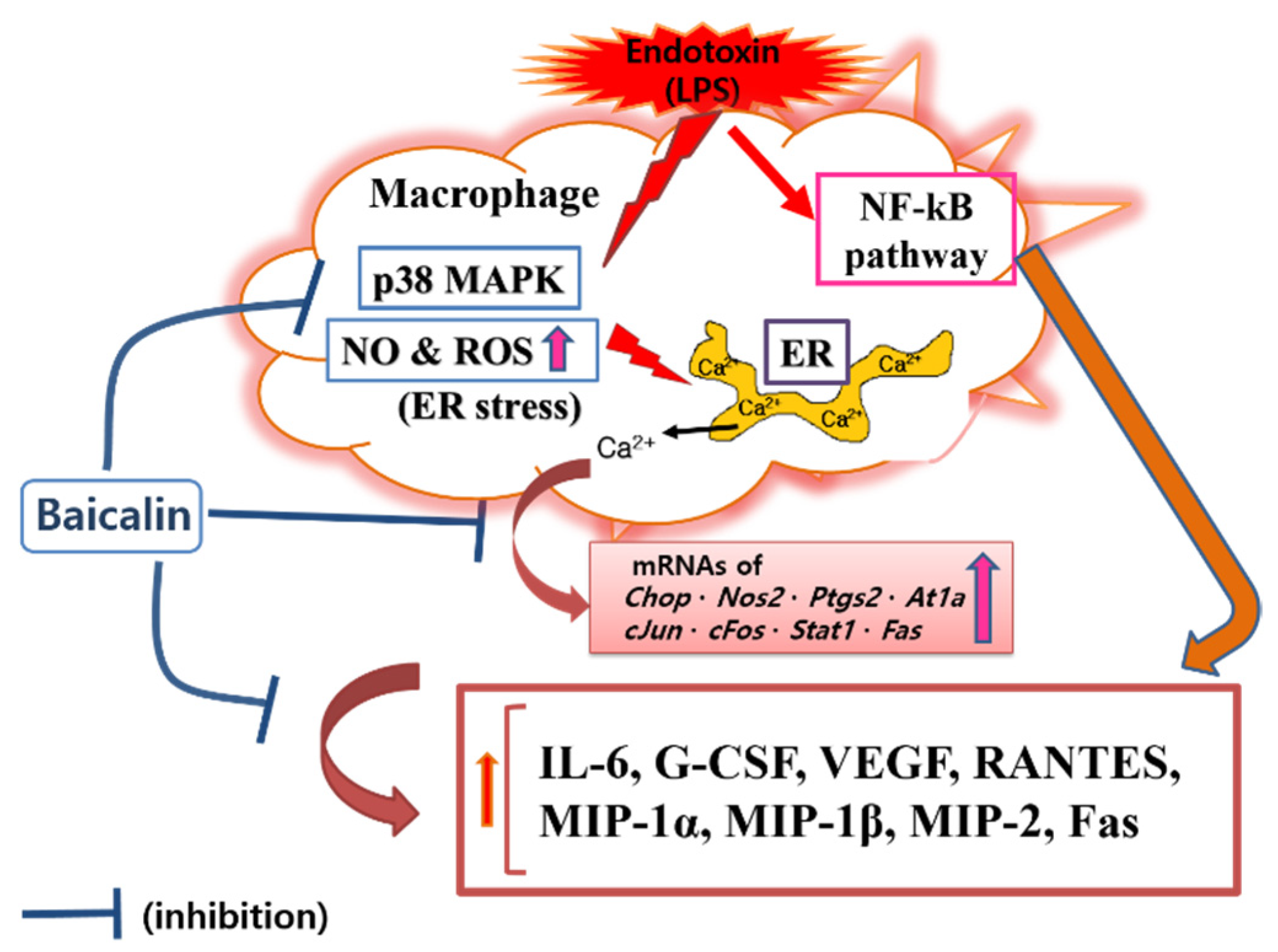
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Lepp, P.W.; Relman, D.A. Archaea and their Potential Role in Human Disease. Infect. Immun. 2003, 71, 591–596. [Google Scholar] [CrossRef]
- Vianna, M.E.; Conrads, G.; Gomes, B.P.; Horz, H.P. T-RFLP-Based mcrA Gene Analysis of Methanogenic Archaea in Association with Oral Infections and Evidence of a Novel Methanobrevibacter Phylotype. Oral Microbiol. Immunol. 2009, 24, 417–422. [Google Scholar] [CrossRef]
- Holmes, C.L.; Anderson, M.T.; Mobley, H.L.T.; Bachman, M.A. Pathogenesis of Gram-Negative Bacteremia. Clin. Microbiol. Rev. 2021, 34, e00234-20. [Google Scholar] [CrossRef]
- Brandenburg, K.; Schromm, A.B.; Weindl, G.; Heinbockel, L.; Correa, W.; Mauss, K.; Martinez de Tejada, G.; Garidel, P. An Update on Endotoxin Neutralization Strategies in Gram-Negative Bacterial Infections. Expert Rev. Anti Infect. Ther. 2021, 19, 495–517. [Google Scholar] [CrossRef]
- Giordano, N.P.; Cian, M.B.; Dalebroux, Z.D. Outer Membrane Lipid Secretion and the Innate Immune Response to Gram-Negative Bacteria. Infect. Immun. 2020, 88, e00920-19. [Google Scholar] [CrossRef]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Seeley, J.J.; Ghosh, S. Molecular Mechanisms of Innate Memory and Tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef]
- Morrison, D.C.; Ryan, J.L. Endotoxins and Disease Mechanisms. Annu. Rev. Med. 1987, 38, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, G.; Cochior, D.; Moldovan, C.; Rusu, E. Molecular Mechanisms in Septic Shock (Review). Exp. Ther. Med. 2021, 22, 1161. [Google Scholar] [CrossRef] [PubMed]
- Adamik, B.; Zielinski, S.; Smiechowicz, J.; Kubler, A. Endotoxin Elimination in Patients with Septic Shock: An Observation Study. Arch. Immunol. Ther. Exp. 2015, 63, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic Reticulum Stress: Cell Life and Death Decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Gao, Y.Q.; Lippton, H.; Hyman, A.; Spitzer, J.J. The Roles of Nitric Oxide and Hydrogen Peroxide Production in Lipopolysaccharide-Induced Intestinal Damage. Shock 1994, 2, 185–191. [Google Scholar] [CrossRef]
- Dahm, P.L.; Thorne, J.; Myhre, E.; Grins, E.; Martensson, L.; Blomquist, S. Intestinal and Hepatic Perfusion and Metabolism in Hypodynamic Endotoxic Shock. Effects of Nitric Oxide Synthase Inhibition. Acta Anaesthesiol. Scand. 1999, 43, 56–63. [Google Scholar] [CrossRef]
- Levi, M.; Dorffler-Melly, J.; Reitsma, P.; Buller, H.; Florquin, S.; van der Poll, T.; Carmeliet, P. Aggravation of Endotoxin-Induced Disseminated Intravascular Coagulation and Cytokine Activation in Heterozygous Protein-C-Deficient Mice. Blood 2003, 101, 4823–4827. [Google Scholar] [CrossRef]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-Inflammatory Effects of Scutellaria Baicalensis Water Extract on LPS-Activated RAW 264.7 Macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef]
- Hui, K.M.; Wang, X.H.; Xue, H. Interaction of Flavones from the Roots of Scutellaria baicalensis with the Benzodiazepine Site. Planta Med. 2000, 66, 91–93. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, F.; Tsang, S.Y.; Ho, K.H.; Zheng, H.; Yuen, C.T.; Chow, C.Y.; Xue, H. Anxiolytic-Like Effect of Baicalin and its Additivity with Other Anxiolytics. Planta Med. 2006, 72, 189–192. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Li, X.; Song, L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm. Biol. 2020, 58, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Park, W. Rubi Fructus Water Extract Alleviates LPS-Stimulated Macrophage Activation Via an ER Stress-Induced Calcium/CHOP Signaling Pathway. Nutrients 2020, 12, 3577. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, J.Y.; Kim, H.J.; Kim, D.H.; Lee, T.H.; Kang, M.S.; Park, W. Anti-Inflammatory Effects of Angelica sinensis (Oliv.) Diels Water Extract on RAW 264.7 Induced with Lipopolysaccharide. Nutrients 2018, 10, 647. [Google Scholar] [CrossRef]
- Yim, S.; Lee, J.; Jo, H.; Scholten, J.; Willingham, R.; Nicoll, J.; Baswan, S.M. Chrysanthemum Morifolium Extract and Ascorbic Acid-2-Glucoside (AA2G) Blend Inhibits UVA-Induced Delayed Cyclobutane Pyrimidine Dimer (CPD) Production in Melanocytes. Clin. Cosmet. Investig. Dermatol. 2019, 12, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zhang, Y.; Yang, Y.; Su, S.; Zhou, L.; Lo, P.C.; Cai, J.; Qiao, Y.; Li, M.; Huang, S.; et al. Baicalin Inhibits Ferroptosis in Intracerebral Hemorrhage. Front. Pharmacol. 2021, 12, 629379. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Endo, M.; Mori, M.; Akira, S.; Gotoh, T. C/EBP Homologous Protein (CHOP) is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammation. J. Immunol. 2006, 176, 6245–6253. [Google Scholar] [CrossRef]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef]
- Wang, X.Z.; Ron, D. Stress-Induced Phosphorylation and Activation of the Transcription Factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef]
- Liu, H.; Xu, R.; Feng, L.; Guo, W.; Cao, N.; Qian, C.; Teng, P.; Wang, L.; Wu, X.; Sun, Y.; et al. A Novel Chromone Derivative with Anti-Inflammatory Property Via Inhibition of ROS-Dependent Activation of TRAF6-ASK1-p38 Pathway. PLoS ONE 2012, 7, e37168. [Google Scholar] [CrossRef] [Green Version]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173 (Suppl. S1), E40–E45. [Google Scholar] [CrossRef]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Klymenko, O.; Huehn, M.; Wilhelm, J.; Wasnick, R.; Shalashova, I.; Ruppert, C.; Henneke, I.; Hezel, S.; Guenther, K.; Mahavadi, P.; et al. Regulation and Role of the ER Stress Transcription Factor CHOP in Alveolar Epithelial Type-II Cells. J. Mol. Med. 2019, 97, 973–990. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Chen, L.; Wang, H.; Wu, S.; Li, S.; Wang, X. Baicalin Inhibits LPS-Induced Inflammation in RAW264.7 Cells through miR-181b/HMGB1/TRL4/NF-kappaB Pathway. Am. J. Transl. Res. 2021, 13, 10127–10141. [Google Scholar]
- Sp, N.; Kang, D.Y.; Kim, H.D.; Rugamba, A.; Jo, E.S.; Park, J.C.; Bae, S.W.; Lee, J.M.; Jang, K.J. Natural Sulfurs Inhibit LPS-Induced Inflammatory Responses through NF-kappaB Signaling in CCD-986Sk Skin Fibroblasts. Life 2021, 11, 427. [Google Scholar] [CrossRef]
- Cardoso, V.G.; Goncalves, G.L.; Costa-Pessoa, J.M.; Thieme, K.; Lins, B.B.; Casare, F.A.M.; de Ponte, M.C.; Camara, N.O.S.; Oliveira-Souza, M. Angiotensin II-Induced Podocyte Apoptosis is Mediated by Endoplasmic Reticulum stress/PKC-delta/p38 MAPK Pathway Activation and Trough Increased Na(+)/H(+) Exchanger Isoform 1 Activity. BMC Nephrol. 2018, 19, 179. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Furusawa, S. Oxidative Stress and Septic Shock: Metabolic Aspects of Oxygen-Derived Free Radicals Generated in the Liver during Endotoxemia. FEMS Immunol. Med. Microbiol. 2006, 47, 167–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene Name | Gene Bank Accession number |
|---|---|
| Chop | NM_007837 |
| Fas | NM_007987 |
| Nos2 | NM_010927.3 |
| Ptgs2 | NM_011198 |
| Stat1 | NM_009283.4 |
| c-Jun | NM_010591 |
| c-Fos | NM_010234 |
| At1a | NM_177322 |
| β-Actin | NM_007393.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.-J.; Lee, J.-Y.; Park, W. Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway. Cells 2022, 11, 3076. https://doi.org/10.3390/cells11193076
An H-J, Lee J-Y, Park W. Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway. Cells. 2022; 11(19):3076. https://doi.org/10.3390/cells11193076
Chicago/Turabian StyleAn, Hyo-Jin, Ji-Young Lee, and Wansu Park. 2022. "Baicalin Modulates Inflammatory Response of Macrophages Activated by LPS via Calcium-CHOP Pathway" Cells 11, no. 19: 3076. https://doi.org/10.3390/cells11193076