The Tolerance, Absorption, and Transport Characteristics of Macleaya cordata in Relation to Lead, Zinc, Cadmium, and Copper under Hydroponic Conditions
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydroponic Experiments
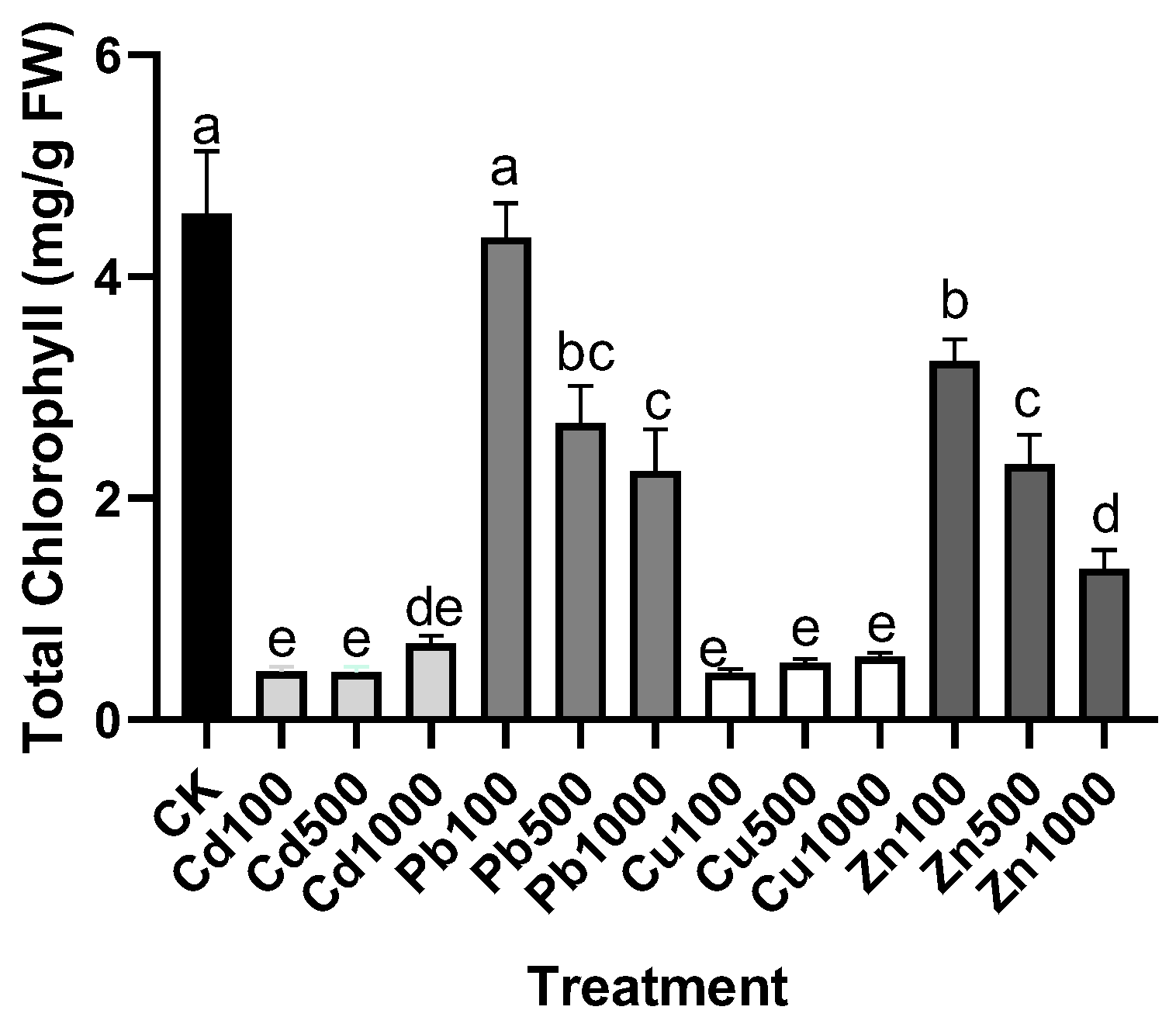
2.2. Estimation of Chlorophyll Content
2.3. Dry Weight and Metal Element Analysis
2.4. Detection of Metals In Situ
2.5. Statistical Analysis
3. Results
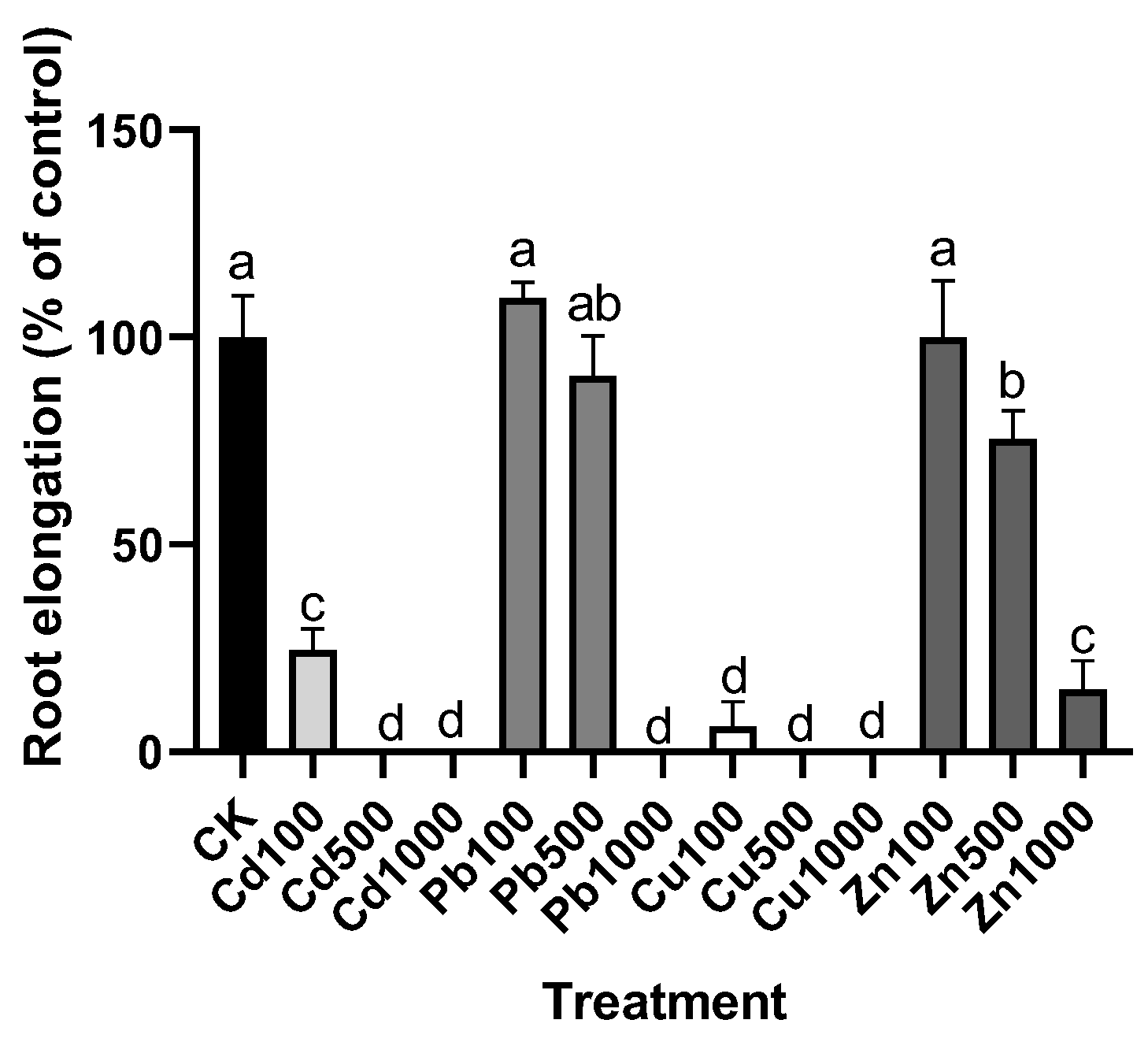
3.1. The Tolerance of M. cordata under the Treatment of Pb, Zn, Cd, and Cu in the Solution
3.2. The Accumulation and Translocation of Pb, Zn, Cd, and Cu in M. cordata
3.3. Content Changes in Pb, Zn, Cd, and Cu in M. cordata Exposed to Compound Heavy Metals in the Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehimb, A.; Hussainef, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Ivanov, V.B. Histochemical investigation of cadmium and lead distribution in plants. Russ. J. Plant Physiol. 1997, 44, 791–796. [Google Scholar]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost-benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, P.; Zhang, S.; Zhang, S.; Sun, Y. Contribution of arbuscular mycorrhizal fungi and soil amendments to remediation of a heavy metal-contaminated soil using sweet sorghum. Pedosphere 2022, 32, 844–855. [Google Scholar] [CrossRef]
- Sai, C.-M.; Li, D.-H.; Xue, C.-M.; Wang, K.-B.; Hu, P.; Pei, Y.-H.; Bai, J.; Jing, Y.-K.; Li, Z.-L.; Hua, H.-M. Two pairs of enantiomeric alkaloid dimers from Macleaya cordata. Org. Lett. 2015, 17, 4102–4105. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Huang, P.; Ma, Y.; Qing, Z.; Tang, Q.; Cao, H.; Cheng, P.; Zheng, Y.; Yuan, Z.; et al. The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol. Plant 2017, 10, 975–989. [Google Scholar] [CrossRef]
- Zhao, L.; Matulka, R.; von Alvensleben, S.; Morlacchini, M. Residue study for a standardized Macleaya cordata extract in growing-finishing swine. Open J. Anim. Sci. 2017, 7, 93–104. [Google Scholar] [CrossRef]
- Li, C.-w.; Hu, N.; Ding, D.-x.; Hu, J.-s.; Li, G.-y.; Wang, Y.-d. Phytoextraction of uranium from contaminated soil by Macleaya cordata before and after application of EDDS and CA. Environ. Sci. Pollut. Res. 2015, 22, 6155–6163. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, J.; Zhang, H.; Xia, Y.; Chen, C.; Shen, Z.G.; Chen, Y. Several newly discovered Mo-enriched plants with a focus on Macleaya cordata. Environ. Sci. Pollut. Res. 2018, 25, 26493–26503. [Google Scholar] [CrossRef]
- Cai, B.; Chen, Y.; Du, L.; Liu, Z.; He, L. Spent mushroom compost and calcium carbonate modification enhances phytoremediation potential of Macleaya cordata to lead-zinc mine tailings. J. Environ. Manag. 2021, 294, 113029. [Google Scholar] [CrossRef]
- Pan, G.; Zhang, H.; Liu, W.; Liu, P. Integrative study of subcellular distribution, chemical forms, and physiological responses for understanding manganese tolerance in the herb Macleaya cordata (papaveraceae). Ecotoxicol. Environ. Saf. 2019, 181, 455–462. [Google Scholar] [CrossRef]
- Zhan, J.; Li, T.; Yu, H.; Zhang, X. Cd and Pb accumulation characteristics of phytostabilizer Athyrium wardii (Hook.) grown in soils contaminated with Cd and Pb. Environ. Sci. Pollut. Res. 2018, 25, 29026–29037. [Google Scholar] [CrossRef]
- Nie, J.; Liu, Y.; Zeng, G.; Zheng, B.; Tan, X.; Liu, H.; Xie, J.; Gan, C.; Liu, W. Cadmium accumulation and tolerance of Macleaya cordata: A newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 10189–10199. [Google Scholar] [CrossRef]
- McBride, M.B.; Zhou, Y.T. Cadmium and zinc bioaccumulation by Phytolacca americana from hydroponic media and contaminated soils. Int. J. Phytoremediat. 2019, 21, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.Q.; Lu, W.L.; Nasar, J.; Zhang, J.J.; Yan, L. Growth responses and accumulation characteristics of three ornamentals under copper and lead contamination in a hydroponic-culture experiment. Bull. Environ. Contam. Toxicol. 2019, 103, 854–859. [Google Scholar] [CrossRef]
- Bonfranceschi, B.A.; Flocco, C.G.; Donati, E.R. Study of the heavy metal phytoextraction capacity of two forage species growing in an hydroponic environment. J. Hazard. Mater. 2009, 165, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhou, J.; Wan, H.; Zhuang, X.; Li, H.; Qin, S.; Lyu, D. Rootstock-scion interaction affects cadmium accumulation and tolerance of Malus. Front. Plant Sci. 2020, 11, 1264. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper-zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta 2008, 227, 465–475. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces production of hydrogen peroxide in the leaf of Elsholtzia haichowensis through apoplastic and symplastic CuZn-superoxide dismutase. J. Hazard. Mater. 2010, 178, 834–843. [Google Scholar] [CrossRef]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z.G. Cadmium-induced oxidative damage and protective effects of N-acetyl-L-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef]
- Ramakrishna, R.S.; Fernandopulle, M. Preparation of o.o’-dichlorodithizone and a study of its metal complexing properties. Anal. Chim. Acta 1968, 41, 35–41. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Lisowska, K.; Romanowska-Duda, Z.; Wolf, W.M. Combined cadmium-zinc interactions alter manganese, lead, copper uptake by Melissa officinalis. Sci. Rep. 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Treatment | Metal Detected | Content in Roots (μg·g−1) | Content in Stems (μg·g−1) | Content in Leaves (μg·g−1) |
|---|---|---|---|---|
| CK | Pb | ND 1 | ND | ND |
| Pb100 | 4678.7 b ± 211.1 | 37.0 a ± 3.9 | 31.2 b ± 4.6 | |
| Pb500 | 5921.3 a ± 260.2 | 32.3 a ± 2.9 | 31.0 b ± 3.9 | |
| Pb1000 | 2030.8 c ± 116.4 | 38.0 a ± 2.1 | 47.1 a ± 3.5 | |
| CK | Zn | 885.5 c ± 54.0 | 82.4 c ± 3.4 | 82.7 b ± 4.1 |
| Zn100 | 1801.2 b ± 138.2 | 708.6 b ± 39.4 | 698.3 a ± 24.4 | |
| Zn500 | 3023.3 a ± 235.3 | 906.1 a ± 75.2.0 | 698.3 a ± 31.9 | |
| Zn1000 | 3322.2 a ± 266.0 | 1032.0 a ± 54.2 | 617.2 a ± 72.6 | |
| CK | Cd | ND | ND | ND |
| Cd100 | 1587.9 c ± 98.7 | 655.6 b ± 31.4 | 44.3 b ± 9.0 | |
| Cd500 | 4179.9 b ± 314.7 | 1014.4 a ± 46.4 | 43.3 b ± 4.6 | |
| Cd1000 | 6180.5 a ± 264.6 | 1133.3 a ± 53.7 | 71.8 a ± 6.4 | |
| CK | Cu | 57.3 d ± 5.1 | 4.1 d ± 0.1 | 10.1 b ± 0.6 |
| Cu100 | 3668.9 c ± 155.7 | 155.9 c ± 3.4 | 16.8 b ± 1.0 | |
| Cu500 | 5164.3 b ± 192.9 | 531.3 b ± 33.7 | 26.0 b ± 0.8 | |
| Cu1000 | 6646.8 a ± 254.1 | 2526.5 a ± 127.8 | 320.8 a ± 21.0 |
| Treatment | Metal Detected | Metal Content in Roots (μg·Plant−1) | Metal Content in Shoots (μg·Plant−1) | TF |
|---|---|---|---|---|
| CK | Pb | ND 1 | ND | ND |
| Pb100 | 738.4 a ± 33.3 | 55.9 b ± 2.8 | 0.08 b | |
| Pb500 | 585.2 b ± 25.7 | 62.7 a ± 7.0 | 0.11 b | |
| Pb1000 | 205.6 c ± 11.8 | 64.0 a ± 4.2 | 0.31 a | |
| CK | Zn | 200.4 b ± 12.2 | 162.6 d ± 4.6 | 0.82 c |
| Zn100 | 508.0 a ± 39.0 | 1430.2 a ± 39.0 | 2.84 b | |
| Zn500 | 223.6 b ± 17.4 | 920.1 b ± 28.7 | 4.16 a | |
| Zn1000 | 139.0 c ± 11.1 | 607.6 c ± 54.8 | 4.44 a | |
| CK | Cd | ND | ND | ND |
| Cd100 | 123.6 c ± 7.7 | 235.6 b ± 8.3 | 1.92 a | |
| Cd500 | 401.0 b ± 30.2 | 382.1 a ± 18.7 | 0.96 b | |
| Cd1000 | 536.5 a ± 23.0 | 266.8 b ± 10.3 | 0.50 b | |
| CK | Cu | 13.0 c ± 1.1 | 16.0 d ± 0.8 | 1.25 a |
| Cu100 | 374.2 b ± 15.9 | 44.2 c ± 0.6 | 0.12 c | |
| Cu500 | 489.4 a ± 18.3 | 97.7 b ± 5.9 | 0.20 c | |
| Cu1000 | 415.4 b ± 15.9 | 192.7 a ± 14.1 | 0.46 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhou, W.; Chen, Y.; Xu, H.; Hou, D.; Lv, S.; Sun, X.; Wang, F.; Yang, L. The Tolerance, Absorption, and Transport Characteristics of Macleaya cordata in Relation to Lead, Zinc, Cadmium, and Copper under Hydroponic Conditions. Appl. Sci. 2022, 12, 9598. https://doi.org/10.3390/app12199598
Zhang H, Zhou W, Chen Y, Xu H, Hou D, Lv S, Sun X, Wang F, Yang L. The Tolerance, Absorption, and Transport Characteristics of Macleaya cordata in Relation to Lead, Zinc, Cadmium, and Copper under Hydroponic Conditions. Applied Sciences. 2022; 12(19):9598. https://doi.org/10.3390/app12199598
Chicago/Turabian StyleZhang, Hongxiao, Wenli Zhou, Yahua Chen, Huawei Xu, Dianyun Hou, Shufang Lv, Xijing Sun, Fayuan Wang, and Liming Yang. 2022. "The Tolerance, Absorption, and Transport Characteristics of Macleaya cordata in Relation to Lead, Zinc, Cadmium, and Copper under Hydroponic Conditions" Applied Sciences 12, no. 19: 9598. https://doi.org/10.3390/app12199598







