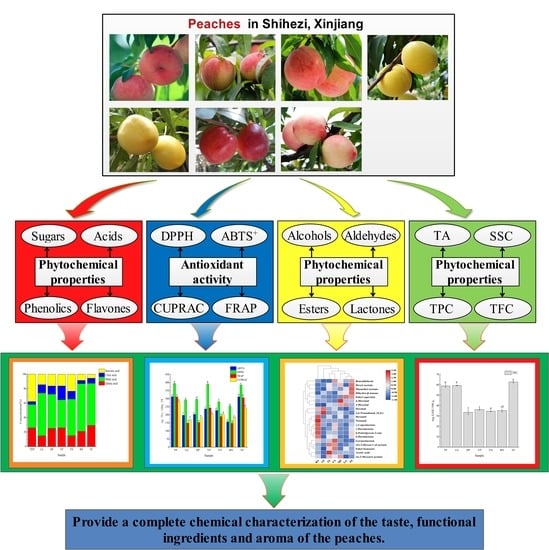
Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals
2.3. Soluble Solids Content (SSC) and Titratable Acidity (TA) Determination
2.4. Total Phenolic and Total Flavonoid Content Determination
2.5. Determination of Sugars and Organic Acids by HPLC
2.6. Phenolic Compound Determination
2.7. In Vitro Antioxidant Activity Determination
2.8. Aroma Compound Determination
2.9. Data Analysis
3. Results and Discussion
3.1. Soluble Solids and Titratable Acidity
3.2. Total Phenolic and Total Flavonoid
3.3. Sugars and Organic Acids
3.4. Phenolic Compounds
3.5. In Vitro Antioxidant Activity
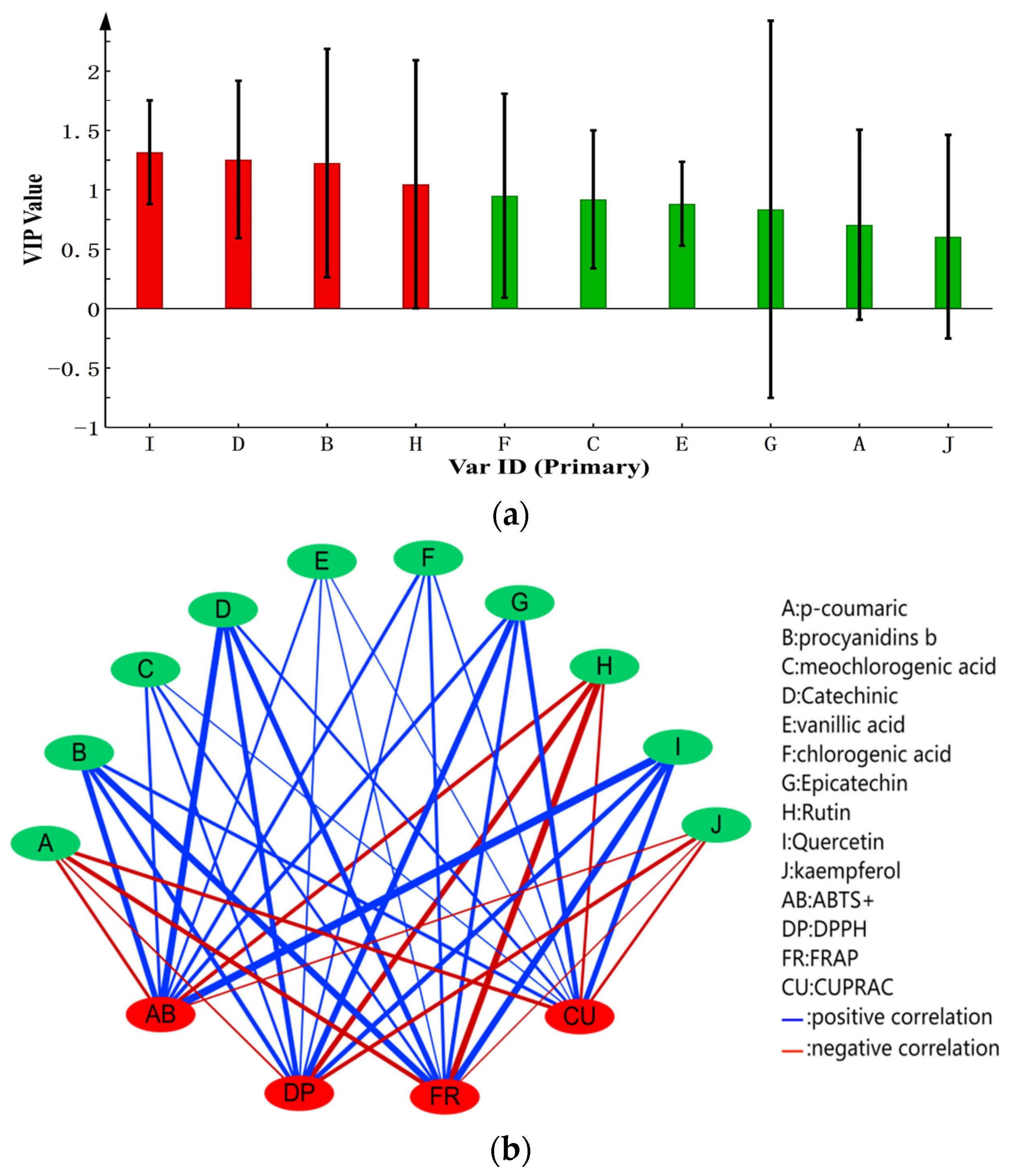
3.6. Correlation Analysis of Phenolic Compounds and Antioxidant Capacity
3.7. Volatile Profiles of Seven Peach Cultivars
3.7.1. Principal Component Analysis
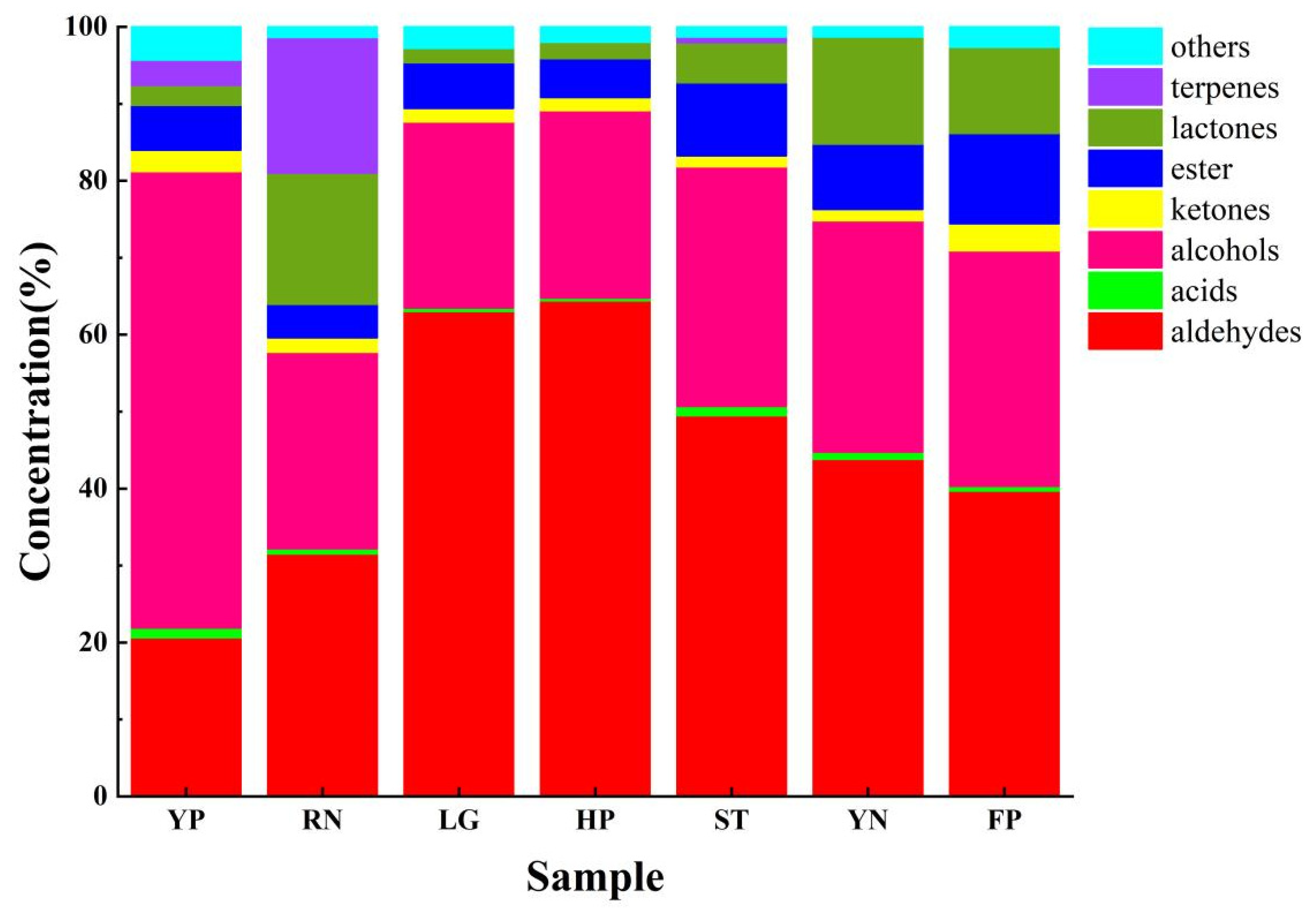
3.7.2. Aroma Compound Composition and Content
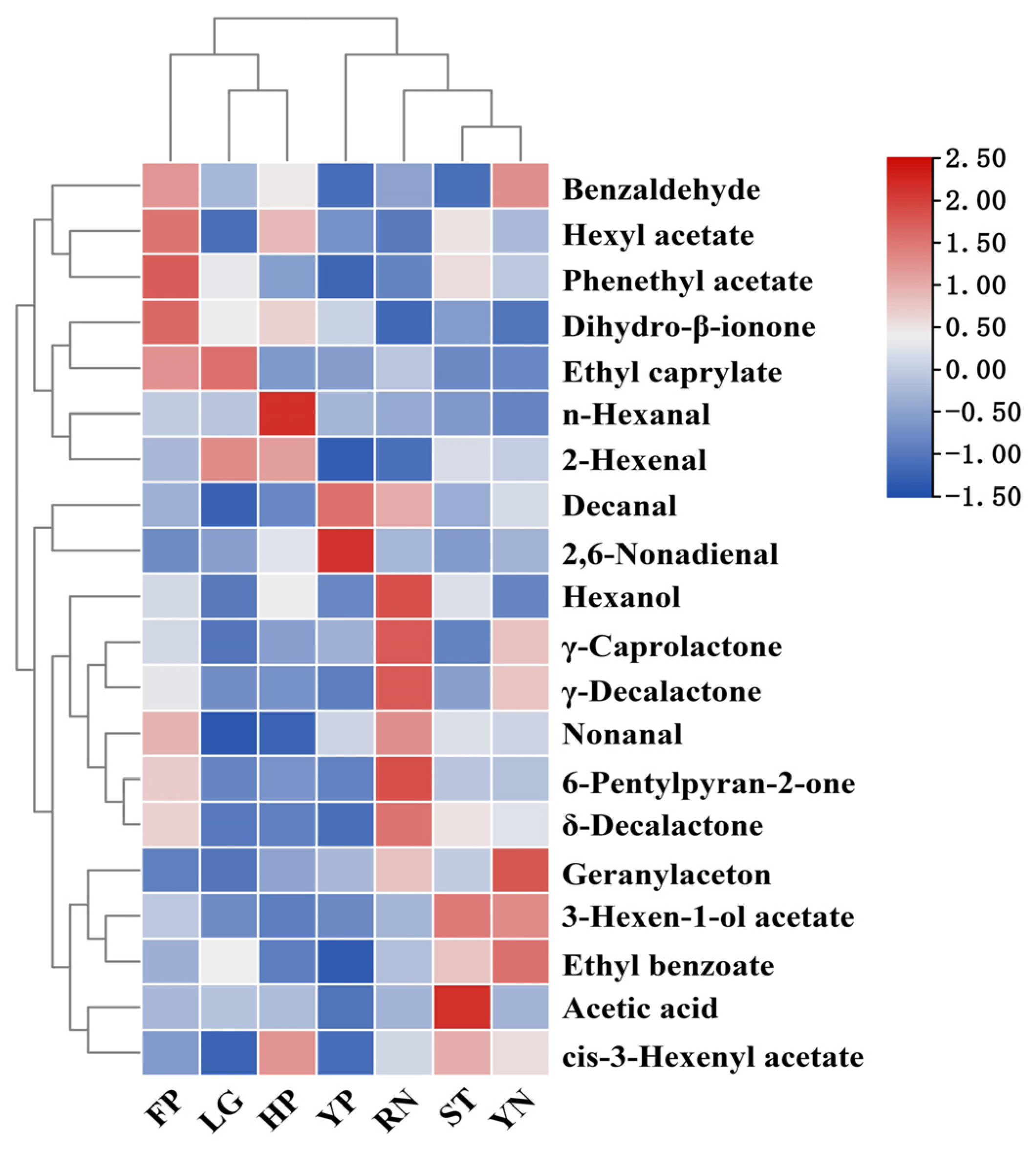
3.7.3. Construction of Peach Aroma Heat Map
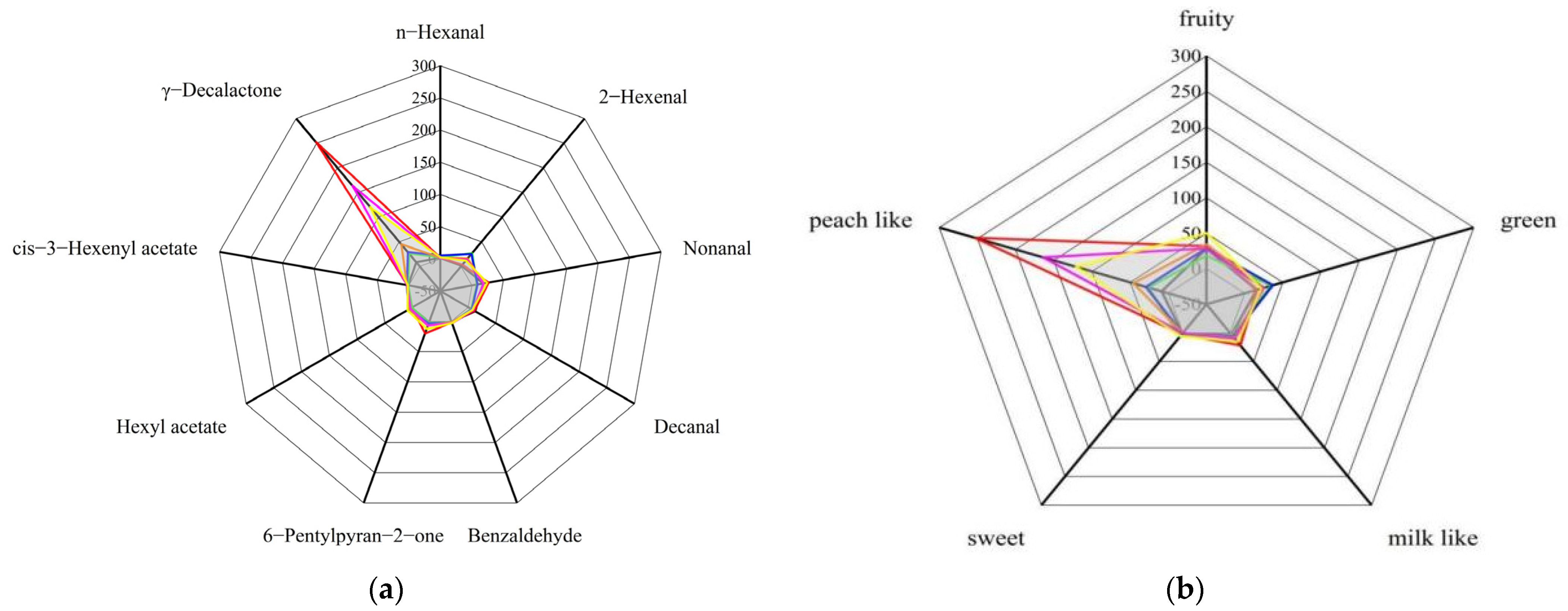
3.7.4. Identification of Peach Aroma Fingerprint
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bavconkralj, M.; Jug, T.; Komel, E.; Fajt, N.; Jarni, K.; Zivkovic, J.; Mujic, I.; Trutic, N. Effects of ripening degree and sample preparation on peach aroma profile characterization by headspace solid-phase microextraction. Turk. J. Agric. For. 2014, 38, 676–687. [Google Scholar] [CrossRef]
- Cao, K.; Yang, X.; Li, Y.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; Wang, L. New high-quality peach (Prunus persica L. Batsch) genome assembly to analyze the molecular evolutionary mechanism of volatile compounds in peach fruits. Plant J. 2021, 108, 281–295. [Google Scholar] [PubMed]
- Aubert, C.; Milhet, C. Distribution of the volatile compounds in the different parts of a white-fleshed peach (Prunus persica L. Batsch). Food Chem. 2007, 102, 375–384. [Google Scholar] [CrossRef]
- Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-Nose and GC-MS reveal a difference in the volatile profiles of white- and red-fleshed peach fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Yuan, Y.; Wu, D.; Chen, M.; Sun, Q.; Zhang, B.; Xu, C.; Chen, K. Improved peach peel color development by fruit bagging. Enhanced expression of anthocyanin biosynthetic and regulatory genes using white non-woven polypropylene as replacement for yellow paper. Sci. Hortic. 2015, 184, 142–148. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G. Physicochemical characteristics, vitamin C, and polyphenolic composition of four European commercial blood-flesh peach cultivars (Prunus persica L. Batsch). J. Food Compos. Anal. 2020, 86, 103337. [Google Scholar] [CrossRef]
- Khomich, L.M.; Perova, I.B.; Eller, K.I. Peach juice-puree nutritional profile. Vopr. Pitan. 2019, 88, 100–109. [Google Scholar]
- Basayigit, L.; Dedeoglu, M.; Akgul, H. The prediction of iron contents in orchards using VNIR spectroscopy. Turk. J. Agric. For. 2015, 39, 123–134. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach Prunus persica (L.) Batsch cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef]
- Kono, R.; Okuno, Y.; Nakamura, M.; Inada, K.-I.; Tokuda, A.; Yamashita, M.; Hidaka, R.; Utsunomiya, H. Peach (Prunus persica) extract inhibits angiotensin II-induced signal transduction in vascular smooth muscle cells. Food Chem. 2013, 139, 371–376. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC-DAD-ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann. Appl. Biol. 2008, 153, 11–23. [Google Scholar] [CrossRef]
- Aubert, C.; Gunata, Z.; Ambid, C.; Baumes, R. Changes in physicochemical characteristics and volatile constituents of yellow- and white-fleshed nectarines during maturation and artificial ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Filizzola, F.; Farina, V.; Lo Bianco, R. Changes in quality parameters and volatile aroma compounds in “fairtime-peach” during fruit development and ripening. Ital. J. Food Sci. 2007, 19, 3–13. [Google Scholar]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Leinen, L.J.; Swenson, V.A.; Juntunen, H.L.; McKay, S.E.; O’Hanlon, S.M.; Videau, P.; Gaylor, M.O. Profiling volatile constituents of homemade preserved foods prepared in early 1950s South Dakota (USA) using solid-phase microextraction (SPME) with gas chromatography-mass spectrometry (GC-MS) determination. Molecules 2019, 24, 660. [Google Scholar] [CrossRef]
- Wang, P.; Zhan, P.; Tian, H.; Zhang, F.; Xi, J. Characterization of the influence of thermal sterilization on the volatiles in flat peach juice. Anal. Lett. 2018, 51, 2340–2350. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Visai, C.; Vanoli, M. Volatile compound production during growth and ripening of peaches and nectarines. Sci. Hortic. 1997, 70, 15–24. [Google Scholar] [CrossRef]
- Mohammed, J.; Belisle, C.E.; Wang, S.; Itle, R.A.; Adhikari, K.; Chavez, D.J. Volatile profile characterization of commercial peach (Prunus persica) cultivars grown in Georgia, USA. Horticulturae 2021, 7, 516. [Google Scholar] [CrossRef]
- Xu, X.Y.; Miao, Y.Y.; Wang, H.; Ye, P.P.; Li, T.; Li, C.Y.; Zhao, R.R.; Wang, B.; Shi, X.W. A Snapshot of Microbial Succession and Volatile Compound Dynamics in Flat Peach Wine During Spontaneous Fermentation. Front. Microbiol. 2022, 13, 919047. [Google Scholar] [CrossRef] [PubMed]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Orazem, P.; Stampar, F.; Hudina, M. Fruit quality of Redhaven and Royal Glory peach cultivars on seven different rootstocks. J. Agric. Food Chem. 2011, 59, 9394–9401. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar]
- Orazem, P.; Stampar, F.; Hudina, M. Quality analysis of ‘Redhaven’ peach fruit grafted on 11 rootstocks of different genetic origin in a replant soil. Food Chem. 2011, 124, 1691–1698. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef]
- Juaniz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Manuel Moreno-Rojas, J.; Cid, C.; De Pena, M.-P. Influence of heat treatment on antioxidant capacity and (poly)phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gomathi, D.; Ravikumar, G.; Kalaiselvi, M.; Vidya, B.; Uma, C. In vitro free radical scavenging activity of ethanolic extract of the whole plant of Evolvulus alsinoides (L.) L. Chin. J. Integr. Med. 2015, 21, 453–458. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie IF, F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gong, Y.; Kerrihard, A.L.; Pegg, R.B. Characterization of the volatile compounds in raw and roasted Georgia Pecans by HS-SPME-GC-MS. J. Food Sci. 2018, 83, 2753–2760. [Google Scholar] [CrossRef]
- Niu, Y.; Deng, J.; Xiao, Z.; Zhu, J. Characterization of the major aroma-active compounds in peach (Prunus persica L. Batsch) by gas chromatography-olfactometry, flame photometric detection and molecular sensory science approaches. Food Res. Int. 2021, 147, 110457. [Google Scholar] [CrossRef]
- Ma, Y.; Li, T.; Xu, X.; Ji, Y.; Jiang, X.; Shi, X.; Wang, B. Investigation of volatile compounds, microbial succession, and their relation during spontaneous fermentation of Petit Manseng. Front. Microbiol. 2021, 12, 717387. [Google Scholar] [CrossRef]
- Su, C.; Zheng, X.; Zhang, D.; Chen, Y.; Xiao, J.; He, Y.; He, J.; Wang, B.; Shi, X. Investigation of sugars, organic acids, phenolic compounds, antioxidant activity and the aroma fingerprint of small white apricots grown in Xinjiang. J. Food Sci. 2020, 85, 4300–4311. [Google Scholar] [CrossRef]
- Delgado, C.; Crisosto, G.M.; Heymann, H.; Crisosto, C.H. Determining the primary drivers of liking to predict consumers’ acceptance of fresh nectarines and peaches. J. Food Sci. 2013, 78, S605–S614. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, C.; Yang, B.; Kallio, H.; Liu, P.; Ou, S. Regulation of phytochemicals in fruits and berries by environmental variation-Sugars and organic acids. J. Food Biochem. 2019, 43, e12642. [Google Scholar] [CrossRef] [PubMed]
- Saidani, F.; Gimeneze, R.; Aubert, C.; Chalot, G.; Betran, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Byrne, D.H.; Bacon, T.A. Founding clones of low-chill fresh market peach germplasm. Fruit Var. J. 1999, 53, 162–171. [Google Scholar]
- Reig, G.; Iglesias, I.; Gatius, F.; Alegre, S. Antioxidant capacity, quality, and anthocyanin and nutrient contents of several peach cultivars Prunus persica (L.) Batsch grown in Spain. J. Agric. Food Chem. 2013, 61, 6344–6357. [Google Scholar] [CrossRef]
- Muradoglu, F.; Kucuk, O. Determination of bioactive composition of some peach cultivars. J. Anim. Plant Sci. 2018, 28, 533–538. [Google Scholar]
- Lyu, J.; Liu, X.; Bi, J.; Jiao, Y.; Wu, X.; Ruan, W. Characterization of Chinese white-flesh peach cultivars based on principle component and cluster analysis. J. Food Sci. Technol. 2017, 54, 3818–3826. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT-Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Scordino, M.; Sabatino, L.; Muratore, A.; Belligno, A.; Gagliano, G. Phenolic characterization of Sicilian yellow flesh peach (Prunus persica L.) cultivars at different ripening stages. J. Food Qual. 2012, 35, 255–262. [Google Scholar] [CrossRef]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Pokorna, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical determination of the antioxidant potential of some less common fruit species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.; Xu, X.; Gan, R.; Zhang, Y.; Xia, E.; Li, H. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.; Wei, W.; Shen, J.; Ferguson, I.; Chen, K. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Montero-Prado, P.; Bentayeb, K.; Nerin, C. Pattern recognition of peach cultivars (Prunus persica L.) from their volatile components. Food Chem. 2013, 138, 724–731. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP-SPME with GC-MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]

| Cultivar | SSC 1 (%) | TA 2 (%) |
|---|---|---|
| FP | 12.20 ± 0.05 b | 0.81 ± 0.02 bc |
| LG | 7.49 ± 0.02 e | 0.89 ± 0.04 a |
| HP | 13.47 ± 0.02 a | 0.79 ± 0.01 c |
| YP | 6.96 ± 0.07 f | 0.82 ± 0.01 b |
| YN | 7.55 ± 0.03 e | 0.57 ± 0.03 e |
| RN | 8.84 ± 0.03 d | 0.67 ± 0.01 d |
| ST | 9.21 ± 0.04 c | 0.69 ± 0.02 d |
| Cultivar | TPC 1 (mg GAE/100 g) | TFC 2 (mg RE/100 g) |
|---|---|---|
| FP | 58.92 ± 1.29 b | 49.30 ± 0.02 a |
| LG | 59.37 ± 0.40 b | 37.28 ± 0.03 b |
| HP | 33.51 ± 2.19 e | 29.16 ± 0.05 d |
| YP | 36.20 ± 0.80 c | 29.05 ± 0.03 e |
| YN | 34.58 ± 0.39 d | 34.53 ± 0.07 c |
| RN | 35.26 ± 1.42 cd | 34.58 ± 0.05 c |
| ST | 62.77 ± 0.95 a | 49.37 ± 0.01 a |
| Cultivar | Fructose | Sorbitol | Glucose | Sucrose | Total |
|---|---|---|---|---|---|
| FP | 6.58 ± 0.49 e | 2.25 ± 0.12 c | 5.64 ± 0.32 d | 90.88 ± 1.15 b | 105.35 ± 1.93 b |
| LG | 9.82 ± 0.43 d | 3.72 ± 0.16 a | 7.52 ± 0.49 c | 52.24 ± 1.07 f | 73.30 ± 1.08 f |
| HP | 12.01 ± 0.30 c | 3.17 ± 0.28 b | 9.90 ± 0.31 b | 93.68 ± 1.33 a | 118.76 ± 0.96 a |
| YP | 13.94 ± 0.32 b | 1.57 ± 0.13 d | 12.27 ± 0.38 a | 41.98 ± 0.51 g | 69.77 ± 0.40 g |
| YN | 9.75 ± 0.33 d | 1.39 ± 0.07 de | 7.92 ± 0.54 c | 68.12 ± 1.40 d | 87.18 ± 0.47 d |
| RN | 6.25 ± 0.48 e | 1.19 ± 0.10 e | 4.77 ± 0.31 e | 76.95 ± 1.35 c | 89.16 ± 0.67 c |
| ST | 14.73 ± 0.37 a | 1.36 ± 0.12 de | 9.83 ± 0.85 b | 54.84 ± 0.72 e | 80.76 ± 0.62 e |
| Cultivar | Quinic Acid | Malic Acid | Citric Acid | Succinic Acid | Total | Sugar/Acid |
|---|---|---|---|---|---|---|
| FP | 2.81 ± 0.21 a | 3.54 ± 0.08 de | 0.44 ± 0.01 f | 4.13 ± 0.13 a | 10.93 ± 0.23 b | 9.64 ± 0.49 c |
| LG | 1.93 ± 0.12 d | 7.73 ± 0.28 a | 1.51 ± 0.10 b | 1.90 ± 0.15 b | 13.07 ± 0.17 a | 5.61 ± 0.62 e |
| HP | 2.00 ± 0.12 cd | 3.79 ± 0.14 d | 0.91 ± 0.03 c | 1.31 ± 0.05 d | 8.01 ± 0.16 d | 14.84 ± 0.25 a |
| YP | 2.34 ± 0.13 b | 3.53 ± 0.13 de | 1.75 ± 0.13 a | 1.48 ± 0.05 c | 9.10 ± 0.18 c | 7.66 ± 0.65 d |
| YN | 0.97 ± 0.09 f | 3.40 ± 0.12 e | 0.73 ± 0.02 d | 1.58 ± 0.05 c | 6.70 ± 0.19 f | 13.02 ± 0.72 b |
| RN | 1.55 ± 0.11 e | 4.93 ± 0.18 b | 0.34 ± 0.01 g | 0.64 ± 0.03 e | 7.47 ± 0.25 e | 11.93 ± 0.31 b |
| ST | 2.24 ± 0.13 bc | 4.52 ± 0.16 c | 0.55 ± 0.02 e | 0.43 ± 0.02 f | 7.74 ± 0.12 de | 10.43 ± 0.09 c |
| Cultivar | FP | LG | HP | YP | YN | RN | ST |
|---|---|---|---|---|---|---|---|
| p-coumaric | 0.63 ± 0.01 b | 1.22 ± 0.10 a | 0.46 ± 0.01 c | 0.30 ± 0.02 d | 0.27 ± 0.01 de | 0.20 ± 0.01 e | 0.19 ± 0.01 e |
| procyanidins b | 1.96 ± 0.13 b | 0.94 ± 0.12 c | 1.03 ± 0.09 c | 1.98 ± 0.06 b | 0.45 ± 0.04 d | 0.60 ± 0.08 d | 6.65 ± 0.37 a |
| neochlorogenic acid | 0.76 ± 0.04 d | 3.60 ± 0.18 b | 0.62 ± 0.01 d | 1.70 ± 0.02 c | 0.17 ± 0.01 e | 1.54 ± 0.04 c | 5.68 ± 0.37 a |
| catechinic | 1.81 ± 0.05 b | 1.00 ± 0.03 c | 1.83 ± 0.05 b | 1.84 ± 0.12 b | 0.39 ± 0.01 d | 0.38 ± 0.01 d | 4.62 ± 0.14 a |
| vanillic acid | 0.08 ± 0.01 c | 0.14 ± 0.01 a | 0.11 ± 0.02 b | 0.05 ± 0.01 de | 0.04 ± 0.01 e | 0.06 ± 0.01 d | 0.16 ± 0.01 a |
| chlorogenic acid | 3.25 ± 0.19 c | 6.55 ± 0.20 b | 0.98 ± 0.08 d | 0.87 ± 0.06 d | 0.22 ± 0.01 e | 2.83 ± 0.30 c | 10.22 ± 0.59 a |
| epicatechin | 0.44 ± 0.01 a | 0.29 ± 0.01 b | 0.17 ± 0.01 e | 0.27 ± 0.01 c | 0.06 ± 0.01 f | n.d | 0.24 ± 0.01 d |
| rutin | 0.34 ± 0.01 d | 0.86 ± 0.06 a | 0.38 ± 0.03 c | 0.08 ± 0.01 f | 0.29 ± 0.01 e | 0.54 ± 0.01 b | 0.12 ± 0.01 f |
| quercetin | 0.22 ± 0.01 b | 0.08 ± 0.01 d | 0.15 ± 0.01 c | 0.14 ± 0.01 c | 0.14 ± 0.01 c | 0.07 ± 0.01 d | 0.35 ± 0.02 a |
| kaempferol | 0.03 ± 0.01 c | n.d | 0.02 ± 0.01 d | n.d | 0.08 ± 0.01 a | n.d | 0.05 ± 0.01 b |
| total | 9.51 ± 0.18 c | 14.68 ± 0.09 b | 5.75 ± 0.28 f | 7.22 ± 0.12 d | 2.11 ± 0.05 g | 6.22 ± 0.21 e | 28.28 ± 0.47 a |
| Cultivar | Radical-Scavenging Capacity | Reducing Capacity | ||
|---|---|---|---|---|
| ABTS+ | DPPH | FRAP | CUPRAC | |
| FP | 312.61 ± 0.23 a | 394.55 ± 0.13 a | 311.79 ± 0.30 a | 282.83 ± 0.21 a |
| LG | 197.56 ± 0.01 f | 293.52 ± 0.34 e | 149.24 ± 0.18 f | 169.83 ± 0.10 f |
| HP | 203.21 ± 0.24 e | 296.42 ± 0.19 d | 153.07 ± 0.08 e | 185.03 ± 0.07 e |
| YP | 236.91 ± 0.03 c | 390.84 ± 0.13 b | 243.57 ± 0.21 c | 204.84 ± 0.04 c |
| YN | 227.46 ± 0.14 d | 280.95 ± 0.07 f | 189.93 ± 0.19 d | 201.49 ± 0.13 d |
| RN | 155.81 ± 0.18 g | 252.68 ± 0.15 g | 147.81 ± 0.11 g | 184.99 ± 0.27 e |
| ST | 311.99 ± 0.04 b | 384.53 ± 0.20 c | 306.86 ± 0.19 b | 243.16 ± 0.17 b |
| Volatile Compounds | RI | FP | LG | HP | YP | YN | RN | ST |
OT 1 (μg/kg) | Flavor Description |
| Esters and lactones | ||||||||||
| Hexyl acetate | 1276 | 9.53 | 1.22 | 7.51 | 2.53 | 1.65 | 4.03 | 6.27 | 2 | Sweet, fruity |
| 3-Hexen-1-ol acetate | 1320 | 1.12 | 0.31 | 0.13 | 0.28 | 0.87 | 2.64 | 2.83 | 7.8 | Banana |
| cis-3-Hexenyl acetate | 1337 | 1.71 | 1.13 | 3.31 | 1.24 | 2.33 | 2.72 | 3.11 | 8 | Fruity, green |
| Ethyl caprylate | 1436 | 5.27 | 6.02 | 0.80 | 0.96 | 2.11 | 0.31 | 0.36 | 5 | Banana, pear |
| Ethyl benzoate | 1658 | 0.07 | 0.08 | 0.06 | 0.05 | 0.07 | 0.11 | 0.09 | 60 | Floral, sweet |
| Phenethyl acetate | 1808 | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 3000 | Rose, sweet |
| γ-Caprolactone | 1709 | 0.04 | <0.01 | 0.02 | 0.02 | 0.07 | 0.05 | 0.01 | 260 | Milk-like, nutty |
| γ-Decalactone | 2109 | 120.36 | 26.15 | 29.15 | 8.77 | 252.17 | 163.58 | 45.21 | 0.7 | Peach-like |
| δ-Decalactone | 2193 | 0.68 | 0.09 | 0.12 | 0.05 | 0.99 | 0.54 | 0.63 | 31 | Cream, peach-like |
| Alcohols and aldehydes | ||||||||||
| Hexanol | 1341 | 0.01 | <0.01 | 0.01 | <0.01 | 0.02 | <0.01 | 0.01 | 500 | Fruity, fatty |
| n-Hexanal | 1093 | 2.34 | 2.24 | 5.14 | 1.99 | 1.83 | 1.27 | 1.57 | 21 | Green, herb |
| 2-Hexenal | 1213 | 11.81 | 26.82 | 25.18 | 1.22 | 3.26 | 14.10 | 15.81 | 30 | Green |
| Nonanal | 1396 | 23.92 | 7.75 | 8.71 | 17.95 | 263.90 | 17.93 | 18.91 | 1 | Orange, rose, fat |
| Decanal | 1506 | 7.83 | 5.57 | 6.64 | 12.53 | 11.15 | 9.04 | 7.76 | 1 | Orange |
| Benzaldehyde | 1546 | 2.96 | 1.76 | 2.32 | 1.05 | 1.57 | 3.01 | 1.07 | 320 | Nutty |
| 2,6-Nonadienal | 1715 | 0.75 | 0.16 | 0.09 | 0.29 | 0.08 | 0.15 | 0.04 | 17.28 | Green, cucumber |
| Others | ||||||||||
| Acetic acid | 1492 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 22,000 | Sour |
| Dihydro-β-ionone | 1825 | 1.38 | 0.12 | 1.74 | 2.00 | 0.74 | 0.27 | 2.98 | 7 | Floral, fruity, wood |
| Geranylaceton | 1840 | 0.04 | 0.03 | 0.05 | 0.06 | 0.09 | 0.13 | 0.07 | 60 | Fruity |
| 6-Pentylpyran-2-one | 2175 | 12.41 | 2.17 | 3.37 | 1.97 | 19.99 | 6.89 | 7.23 | 0.9 | Cheese, milk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods 2022, 11, 2944. https://doi.org/10.3390/foods11192944
Wu H, Xu Y, Wang H, Miao Y, Li C, Zhao R, Shi X, Wang B. Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods. 2022; 11(19):2944. https://doi.org/10.3390/foods11192944
Chicago/Turabian StyleWu, Huimin, Youyou Xu, Huan Wang, Yuanyuan Miao, Chunyan Li, Ruirui Zhao, Xuewei Shi, and Bin Wang. 2022. "Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang" Foods 11, no. 19: 2944. https://doi.org/10.3390/foods11192944
APA StyleWu, H., Xu, Y., Wang, H., Miao, Y., Li, C., Zhao, R., Shi, X., & Wang, B. (2022). Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods, 11(19), 2944. https://doi.org/10.3390/foods11192944