Malachite Green Dye Decoloration over Au/TiO2-Nanotubes Photocatalyst under Simulate Visible-Light Irradiation
Abstract
1. Introduction
2. Materials and Methods
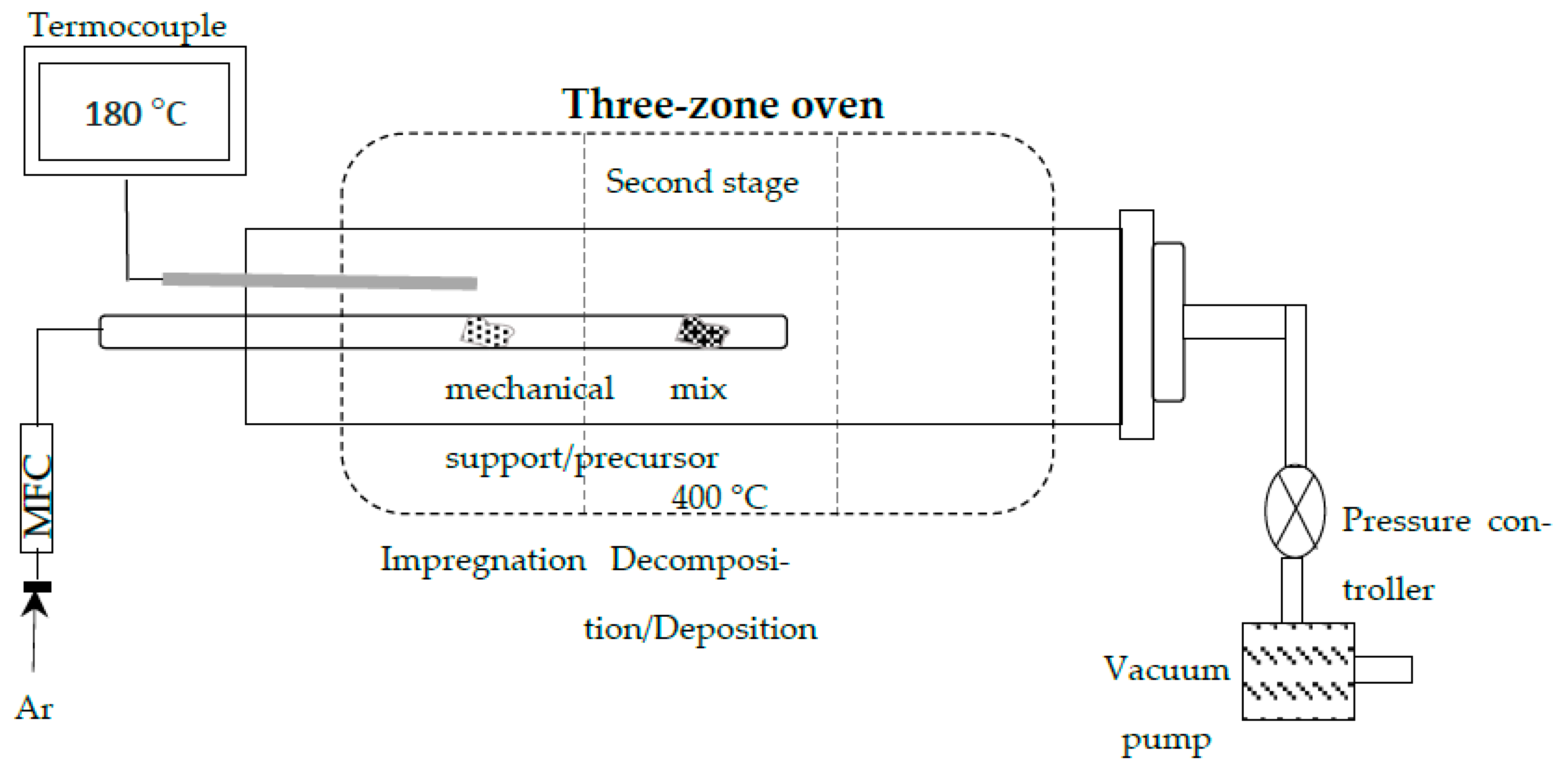
Materials Synthesis
3. Materials Characterization
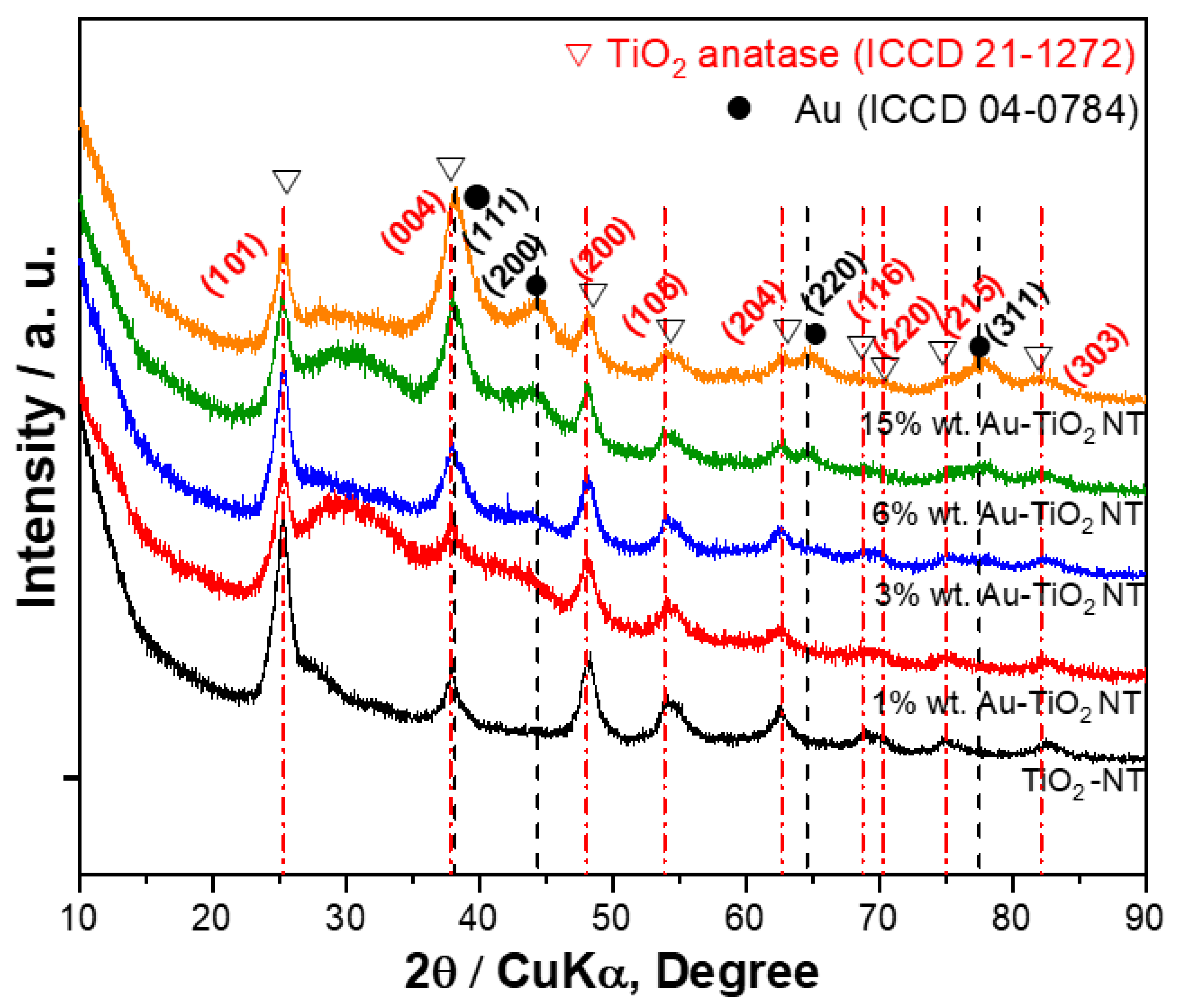
3.1. X-ray Diffraction
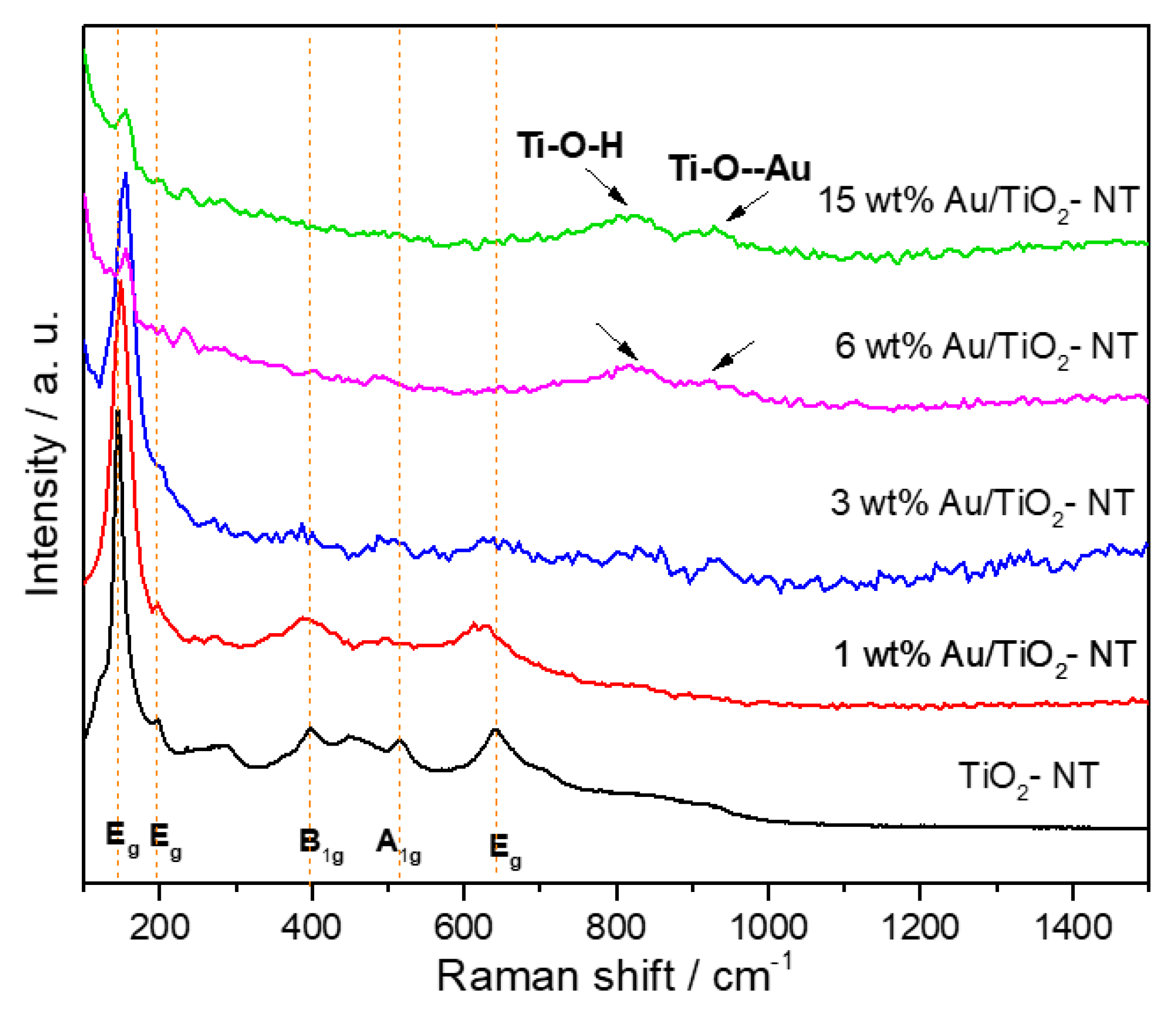
3.2. Raman Spectroscopy
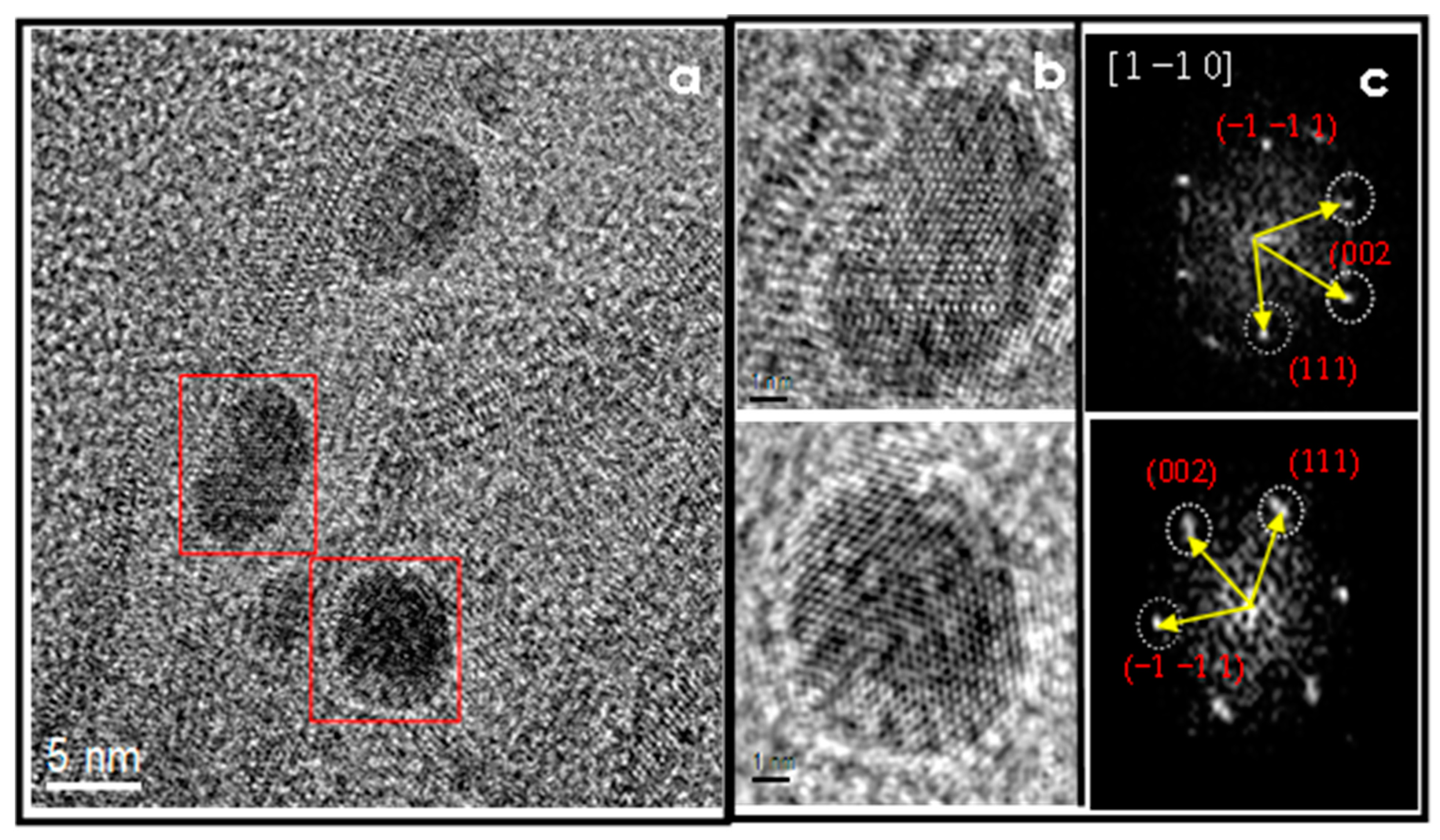
3.3. HAADF-STEM and HRTEM
3.4. X-ray Photoelectronic Spectroscopy (XPS)
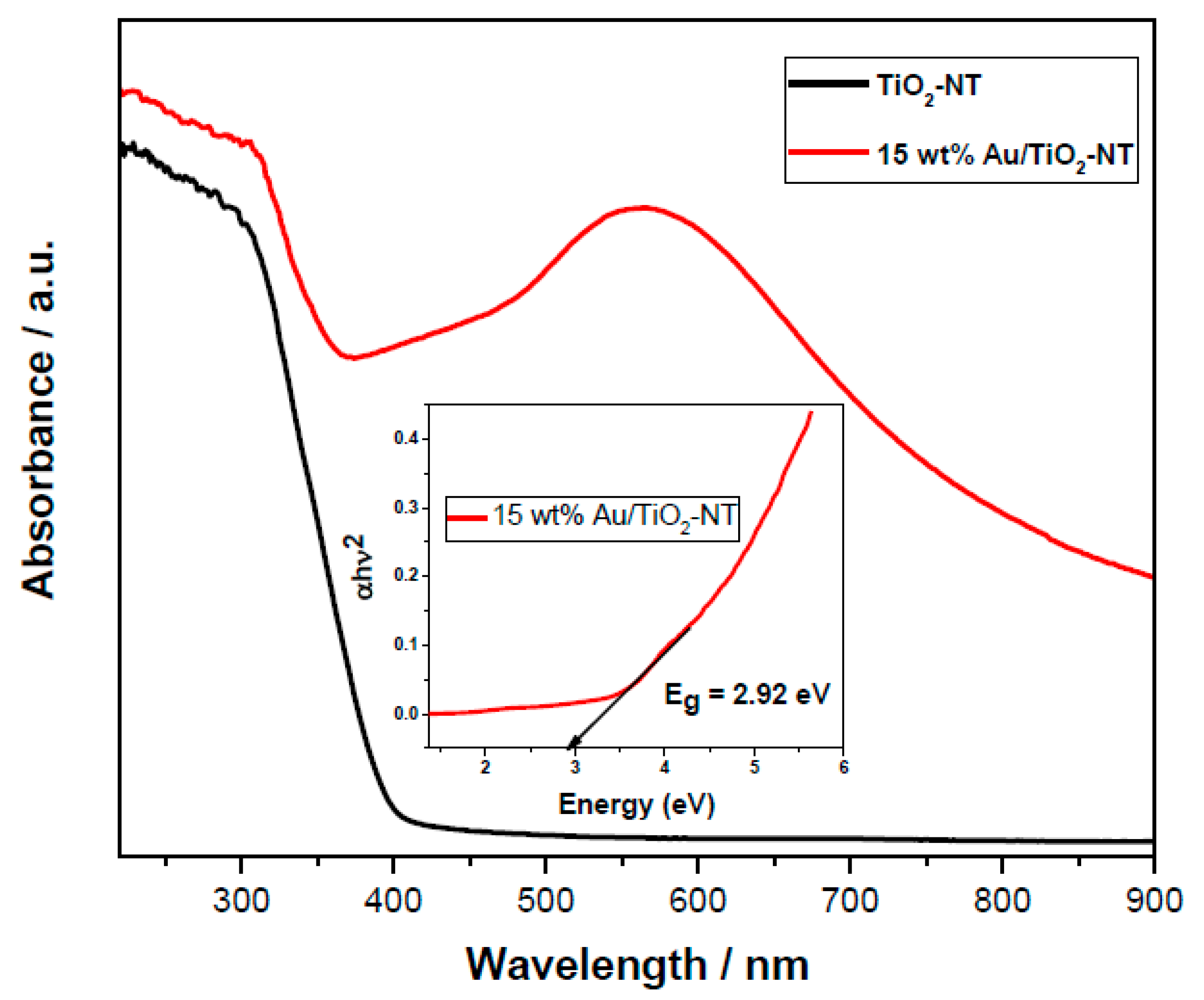
3.5. UV–Vis Spectroscopy
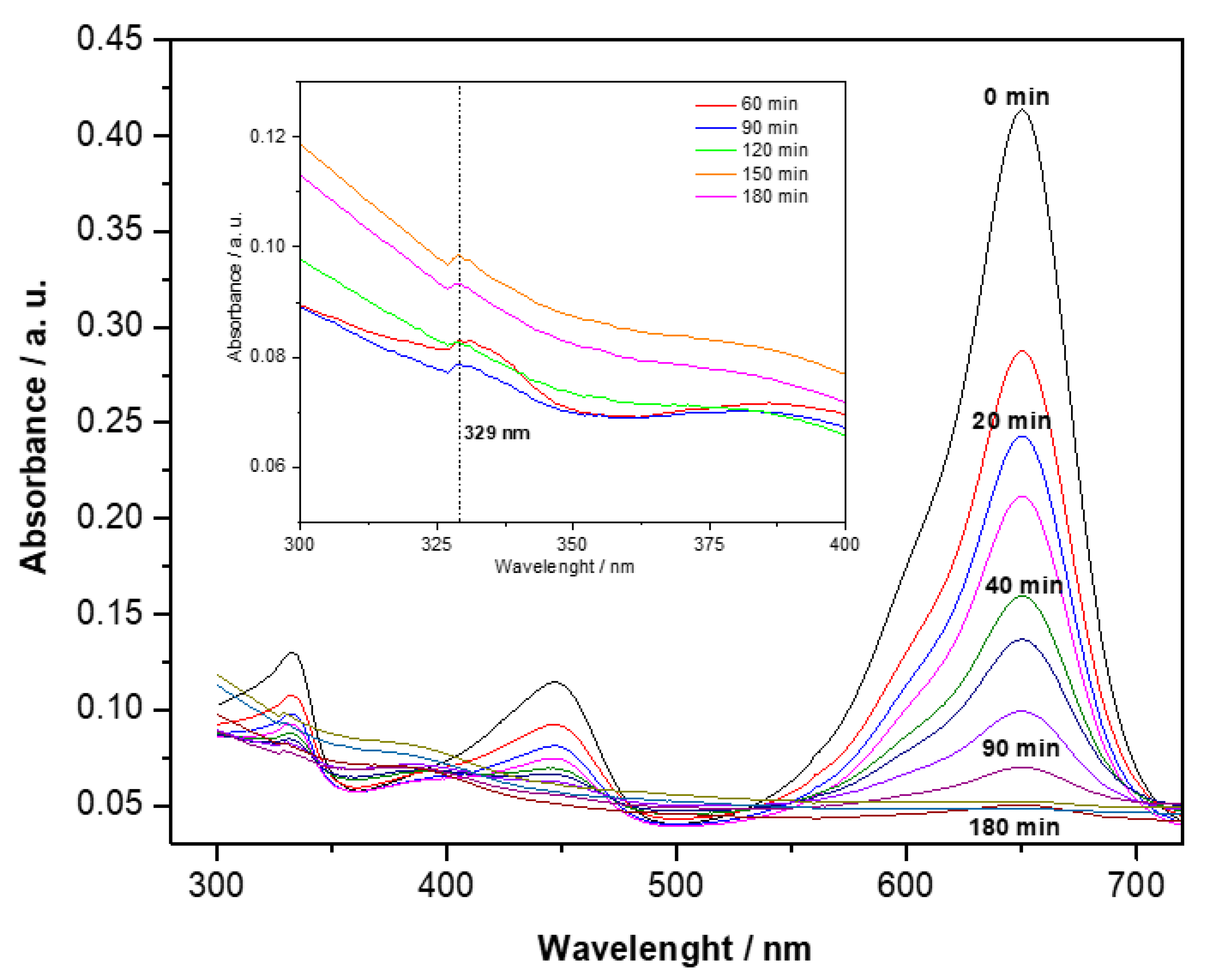
3.6. Photocatalytic Evaluation
4. Results
4.1. X-ray Diffraction
4.2. Raman Spectroscopy
4.3. Scanning Transmission Electron Microscopy (STEM)
4.4. HAADF-STEM
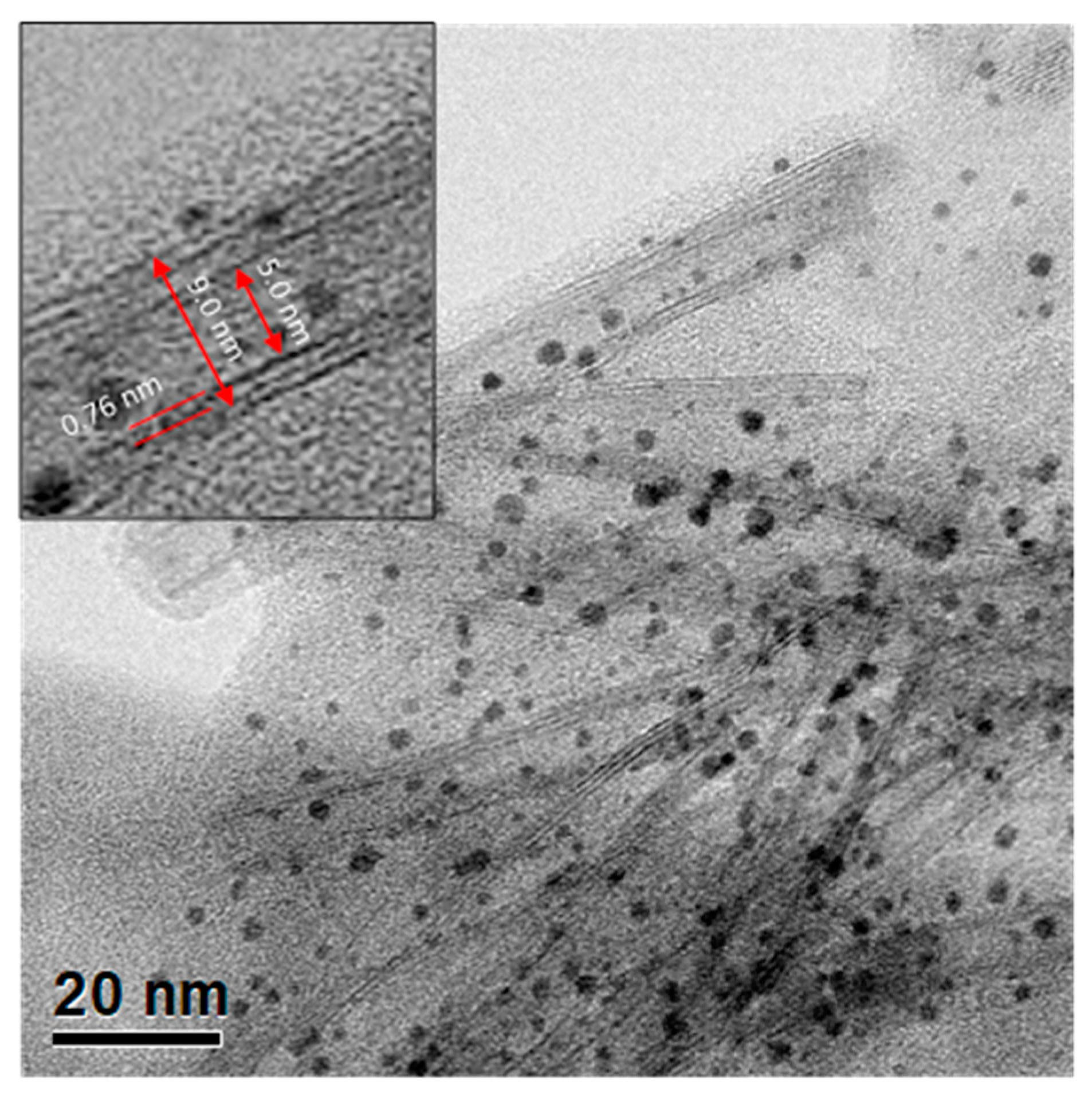
4.5. HR-TEM
4.6. X-ray Photoelectronic Spectroscopy
4.7. UV–Vis Spectroscopy
4.8. Photocatalytic Evaluation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahoo, R.; Das, S.K. Ultraviolet photocatalytic dye decomposition of malachite green dye by using cost effective ZnO nanoparticles. AIP Conf. Proc. 2019, 2166, 020021. [Google Scholar]
- Wang, Q.; Cai, C.; Wang, M.; Guo, Q.; Wang, B.; Luo, W.; Wang, Y.; Zhang, C.; Zhou, L.; Zhang, D.; et al. Efficient photocatalytic decoloration of Malachite Green in seawater by the hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) reduced graphene oxide (RGO)/Ni foam. Materials 2018, 11, 1004. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Z.; Ji, Y.; Hu, X.; Sun, C.; Yang, S.; Wang, L.; Wang, Q.; Fang, D. Photodecoloration of malachite green under simulated and natural irradiation: Kinetics, products, and pathways. J. Hazard. Mater. 2015, 285, 127–136. [Google Scholar] [CrossRef]
- Baeissa, E.S. Photocatalytic decoloration of malachite green dye using Au/NaNbO3 nanoparticles. J. Alloys Compd. 2016, 672, 564–570. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Abdel-Kader Ahmed, S.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic decoloration of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef] [PubMed]
- Amar, I.A.; Harara, H.M.; Baqul, Q.A.; Abdulqadir, M.A.; Altohami, F.A.; Ahwidi, M.M.; Abdalsamed, I.A.; Saleh, F.A. Nanoscience and Materials Photocatalytic decoloration of malachite green dye under UV light irradiation using calcium-doped ceria nanoparticles. Asian J. Nanosci. Mater. 2020, 3, 1–14. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, T.; Qu, C.; Zhang, S.; Li, X.; Qu, Y. Photolyase-Like Catalytic Behavior of CeO2. Nano Lett. 2019, 19, 8270–8277. [Google Scholar] [CrossRef]
- Kgoetlana, C.M.; Malinga, S.P.; Dlamini, L.N. Photocatalytic decoloration of chlorpyrifos with Mn-WO3/SnS2 heterostructure. Catalysts 2020, 10, 699. [Google Scholar] [CrossRef]
- Yao, S.; Liu, J.; Liu, F.; Wang, B.; Ding, Y.; Li, L.; Liu, C.; Huang, F.; Fang, J.; Lin, Z.; et al. Robust route to photocatalytic nitrogen fixation mediated by capitalizing on defect-tailored InVO4 nanosheets. Environ. Sci. Nano 2022, 9, 1996–2005. [Google Scholar] [CrossRef]
- Gu, J.; Peng, Y.; Zhou, T. Porphyrin-based framework materials for energy conversion. Nano Res. Energy 2022, 1, e9120009. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent developments of TiO2-based photocatalysis in the hydrogen evolution and photodecoloration: A review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic decoloration of pharmaceutical and pesticide compounds (PPCs) using doped TiO2 nanomaterials: A review. Water-Energy Nexus. 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Prado, A.G.S.; Costa, L.L. Photocatalytic decouloration of malachite green dye by application of TiO2 nanotubes. J. Hazard. Mater. 2009, 169, 297–301. [Google Scholar] [CrossRef]
- Palcheva, R.; Dimitrov, L.; Tyuliev, G.; Spojakina, A.; Jiratova, K. TiO2 nanotubes supported NiW hydrodesulphurization catalysts: Characterization and activity. Appl. Surf. Sci. 2013, 265, 309–316. [Google Scholar] [CrossRef]
- Toledo-Antonio, J.A.; Cortés-Jácome, M.A.; Angeles-Chávez, C.; Escobar, J.; Barrera, M.C.; López-Salinas, E. Highly active CoMoS phase on titania nanotubes as new hydrodesulfurization catalysts. Appl. Catal. B Environ. 2009, 90, 213–223. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, X.; Zhu, B.; Wang, S.; Zhang, S.; Huang, W. Synthesis and characterization of TiO2 nanotube supported Rh-nanoparticle catalysts for regioselective hydroformylation of vinyl acetate. RSC Adv. 2014, 4, 62215–62222. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 Nanotubes: “Colorful” Reactivity and Designing Site-Specific Photocatalytic Centers into TiO2 Nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef]
- Camposeco, R.; Castillo, S.; Navarrete, J.; Gomez, R. Synthesis, characterization and photocatalytic activity of TiO2 nanostructures: Nanotubes, nanofibers, nanowires and nanoparticles. Catal. Today 2016, 266, 90–101. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Bamoharram, F.F.; Ahmadpour, A.; Maydannik, P.; Sillanpää, M. Photocatalytic decoloration of nitrobenzene by gold nanoparticles decorated polyoxometalate immobilized TiO2 nanotubes. Sep. Purif. Technol. 2016, 171, 62–68. [Google Scholar] [CrossRef]
- Khawaji, M.; Chadwick, D. Au-Pd Bimetallic Nanoparticles Immobilised on Titanate Nanotubes: A Highly Active Catalyst for Selective Oxidation. ChemCatChem 2017, 9, 4353–4363. [Google Scholar] [CrossRef]
- Sandoval, A.; Zanella, R.; Klimova, T.E. Titania nanotubes decorated with anatase nanocrystals as support for active and stable gold catalysts for CO oxidation. Catal. Today 2017, 282, 140–150. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Han, J.K.; Lee, Y.B.; Thumthan, O.; An, K.S. Au NPs decorated TiO2 nanotubes array candidate for UV photodetectors. Prog. Nat. Sci. Mater. Int. 2017, 27, 641–646. [Google Scholar] [CrossRef]
- Li, L.; Hasan, I.M.; Qiao, J. Copper as a single metal atom based photo-, electro- and photoelectrochemical cata-lyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res. Energy 2022, 2790–8119. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Salama, R.S. Gold-selenide quantum dots supported onto cesium ferrite nanocompo-sites for the efficient degradation of rhodamine B. Heliyon 2022, 8, e09652. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional Synergistic Effect of Cs–Au NPs on the Performance of Cs–Au/MgFe2O4 Catalysts in Catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. 2022. [Google Scholar] [CrossRef]
- Pandikumar, A.; Ramaraj, R. Photocatalytic reduction of hexavalent chromium at gold nanoparticles modified titania nanotubes. Mater. Chem. Phys. 2013, 141, 629–635. [Google Scholar] [CrossRef]
- Gao, Y.; Fan XBin Zhang, W.F.; Zhao, Q.S.; Zhang, G.L.; Zhang, F.B.; Li, Y. Enhanced photocatalytic decoloration of methyl orange by Au/TiO2 nanotubes. Mater. Lett. 2014, 130, 1–4. [Google Scholar] [CrossRef]
- Xiao, F. Self-assembly preparation of gold nanoparticles-TiO2 nanotube arrays binary hybrid nanocomposites for photocatalytic applications. J. Mater. Chem. 2012, 22, 7819–7830. [Google Scholar] [CrossRef]
- Xu, W.; Qi, M.; Li, X.; Liu, X.; Wang, L.; Yu, W.; Liu, M.; Lan, A.; Zhou, Y.; Song, Y. TiO2 nanotubes modified with Au nanoparticles for visible-light enhanced antibacterial and anti-inflammatory capabilities. J. Electroanal. Chem. 2019, 842, 66–73. [Google Scholar] [CrossRef]
- Zhang, X.; Yue, D.; Zhang, L.; Lin, S. Three-dimensional flexible Au nanoparticles-decorated TiO2 nanotube arrays for photoelectrochemical biosensing. J. Mater. Sci. Technol. 2020, 56, 162–169. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Faraji, M.; Momeni, M.M.; Ershad, S. An innovative electrochemical approach for voltammetric determination of levodopa using gold nanoparticles doped on titanium dioxide nanotubes. Microchim. Acta 2011, 172, 103–108. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, W.; Liang, W.; Yang, F. Au nanocrystals decorated TiO2 nanotube arrays as anode material for lithium ion batteries. Appl. Surf. Sci. 2019, 476, 948–958. [Google Scholar] [CrossRef]
- Maria Dalcin Fornari, A.; de Araujo, M.B.; Bergamin Duarte, C.; Machado, G.; Teixeira, S.R.; Weibel, D.E. Photocatalytic reforming of aqueous formaldehyde with hydrogen generation over TiO2 nanotubes loaded with Pt or Au nanoparticles. Int. J. Hydrogen Energy 2016, 41, 11599–11607. [Google Scholar] [CrossRef]
- Pandikumar, A.; Manonmani, S.; Ramaraj, R. TiO2-Au nanocomposite materials embedded in polymer matrices and their application in the photocatalytic reduction of nitrite to ammonia. Catal. Sci. Technol. 2012, 2, 345–353. [Google Scholar] [CrossRef]
- Méndez-Cruz, M.; Ramírez-Solís, J.; Zanella, R. CO oxidation on gold nanoparticles supported over titanium oxide nanotubes. Catal. Today 2011, 166, 172–179. [Google Scholar] [CrossRef]
- Liu, X.; Jaramillo, T.F.; Kolmakov, A.; Baeck, S.H.; Moskovits, M.; Stucky, G.D.; McFarland, E.W. Synthesis of Au nanoclusters supported upon a TiO2 nanotube array. J. Mater. Res. 2005, 20, 1093–1096. [Google Scholar] [CrossRef]
- Encarnación-Gómez, C.; Cortés-Jácome, M.A.; Medina-Mendoza, A.K.; Chávez, C.A.; Hernández-Cruz, M.G.; López, I.C.; Hernández-Cortéz, J.; Salinas, E.L.; García, J.V.; Toledo-Antonio, J. Uniformly sized Pt nanoparticles dispersed at high loading on Titania nanotubes. Appl. Catal. A Gen. 2020, 600, 117631. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Massard, C.; Pairis, S.; Sibaud, Y.; Blavignac, C.; Awitor, O.K. Boosted Photoactivity of Titania Nanotube Layers Doped with a Suspension of Gold Nanoparticles. Adv. Nanoparticles 2017, 6, 114–127. [Google Scholar] [CrossRef]
- Smith, K.A.; Savva, A.I.; Deng, C.; Wharry, J.P.; Hwang, S.; Su, D.; Wang, Y.; Gong, J.; Xu, T.; Butt, D.P.; et al. Effects of proton irradiation on structural and electrochemical charge storage properties of TiO2 nanotube electrodes for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 11815–11824. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Torrente-Murciano, L.; Lapkin, A.A.; Chadwick, D. Synthesis of high aspect ratio titanate nanotubes. J. Mater. Chem. 2010, 20, 6484–6489. [Google Scholar] [CrossRef]
- Pótári, G.; Madarász, D.; Nagy, L.; László, B.; Sápi, A.; Oszkó, A.; Kukovecz, A.; Erdőhelyi, A.; Kónya, Z.; Kiss, J. Rh-induced support transformation phenomena in titanate nanowire and nanotube catalysts. Langmuir 2013, 29, 3061–3072. [Google Scholar] [CrossRef]
- Gao, T.; Fjellvåg, H.; Norby, P. Crystal structures of titanate nanotubes: A Raman scattering study. Inorg. Chem. 2009, 48, 1423–1432. [Google Scholar] [CrossRef]
- Pusztai, P.; Puskás, R.; Varga, E.; Erdőhelyi, A.; Kukovecz, A.; Kónya, Z.; Kiss, J. Influence of gold additives on the stability and phase transformation of titanate nanostructures. Phys. Chem. Chem. Phys. 2014, 16, 26786–26797. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Synthesis and characterization of ion-exchangeable titanate nanotubes. Chem.-A Eur. J. 2003, 9, 2229–2238. [Google Scholar] [CrossRef]
- Rendón-Rivera, A.; Toledo-Antonio, J.A.; Cortés-Jácome, M.A.; Angeles-Chávez, C. Generation of highly reactive OH groups at the surface of TiO2 nanotubes. Catal. Today 2011, 166, 18–24. [Google Scholar] [CrossRef]
- Cortes-Jácome, M.A.; Morales, M.; Angeles Chavez, C.; Ramírez-Verduzco, L.F.; López-Salinas, E.; Toledo-Antonio, J.A. WOx/TiO2 catalysts via titania nanotubes for the oxidation of dibenzothiophene. Chem. Mater. 2007, 19, 6605–6614. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, srep32355. [Google Scholar] [CrossRef]
- Veziroglu, S.; Obermann, A.L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Decoloration over TiO2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Hu, Y.; Jiang, H.; Li, C. In situ surface hydrogenation synthesis of Ti3+ self-doped TiO2 with enhanced visible light photoactivity. Nanoscale 2014, 6, 9078–9084. [Google Scholar] [CrossRef] [PubMed]
- Sathasivam, S.; Bhachu, D.S.; Lu, Y.; Chadwick, N.; Althabaiti, S.A.; Alyoubi, A.O.; Basahel, S.N.; Carmalt, C.; Parkin, I.P. Tungsten doped TiO2 with enhanced photocatalytic and optoelectrical properties via aerosol assisted chemical vapor deposition. Sci. Rep. 2015, 5, 10952. [Google Scholar] [CrossRef]
- Tóth, M.; Kiss, J.; Oszkó, A.; Pótári, G.; László, B.; Erdohelyi, A. Hydrogenation of carbon dioxide on Rh, Au and Au-Rh bimetallic clusters supported on titanate nanotubes, nanowires and TiO2. Top. Catal. 2012, 55, 747–756. [Google Scholar] [CrossRef]
- Gandla, S.; Gollu, S.R.; Sharma, R.; Sarangi, V.; Gupta, D. Dual role of boron in improving electrical performance and device stability of low temperature solution processed ZnO thin film transistors. Appl. Phys. Lett. 2015, 107, 152102. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Guo, H.; Saghayezhian, M.; Wang, G.; Chen, L.; Chen, W.; Zhang, J.; Plummer, E. Unusual Fe–H bonding associated with oxygen vacancies at the (001) surface of Fe3O4. Surf. Sci. 2017, 655, 25–30. [Google Scholar] [CrossRef]
- Feil, A.F.; Migowski, P.; Scheffer, F.R.; Pierozan, M.D.; Corsetti, R.R.; Rodrigues, M.; Pezzi, R.P.; Machado, G.; Amaral, L.; Teixeira, S.R.; et al. Growth of TiO2 nanotube arrays with simultaneous Au nanoparticles impregnation: Photocatalysts for hydrogen production. J. Braz. Chem. Soc. 2010, 21, 1359–1365. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef] [PubMed]
- Pandikumar, A.; Ramaraj, R. Titanium dioxide-gold nanocomposite materials embedded in silicate sol-gel film catalyst for simultaneous photodecoloration of hexavalent chromium and methylene blue. J. Hazard. Mater. 2012, 203–204, 244–250. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Juárez, R.; Marino, T. Influence of excitation wavelength (UV or visible light) on the photocatalytic activity of titania containing gold nanoparticles for the generation of hydrogen or oxygen from water. J. Am. Chem. 2011, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Corma, A.; García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, D.; Shiraishi, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Gold nanoparticles located at the interface of anatase/rutile TiO2 particles as active plasmonic photocatalysts for aerobic oxidation. J. Am. Chem. Soc. 2012, 134, 6309–6315. [Google Scholar] [CrossRef]
- Kiss, J.; Pusztai, P.; Ovari, L.; Baan, K.; Merza, G.; Erdohelyi, A.; Kukovecz, A.; Konya, Z. Decoration of Titanate Nanowires and Nanotubes by Gold Nanoparticles: XPS, HRTEM and XRD Characterization. E-J. Surf. Sci. Nanotechnol. 2014, 12, 252–258. [Google Scholar] [CrossRef][Green Version]
- Lavand, A.B.; Bhatu, M.N.; Malghe, Y.S. Visible light photocatalytic decoloration of malachite green using modified titania. J. Mater. Res. Technol. 2019, 8, 299–308. [Google Scholar] [CrossRef]
- Navarro, P.; Zapata, J.P.; Gotor, G.; Gonzalez-Olmos, R.; Gómez-López, V.M. Decoloration of malachite green by a pulsed light/H2O2 process. Water Sci. Technol. 2019, 79, 260–269. [Google Scholar] [CrossRef]
- Afshar, S.; Samari Jahromi, H.; Jafari, N.; Ahmadi, Z.; Hakamizadeh, M. Decoloration of malachite green oxalate by UV and visible lights irradiation using Pt/TiO2/SiO2 nanophotocatalyst. Sci. Iran. 2011, 18, 772–779. [Google Scholar] [CrossRef]
- Kumar, A. A Review on the Factors Affecting the Photocatalytic Decoloration of Hazardous Materials. Mater. Sci. Eng. Int. J. 2017, 1, 106–114. [Google Scholar]
- Murugan, R.; Kashinath, L.; Subash, R.; Sakthivel, P.; Byrappa, K.; Rajendran, S.; Ravi, G. Pure and alkaline metal ion (Mg, Ca, Sr, Ba) doped cerium oxide nanostructures for photo decoloration of methylene blue. Mater. Res. Bull. 2018, 97, 319–325. [Google Scholar] [CrossRef]
- Paramasivam, I.; Jha, H.; Liu, N.; Schmuki, P. A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 2012, 8, 3073–3103. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. The Formation of Colloidal. Gold J. Phys. Chem. 1953, 57, 670–673. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y. Investigation of the NaBH4-Induced Aggregation of Au Nanoparticles. Langmuir 2010, 26, 9214–9223. [Google Scholar] [CrossRef]
- Zhang, Q.; Lima, D.Q.; Lee, I.; Zaera, F.; Chi, M.; Yin, Y. A Highly Active Titanium Dioxide Based Visible-Light Photocatalyst with Nonmetal Doping and Plasmonic Metal Decoration. Angew. Chem. Int. 2011, 50, 7088–7092. [Google Scholar] [CrossRef]

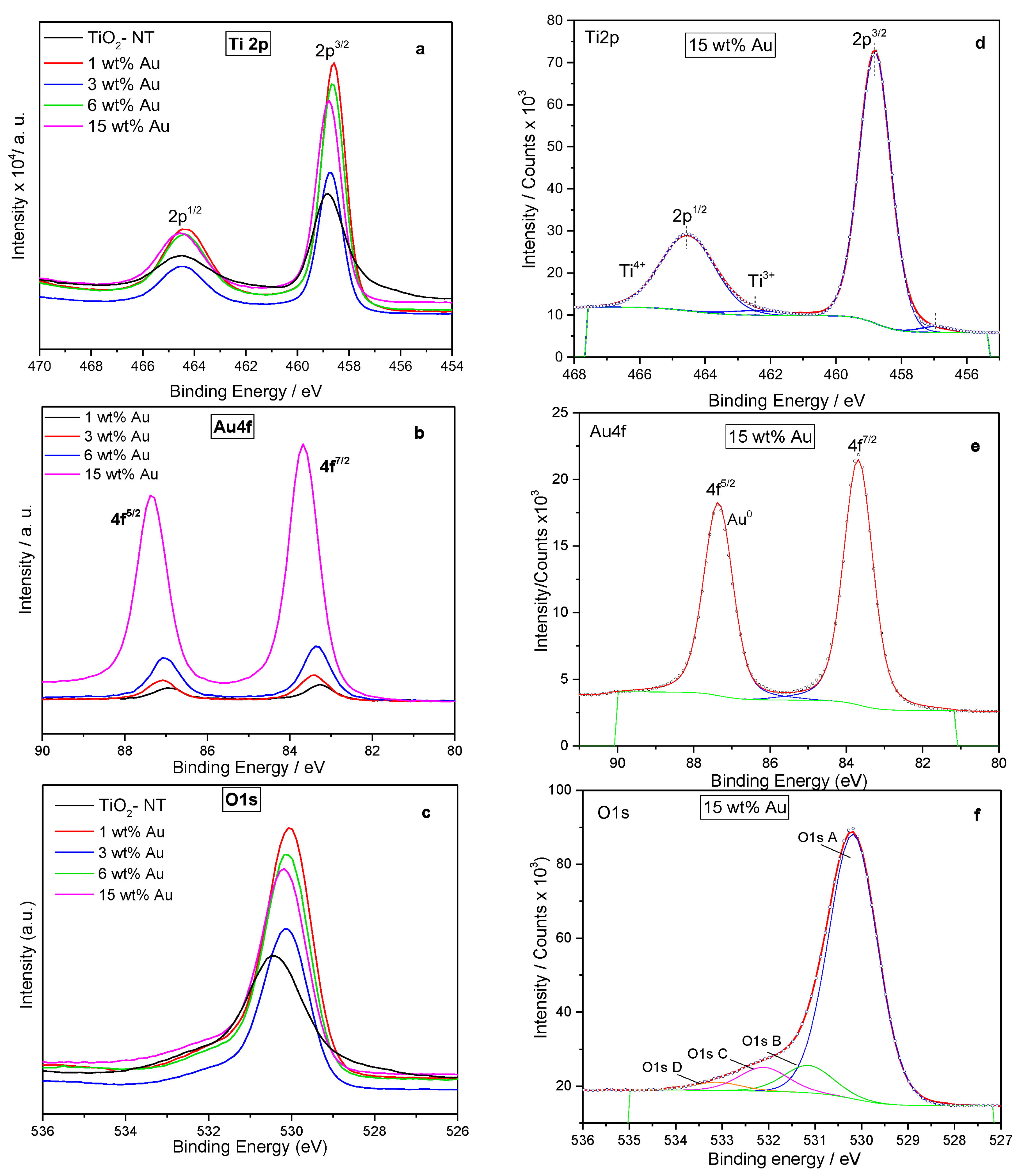
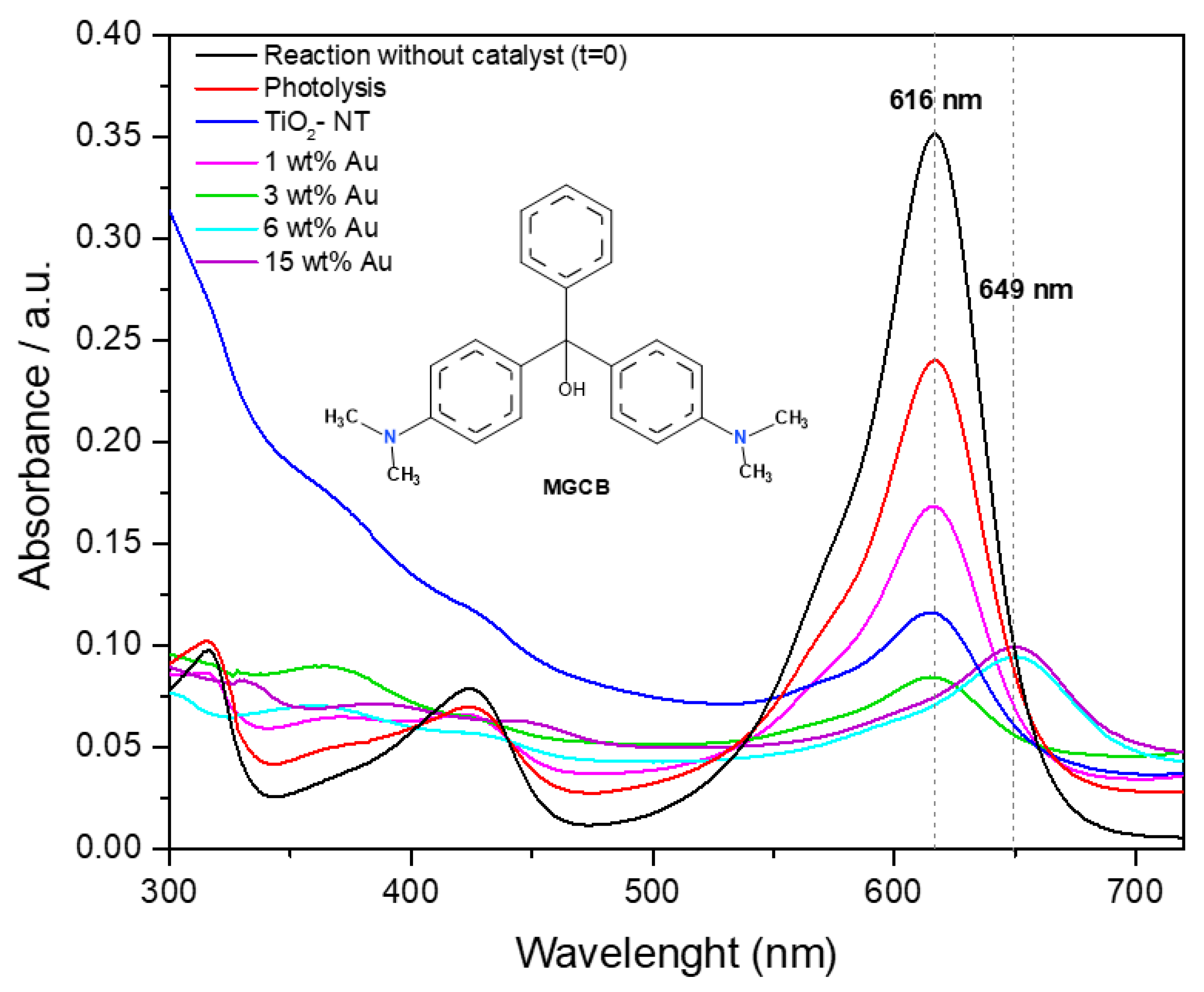
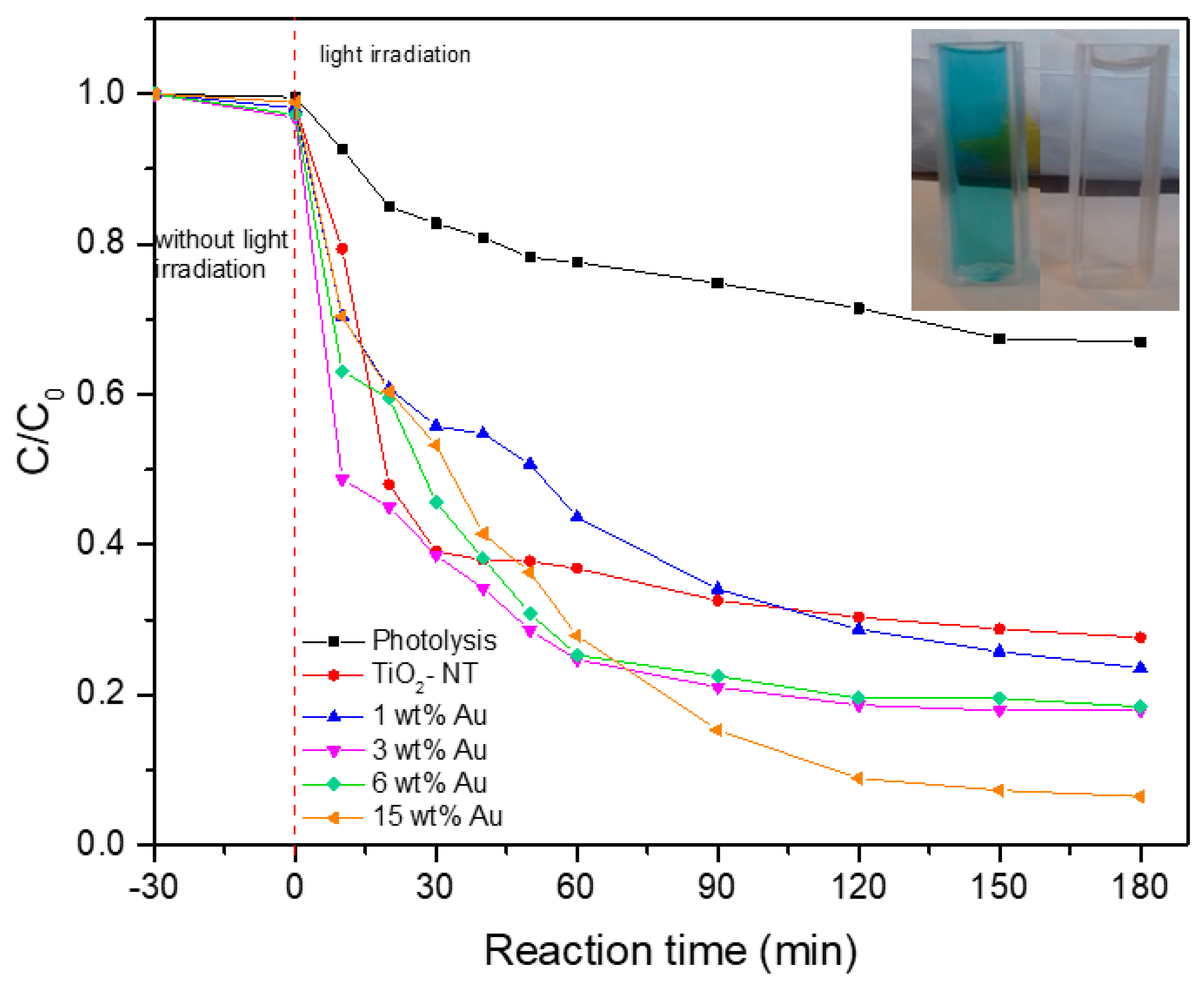
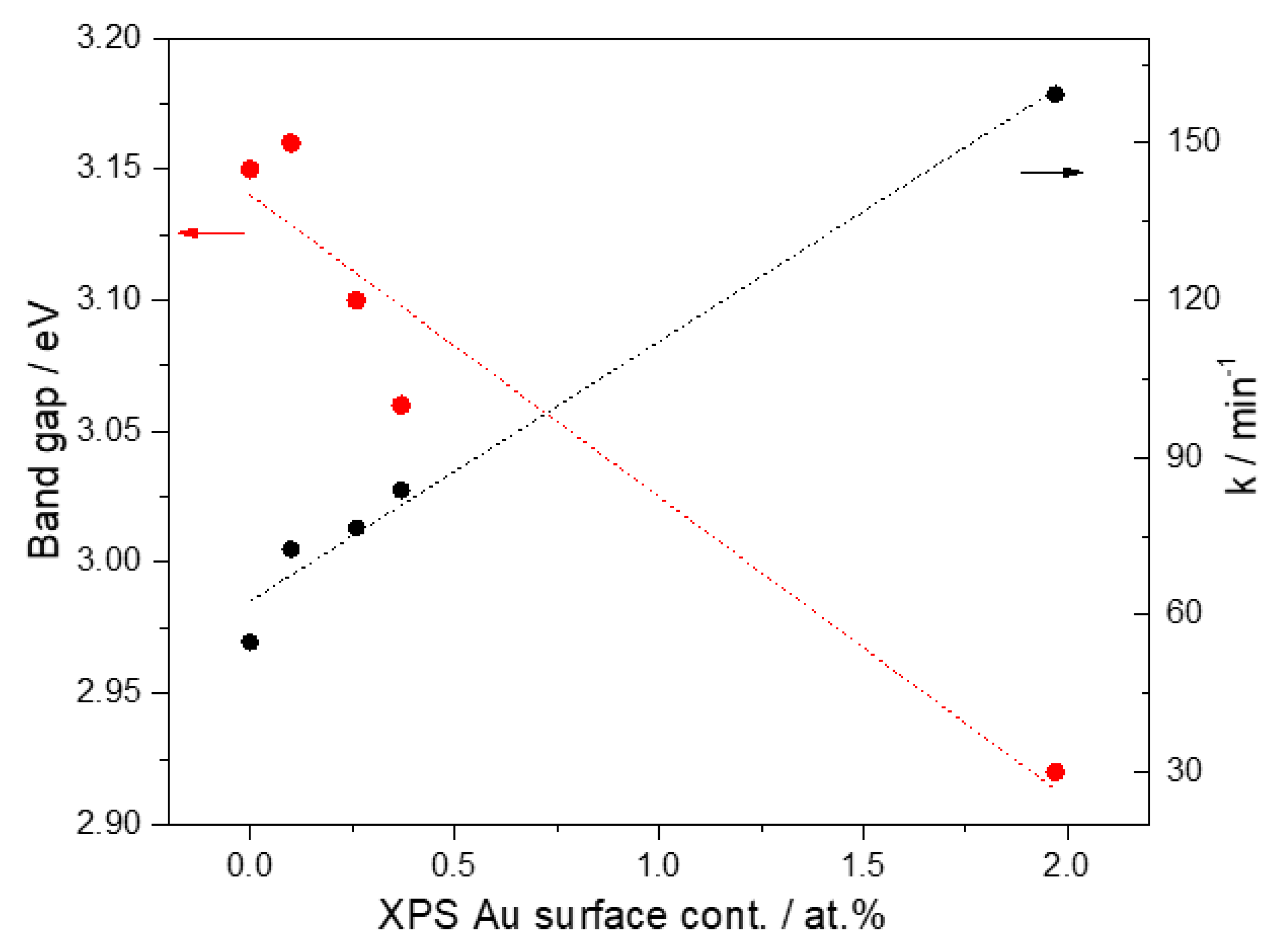
| Photocatalyst | Binding Energy (eV) | FWHM (eV) | Assignment | % at. |
|---|---|---|---|---|
| TiO2-NT | 458.8 457.5 | 1.3 2.2 | Ti4+ Ti3+ | 16.7 4.1 |
| 1 wt% Au/TiO2-NT | 458.6 457.0 83.3 | 1.1 1.1 0.8 | Ti4+ Ti3+ Au0 | 25.4 0.7 0.1 |
| 3 wt% Au/TiO2-NT | 458.7 456.9 83.4 | 1.1 1.1 0.9 | Ti4+ Ti3+ Au0 | 23.3 0.5 0.26 |
| 6 wt% Au/TiO2-NT | 458.6 456.8 83.4 | 1.1 1.1 0.9 | Ti4+ Ti3+ Au0 | 25.4 0.6 0.37 |
| 15 wt% Au/TiO2-NT | 458.8 457.0 83.7 | 1.1 1.1 0.9 | Ti4+ Ti3+ Au0 | 24.4 0.5 1.97 |
| Catalysts | Mean Particle Size (nm) | Band Gap Energy (eV) | Rate Constant a, k × 10−4 (min−1) | % Decoloration a |
|---|---|---|---|---|
| Photolysis | -- | -- | 17.55 | 31.19 |
| TiO2-NT | -- | 3.15 | 54.76 | 72.15 |
| 1 wt%Au/TiO2-NT | 3.1 | 3.16 | 72.54 | 75.98 |
| 3 wt% Au/TiO2-NT | 3.1 | 3.10 | 76.54 | 81.51 |
| 6 wt% Au/TiO2-NT | 2.9 | 3.06 | 83.77 | 81.05 |
| 15 wt% Au/TiO2-NT | 3.2 | 2.92 | 159.30 | 93.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cruz, M.G.; Solís-Casados, D.A.; Toledo-Antonio, J.A.; Vargas-García, J.R.; Estrada-Flores, M.; Ángeles-Chávez, C.; Cortés-Jácome, M.A.; Encarnación-Gómez, C. Malachite Green Dye Decoloration over Au/TiO2-Nanotubes Photocatalyst under Simulate Visible-Light Irradiation. Materials 2022, 15, 6209. https://doi.org/10.3390/ma15186209
Hernández-Cruz MG, Solís-Casados DA, Toledo-Antonio JA, Vargas-García JR, Estrada-Flores M, Ángeles-Chávez C, Cortés-Jácome MA, Encarnación-Gómez C. Malachite Green Dye Decoloration over Au/TiO2-Nanotubes Photocatalyst under Simulate Visible-Light Irradiation. Materials. 2022; 15(18):6209. https://doi.org/10.3390/ma15186209
Chicago/Turabian StyleHernández-Cruz, María Guadalupe, Dora Alicia Solís-Casados, José Antonio Toledo-Antonio, Jorge Roberto Vargas-García, Miriam Estrada-Flores, Carlos Ángeles-Chávez, María Antonia Cortés-Jácome, and Cecilia Encarnación-Gómez. 2022. "Malachite Green Dye Decoloration over Au/TiO2-Nanotubes Photocatalyst under Simulate Visible-Light Irradiation" Materials 15, no. 18: 6209. https://doi.org/10.3390/ma15186209
APA StyleHernández-Cruz, M. G., Solís-Casados, D. A., Toledo-Antonio, J. A., Vargas-García, J. R., Estrada-Flores, M., Ángeles-Chávez, C., Cortés-Jácome, M. A., & Encarnación-Gómez, C. (2022). Malachite Green Dye Decoloration over Au/TiO2-Nanotubes Photocatalyst under Simulate Visible-Light Irradiation. Materials, 15(18), 6209. https://doi.org/10.3390/ma15186209







