Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective
Abstract
:1. Introduction
2. Designing for the Circular Economy
2.1. The Performance Economy and the Inertia Principle
2.2. Cradle-to-Cradle Design
- Waste equals food;
- Use of current solar income;
- Celebrate diversity.
2.3. The 12 Principles of Green Engineering
3. Methods
- What characteristics does a polymer need to have for successful foaming?
- Which are the main morphology–properties relationships of cellular plastics and the link to processing conditions?
- Which strategies can be followed to use these relationships and what research needs to arise when developing insulating polymeric foams according to circular product design?
4. Results
4.1. Selection of Raw Materials: Need for Material Characterization
4.2. Optimization of Polymer Foamability
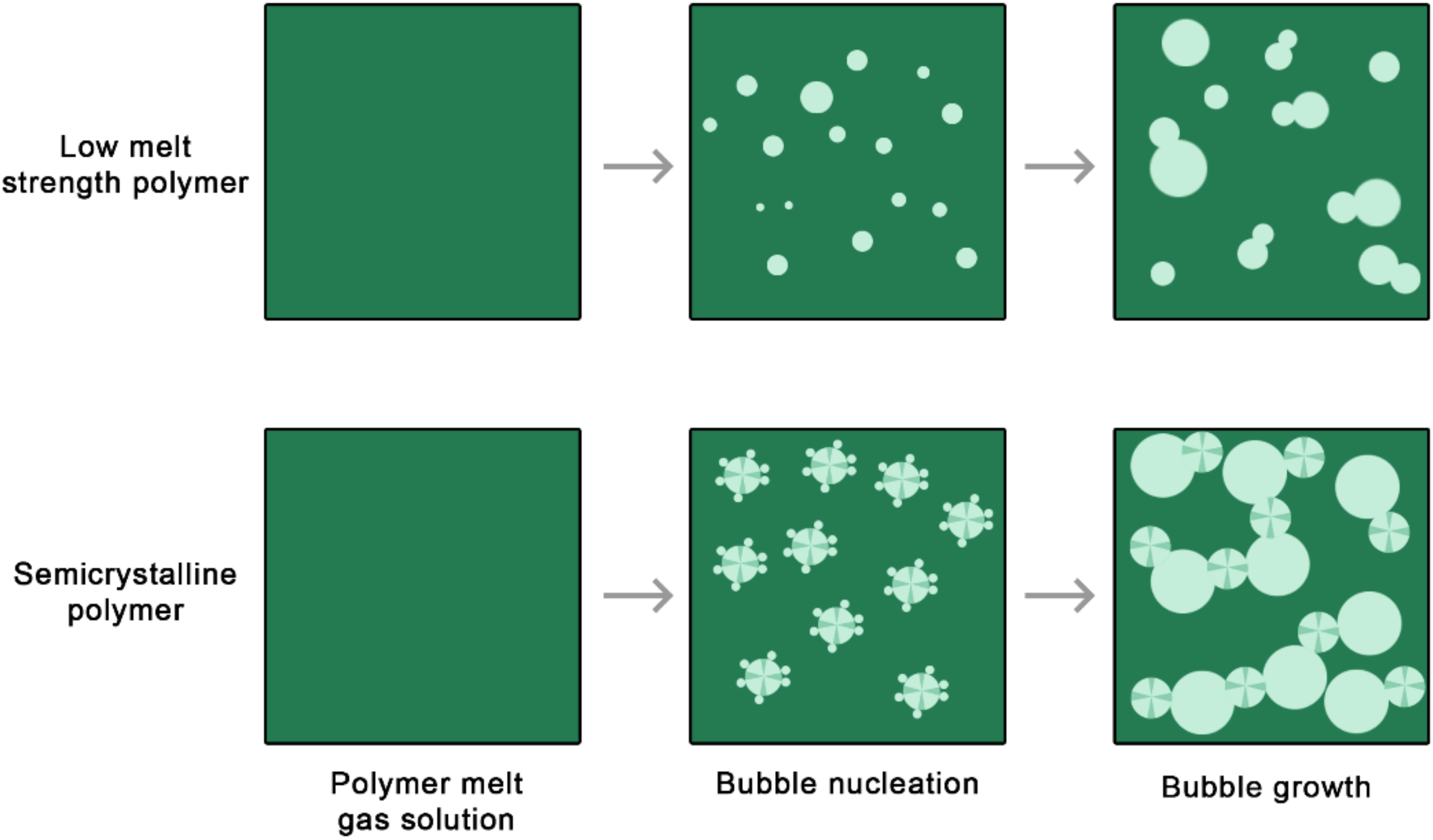
4.2.1. Overcoming Poor Melt Strength
- Reactive extrusion: branching and chain extending.
- Improving foamability properties during the resin manufacturing phase.
- Optimizing the processing tools.
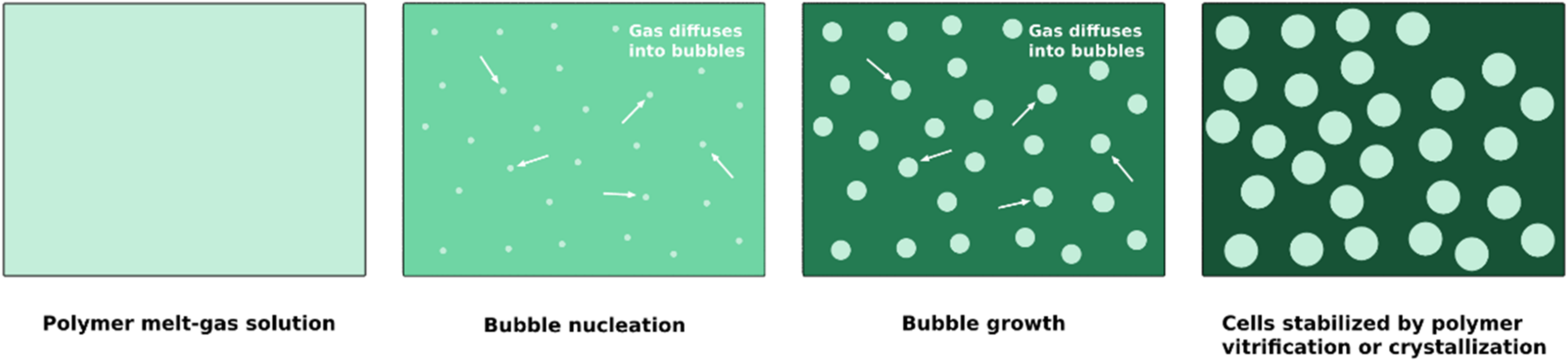
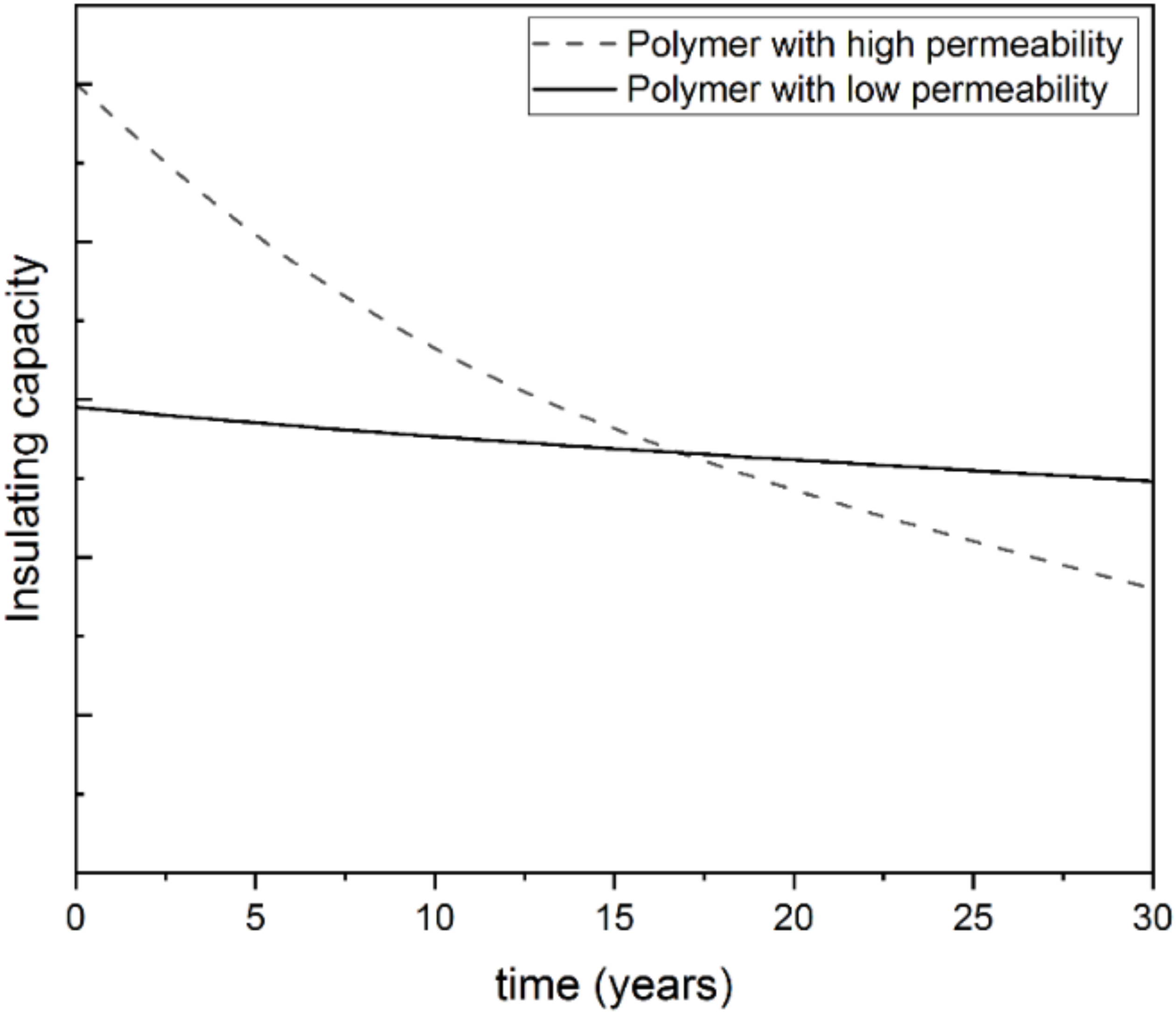
4.2.2. Processing Strategies towards Cell Stabilization
4.3. Tailoring Properties through Foam Processing and Cellular Structure
5. Conclusions
- To broaden the selection of polymeric foams, research on material characterization is needed, on (i) polymer melt–gas systems, including extensional rheology, solubility and diffusivity of the gas in the melt, superficial tension, plasticization effects and crystallization kinetics to assess foamability and optimize foaming, and (ii) ageing of foams, to assess their service life and benchmark foams according to their entire lifecycle;
- Chain extending and branching through reactive extrusion has been found a successful technique to improve the melt strength and enable successful foaming of polymers. Research and development efforts are required to identify and/or develop nontoxic chain extenders and branching agents;
- Polymer chain configuration could be tailored for foaming applications during the manufacturing phase. It is expected that the demand will drive the tailoring of resin properties of alternative polymers as they become the focus of foaming research;
- Optimizing the processing tools is an effective strategy to advance on polymer foaming. The breaker plate could be used for the foaming of low melt strength polymers. Significant research efforts are required in this area, to allow the commercial manufacturing of, i.e., nanocellular foams;
- Identified strategies to control the dimensional stability of cellular structures include the foaming pressure and temperature and the use of molds. The impact of these strategies on the obtained foam morphology and skin formation, and the optimization and potential trade-offs between the different parameters in play need to be assessed and adjusted for each practical case;
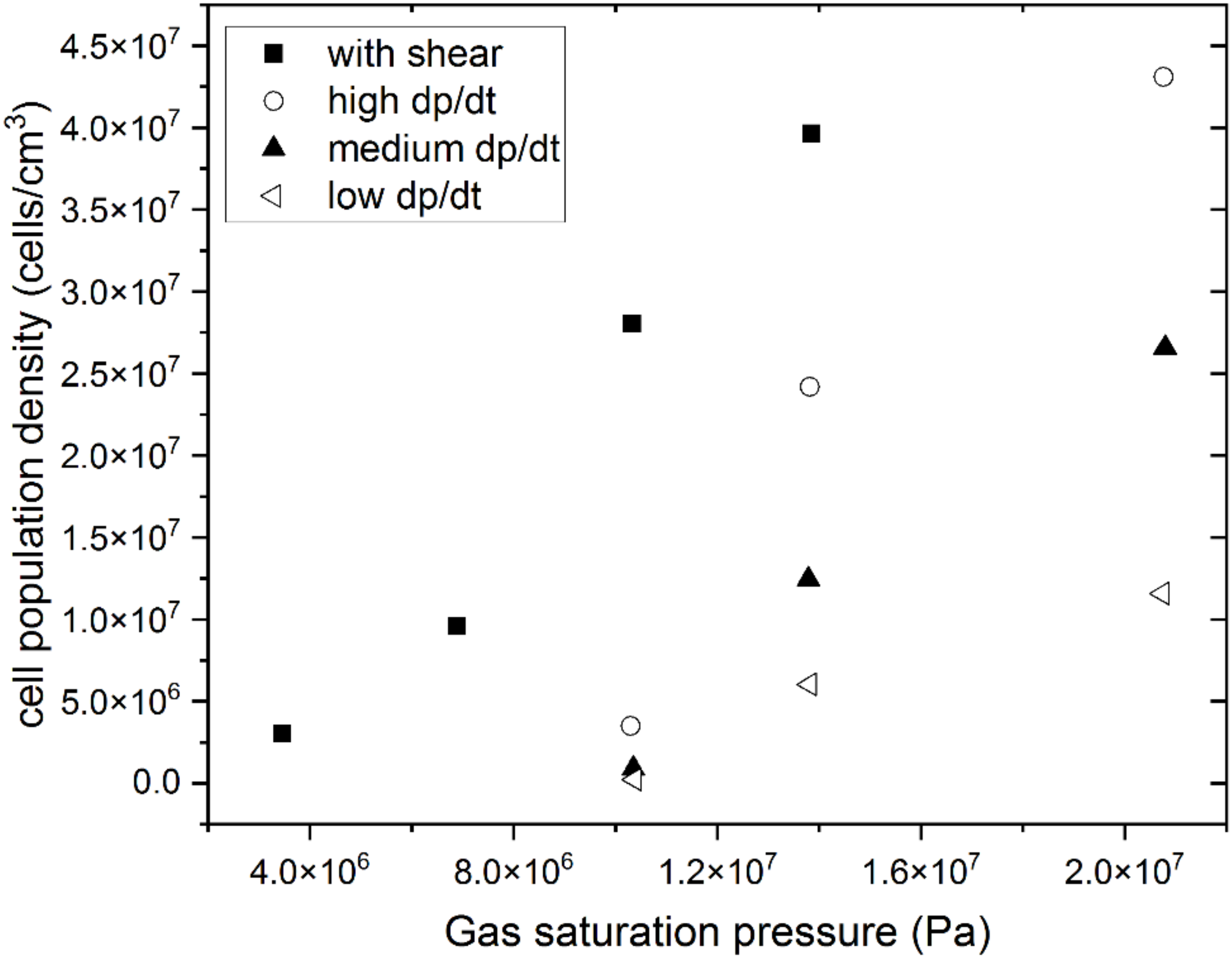
- Mechanical and thermal properties of polymeric foams are generally improved with the reduction of cell size and can be fine-tuned through cell size variability. The pressure drop rate and acting shear have been confirmed as processing parameters that contribute to the obtention of microcells by promoting cell nucleation. Research is needed to gain a more detailed understanding of the effects of shear on cell nucleation. R&D efforts are needed to scale up manufacturing tools to obtain high pressure drop rates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Directive 2009/125/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for the Setting of Ecodesign Requirements for Energy-Related Product. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009L0125 (accessed on 1 September 2022).
- Faber, N.; Jorna, R.; van Engelen, J. The Sustainability of “Sustainability”—A Study into the Conceptual Foundations of the Notion of “Sustainability”. J. Environ. Assess. Policy Manag. 2005, 7, 1–33. [Google Scholar] [CrossRef]
- Den Hollander, M.C.; Bakker, C.A.; Hultink, E.J. Product Design in a Circular Economy: Development of a Typology of Key Concepts and Terms. J. Ind. Ecol. 2017, 21, 517–525. [Google Scholar] [CrossRef]
- McDonough, W.; Braungart, M. Cradle to Cradle: Remaking the Way We Make Things, 1st ed.; North Point Press: New York, NY, USA, 2002; ISBN 978-0865475878. [Google Scholar]
- Sprinckx, C. Exploratory Study with Regard to Ecodesign of Thermal Insulation in Buildings (Lot 36): MEErP Tasks 0, 1 and 7-MEErP Study on Insulation Materials, Brussels. 2014. Available online: https://www.eup-network.de/fileadmin/user_upload/Produktgruppen/MEErP_exploratory_study_insulation_materials_final_report.pdf (accessed on 1 September 2022).
- Lee, S.-T.; Park, C.B. (Eds.) Foam Extrusion; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429184703. [Google Scholar]
- Leslie, H.A.; Leonards, P.E.G.; Brandsma, S.H.; Boer, J.D.; Jonkers, N. Propelling plastics into the circular economy-weeding out the toxics first. Environ. Int. 2016, 94, 230–234. [Google Scholar] [CrossRef]
- Chian, K.S.; Gan, L.H. Development of a rigid polyurethane foam from palm oil. J. Appl. Polym. Sci. 1998, 68, 509–515. [Google Scholar] [CrossRef]
- Pillai, P.K.; Li, S.; Bouzidi, L.; Narine, S.S. Metathesized palm oil & novel polyol derivatives: Structure, chemical composition and physical properties. Ind. Crops Prod. 2016, 84, 205–223. [Google Scholar] [CrossRef]
- Tanaka, R.; Hirose, S.; Hatakeyama, H. Preparation and characterization of polyurethane foams using a palm oil-based polyol. Bioresour. Technol. 2008, 99, 3810–3816. [Google Scholar] [CrossRef]
- Kairytė, A.; Vėjelis, S. Evaluation of forming mixture composition impact on properties of water blown rigid polyurethane (PUR) foam from rapeseed oil polyol. Ind. Crops Prod. 2015, 66, 210–215. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Zhang, L.; Jeon, H.K.; Malsam, J.; Herrington, R.; Macosko, C.W. Substituting soybean oil-based polyol into polyurethane flexible foams. Polymer 2007, 48, 6656–6667. [Google Scholar] [CrossRef]
- Khoe, T.H.; Otey, F.H.; Frankel, E.N. Rigid urethane foams from hydroxymethylated linseed oil and polyol esters. J. Am. Oil Chem. Soc. 1972, 49, 615–618. [Google Scholar] [CrossRef]
- Akram, D.; Sharmin, E.; Ahmad, S. Synthesis and Characterization of Boron Incorporated Polyester Polyol from Linseed Oil: A Sustainable Material. Macromol. Symp. 2009, 277, 130–137. [Google Scholar] [CrossRef]
- CLP Regulation: (EC) No 1272/2008. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 1 September 2022).
- European Union. Commission Regulation (EU) 2020/1149 of 3 August 2020 amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards diisocyanates: (EU) 2020/1149. Off. J. Eur. Union 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32020R1149 (accessed on 7 September 2020).
- Attia, S.; Beney, J.F.; Andersen, M. Application of the Cradle to Cradle paradigm to a housing unit in Switzerland: Findings from a prototype design. In Proceedings of the PLEA 2013—29th Conference on Passive-Low Energy Architecture Conference—Sustainable Architecture for a Renewable Future, Munich, Germany, 10–12 September 2013. [Google Scholar]
- Finch, G.; Marriage, G.; Pelosi, A.; Gjerde, M. Building envelope systems for the circular economy; Evaluation parameters, current performance and key challenges. Sustain. Cities Soc. 2021, 64, 102561. [Google Scholar] [CrossRef]
- Minunno, R.; O’Grady, T.; Morrison, G.; Gruner, R.; Colling, M. Strategies for Applying the Circular Economy to Prefabricated Buildings. Buildings 2018, 8, 125. [Google Scholar] [CrossRef]
- Ketekaars, M.; Venmans, A. Making Cradle-to-Cradle Work: First Steps for Dutch Infrastructure. 2010. Available online: https://publications.deltares.nl/Deltares107.pdf (accessed on 1 September 2022).
- Giezen, M. Shifting Infrastructure Landscapes in a Circular Economy: An Institutional Work Analysis of the Water and Energy Sector. Sustainability 2018, 10, 3487. [Google Scholar] [CrossRef]
- Mignacca, B.; Locatelli, G.; Velenturf, A. Modularisation as enabler of circular economy in energy infrastructure. Energy Policy 2020, 139, 111371. [Google Scholar] [CrossRef]
- Jensen, P.D.; Purnell, P.; Velenturf, A.P. Highlighting the need to embed circular economy in low carbon infrastructure decommissioning: The case of offshore wind. Sustain. Prod. Consum. 2020, 24, 266–280. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Package: Options to Address the Interface between Chemical, Product and Waste Legislation (Text with EEA Relevance) Options to Address the Interface between Chemical, Product and Waste Legislation: COM (2018) 32 Final. 2018. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A52018DC0032 (accessed on 1 September 2022).
- Anastas, P.T.; Zimmerman, J.B. Design through the 12 principles of green engineering. Environ. Sci. Technol. 2003, 37, 94A–101A. [Google Scholar] [CrossRef]
- Stahel, W.R. The Performance Economy, 2nd ed.; Palgrave Macmillan: Basingstoke, UK; New York, NY, USA, 2010; ISBN 978-0-230-58466-2. [Google Scholar]
- Lamberts, W.M. 1,1,1,4,4,4 Hexafluorobutane, a New Non-Ozone-Depleting Blowing Agent for Rigid PUR Foams. J. Cell. Plast. 1992, 28, 584–595. [Google Scholar] [CrossRef]
- Barthelemy, P.P.; Leroy, A.; Franklin, J.A.; Zipfel, L.; Krücke, W. 1,1,2-Trifluoroethane (HFC-143): Zero-ODP Blowing Agent for Rigid Polyurethane Foams Using Conventional Dispensing Equipment. J. Cell. Plast. 1995, 31, 513–531. [Google Scholar] [CrossRef]
- Howard, P.; Tunkel, J. Identification of CFC and HCFC Substitutes for Blowing Polyurethane Foam Insulation Products, Washington, DC. 1995. Available online: https://cfpub.epa.gov/si/si_public_record_Report.cfm?Lab=NRMRL&dirEntryId=115653&CFID=3363171&CFTOKEN=40126298&jsessionid=3830744124ae48e33d8f4245575a1162753a (accessed on 1 September 2022).
- Ohara, Y.; Tanaka, K.; Hayashi, T.; Tomita, H.; Motani, S. The Development of a Non-Fluorocarbon-Based Extruded Polystyrene Foam Which Contains a Halogen-Free Blowing Agent. Bull. Chem. Soc. Jpn. 2004, 77, 599–605. [Google Scholar] [CrossRef]
- Kaewmesri, W.; Lee, P.C.; Park, C.B.; Pumchusak, J. Effects of CO2 and Talc Contents on Foaming Behavior of Recyclable High-melt-strength PP. J. Cell. Plast. 2006, 42, 405–428. [Google Scholar] [CrossRef]
- Di Maio, E.; Mensitieri, G.; Iannace, S.; Nicolais, L.; Li, W.; Flumerfelt, R.W. Structure optimization of polycaprolactone foams by using mixtures of CO2 and N2 as blowing agents. Polym. Eng. Sci. 2005, 45, 432–441. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Extrusion Foaming of Poly(lactic acid) Blown with CO2: Toward 100% Green Material. Cell. Polym. 2007, 26, 83–115. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Production of controlled polymeric foams by supercritical CO2. J. Supercrit. Fluids 2007, 40, 144–152. [Google Scholar] [CrossRef]
- Gong, P.; Zhai, S.; Lee, R.; Zhao, C.; Buahom, P.; Li, G.; Park, C.B. Environmentally Friendly Polylactic Acid-Based Thermal Insulation Foams Blown with Supercritical CO2. Ind. Eng. Chem. Res. 2018, 57, 5464–5471. [Google Scholar] [CrossRef]
- Nam, G.J.; Yoo, J.H.; Lee, J.W. Effect of long-chain branches of polypropylene on rheological properties and foam-extrusion performances. J. Appl. Polym. Sci. 2005, 96, 1793–1800. [Google Scholar] [CrossRef]
- Petrone, G.; D′Alessandro, V.; Franco, F.; Mace, B.; Rosa, S.d. Modal characterisation of recyclable foam sandwich panels. Compos. Struct. 2014, 113, 362–368. [Google Scholar] [CrossRef]
- Grünewald, J.; Parlevliet, P.; Altstädt, V. Manufacturing of thermoplastic composite sandwich structures. J. Thermoplast. Compos. Mater. 2017, 30, 437–464. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Polylactide Foams: Fundamentals, Manufacturing, and Applications; Elsevier/William Andrew: Oxford, UK, 2018; ISBN 9780128139912. [Google Scholar]
- Chen, X.; Li, J.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Du, G. Tannin-furanic foams modified by soybean protein isolate (SPI) and industrial lignin substituting formaldehyde addition. Ind. Crops Prod. 2021, 168, 113607. [Google Scholar] [CrossRef]
- Ye, X.; Capezza, A.J.; Gowda, V.; Olsson, R.T.; Lendel, C.; Hedenqvist, M.S. High-Temperature and Chemically Resistant Foams from Sustainable Nanostructured Protein. Adv. Sustain. Syst. 2021, 5, 2100063. [Google Scholar] [CrossRef]
- Reignier, J.; Gendron, R.; Champagne, M.F. Autoclave Foaming of Poly (ε-Caprolactone) Using Carbon Dioxide: Impact of Crystallization on Cell Structure. J. Cell. Plast. 2007, 43, 459–489. [Google Scholar] [CrossRef]
- Frerich, S.C. Biopolymer foaming with supercritical CO2—Thermodynamics, foaming behaviour and mechanical characteristics. J. Supercrit. Fluids 2015, 96, 349–358. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Jin, F.-L.; Zhao, M.; Park, M.; Park, S.-J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.A.; Kiran, E. Foaming of polymers with carbon dioxide—The year-in-review—2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Homrich, A.S.; Galvão, G.; Abadia, L.G.; Carvalho, M.M. The circular economy umbrella: Trends and gaps on integrating pathways. J. Clean. Prod. 2018, 175, 525–543. [Google Scholar] [CrossRef]
- Bjørn, A.; Hauschild, M.Z. Cradle to Cradle and LCA. In Life Cycle Assessment; Hauschild, M.Z., Rosenbaum, R.K., Olsen, S.I., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 605–631. ISBN 978-3-319-56474-6. [Google Scholar]
- Bakker, C.A.; Wever, R.; Teoh, C.; Clercq, S.d. Designing cradle-to-cradle products: A reality check. Int. J. Sustain. Eng. 2010, 3, 2–8. [Google Scholar] [CrossRef]
- Lee, S.-T. Shear effects on thermoplastic foam nucleation. Polym. Eng. Sci. 1993, 33, 418–422. [Google Scholar] [CrossRef]
- McDonough, W.; Braungart, M.; Anastas, P.T.; Zimmerman, J.B. Applying the principles of Green Engineering to cradle-to-cradle design. Environ. Sci. Technol. 2003, 37, 434A–441A. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids; Cambridge University Press: Cambridge, UK, 1997; ISBN 0 521 49911 9. [Google Scholar]
- Di Maio, E.; Coccorullo, I.; Montesano, S.; Incarnato, L. Chain Extension and Foaming of Recycled PET in Extrusion Equipment. Macromol. Symp. 2005, 228, 185–200. [Google Scholar] [CrossRef]
- Walker, S.; Rothman, R. Life cycle assessment of bio-based and fossil-based plastic: A review. J. Clean. Prod. 2020, 261, 121158. [Google Scholar] [CrossRef]
- Van der Ven, E.; Bout, H.; De Bell, H.; Baars, G. Thermal Insulation Metallocene Polyethylene Foam and Manufacturing Method Therefor. European Patent EP1336064B1, 26 January 2005. [Google Scholar]
- Knaus, D.A. Stability Control Agent Composition for Polyolefin Foam. U.S. Patent US005750584A, 12 May 1998. [Google Scholar]
- Tabacchiera, A. Concentrate of Polyfunctional Compounts Usable for the Preparation of Foamed Polyester Materials. European Patent EP2009043A1, 31 December 2008. [Google Scholar]
- Meller, M.; Li, J.; Dolega, J.; Graeter, H. Cellular Polyester Made of Post-Consumer Flakes and the Use F Products Made Thereof. European Patent EP2383309A1, 2 November 2011. [Google Scholar]
- Laguna-Gutierrez, E.; Escudero, J.; Kumar, V.; Rodriguez-Perez, M.A. Microcellular foaming by using subcritical CO2 of crosslinked and non-crosslinked LDPE/clay nanocomposites. J. Cell. Plast. 2018, 54, 257–282. [Google Scholar] [CrossRef]
- Velasco, J.I.; Antunes, M.; Realinho, V.; Ardanuy, M. Characterization of rigid polypropylene-based microcellular foams produced by batch foaming processes. Polym. Eng. Sci. 2011, 51, 2120–2128. [Google Scholar] [CrossRef]
- Doyle, L. Extrusion foaming behavior of polybutene-1. Toward single-material multifunctional sandwich structures. J. Appl. Polym. Sci. 2021, 139, 51816. [Google Scholar] [CrossRef]
- Rotter, G.E.; Melquist, J.L.; Chiang, W.; Tsai, B.C.; Kelly, J.J. Increased Throughput in Foaming and Other Melt Fabrication of Polyester. European Patent EP0636158B1, 31 March 1999. [Google Scholar]
- Xanthos, M.; Zhang, Q.; Dey, S.K.; Li, Y.; Yilmazer, U.; O’Shea, M. Effects of Resin Rheology on the Extrusion Foaming Characteristics of PET. J. Cell. Plast. 1998, 34, 498–510. [Google Scholar] [CrossRef]
- Xanthos, M.; Dey, S.K.; Zhang, Q.; Quintans, J. Parameters Affecting Extrusion Foaming of PET by Gas Injection. J. Cell. Plast. 2000, 36, 102–111. [Google Scholar] [CrossRef]
- Olabisi, O.; Adewale, K. Handbook of Thermoplastics; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1466577223. [Google Scholar]
- Wypych, G. Handbook of Plasticizers, 3rd ed.; Chemtec: Toronto, ON, Canada, 2017; ISBN 9781895198973. [Google Scholar]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
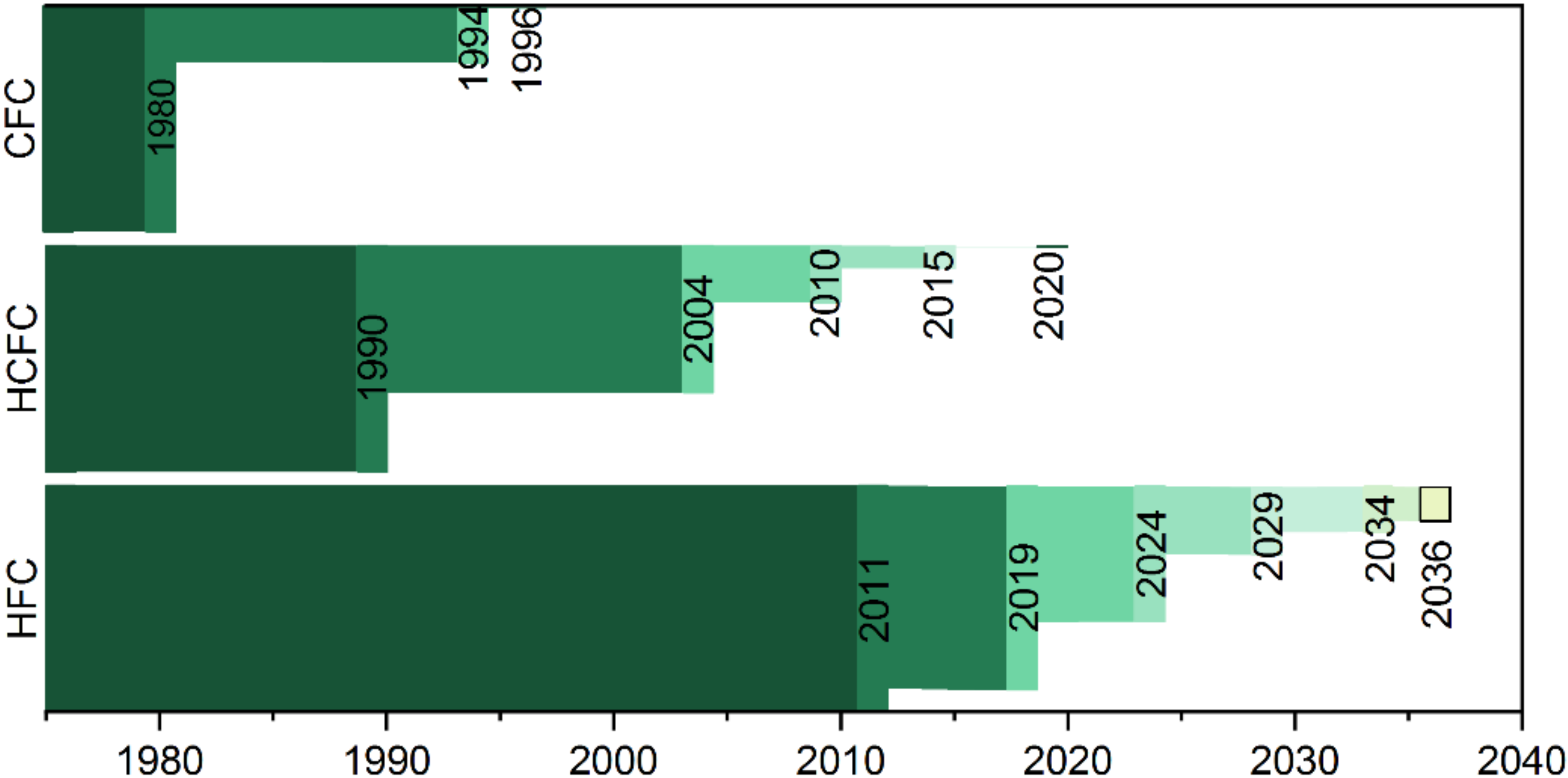
- Montereal Protocol on Substances That Deplate the Ozone Layer. Chapter XXVII Environment. 1987. Available online: https://treaties.un.org/Pages/ViewDetails.aspx?src=IND&mtdsg_no=XXVII-2-a&chapter=27&clang=_en (accessed on 1 September 2022).
- Chapter XXVII 2.f Amendment to the Montreal Protocol on Substances That Deplete the Ozone Layer. 2016. Available online: https://treaties.un.org/Pages/ViewDetails.aspx?src=IND&mtdsg_no=XXVII-2-f&chapter=27&clang=_en (accessed on 1 September 2022).
- Zhang, H.; Fang, Z.; Liu, T.; Li, B.; Li, H.; Cao, Z.; Jin, G.; Zhao, L.; Xin, Z. Dimensional Stability of LDPE Foams with CO2 + i-C4H10 Mixtures as Blowing Agent: Experimental and Numerical Simulation. Ind. Eng. Chem. Res. 2019, 58, 13154–13162. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Li, B.; Li, H.; Cao, Z.; Jin, G.; Zhao, L.; Xin, Z. Foaming and dimensional stability of LDPE foams with N2, CO2, i-C4H10 and CO2-N2 mixtures as blowing agents. J. Supercrit. Fluids 2020, 164, 104930. [Google Scholar] [CrossRef]
- Dey, S.K.; Natarajan, P.; Xanthos, M.; Braathen, M.D. Use of inert gases in extruded medium density polypropylene foams. J. Vinyl Addit. Technol. 1996, 2, 339–344. [Google Scholar] [CrossRef]
- Gendron, R.; Daigneault, L.E. Rheology of Thermoplastic Foam Extrusion Process. In Foam Extrusion; Lee, S.-T., Park, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 56–99. ISBN 9780429184703. [Google Scholar]
- Alsoy, S. Modeling of Diffusion in Closed Cell Polymeric Foams. J. Cell. Plast. 1999, 35, 247–271. [Google Scholar] [CrossRef]
- Sarver, J.A.; Sumey, J.L.; Whitfield, R.M.; Kiran, E. Confined batch foaming of semi-crystalline rubbery elastomers with carbon dioxide using a mold. J. Appl. Polym. Sci. 2021, 138, 50698. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhang, H.; Ling, Y.; Wu, K.; Liu, T.; Hu, D.; Zhao, L. Antishrinking Strategy of Microcellular Thermoplastic Polyurethane by Comprehensive Modeling Analysis. Ind. Eng. Chem. Res. 2021, 60, 7155–7166. [Google Scholar] [CrossRef]
- Lee, Y.; Youngchul, Y. Properties of Polyurethane Foam Blown by Environment Friendly Blowing Agent. In Proceedings of the 28th (2018) International Ocean and Polar Engineering Conference, Sapporo, Japan, 10–15 June 2018; International Society of Offshore and Polar Engineers: Cupertino, CA, USA, 2018. ISBN 9781880653876. [Google Scholar]
- Nair, V. HFO refrigerants: A review of present status and future prospects. Int. J. Refrig. 2021, 122, 156–170. [Google Scholar] [CrossRef]
- Vo, C.V.; Fox, R.T. Assessment of hydrofluoropropenes as insulating blowing agents for extruded polystyrene foams. J. Cell. Plast. 2013, 49, 423–438. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents; ChemTec Publishing: Toronto, ON, Canada, 2017; ISBN 978-1-895198-99-7. [Google Scholar]
- Yakushin, V.; Cabulis, U.; Fridrihsone, V.; Kravchenko, S.; Pauliks, R. Properties of polyurethane foam with fourth-generation blowing agent. e-Polymers 2021, 21, 763–769. [Google Scholar] [CrossRef]
- Bowman, J.M.; Williams, D.J. Thermal Insulating Foam Comprising HFO-1233zd as Blowing Agent. European Patent EP2154223B3, 26 June 2006. [Google Scholar]
- Hendriks, S.; Hopmann, C.; Zepnik, S. Extrusion foaming of thermoplastic cellulose acetate sheets with HFO-1234ze and co-blowing agents. Polym. Eng. Sci. 2018, 58, E182–E188. [Google Scholar] [CrossRef]
- Lindley, A.A.; Noakes, T.J. Consideration F Hydrofluoroolefins (HFOs) as Potential Candidate Medical Propellants. 2010. Available online: http://mexfluor.com/pdf/hfos-as-candidate-medical-propellants.pdf (accessed on 26 May 2022).
- Fleet, D.; Hanlon, J.; Osborne, K.; La Vendrine, M.L.; Ashford, P. Study on Environmental and Health Effects of HFO Refrigerants. Report Prepared for the Norwegian Environment Agency M-917/2017. 2017. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/M917/M917.pdf (accessed on 26 May 2022).
- Pontiff, T. Foaming Agents for Foam Extrusion. In Foam Extrusion; Lee, S.-T., Park, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429184703. [Google Scholar]
- Wegner, J.-E.; Groeseling, M. Stable PET foaming. Kunstst. Int. 2010, 9, 144–146. [Google Scholar]
- Laguna-Gutierrez, E.; van Hooghten, R.; Moldenaers, P.; Rodriguez-Perez, M.A. Understanding the foamability and mechanical properties of foamed polypropylene blends by using extensional rheology. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Spitael, P.; Macosko, C.W. Strain hardening in polypropylenes and its role in extrusion foaming. Polym. Eng. Sci. 2004, 44, 2090–2100. [Google Scholar] [CrossRef]
- Park, C.B.; Cheung, L.K. A study of cell nucleation in the extrusion of polypropylene foams. Polym. Eng. Sci. 1997, 37, 1–10. [Google Scholar] [CrossRef]
- Naguib, H.E.; Park, C.B.; Reichelt, N. Fundamental foaming mechanisms governing the volume expansion of extruded polypropylene foams. J. Appl. Polym. Sci. 2004, 91, 2661–2668. [Google Scholar] [CrossRef]
- Gotsis, A.D.; Zeevenhoven, B.L.F.; Tsenoglou, C. Effect of long branches on the rheology of polypropylene. J. Rheol. 2004, 48, 895–914. [Google Scholar] [CrossRef]
- Yamaguchi, M. Material Strength in Molten State for Foam. In Foam Extrusion; Lee, S.-T., Park, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 83–117. ISBN 9780429184703. [Google Scholar]
- Xu, Z.; Zhang, Z.; Guan, Y.; Wei, D.; Zheng, A. Investigation of extensional rheological behaviors of polypropylene for foaming. J. Cell. Plast. 2013, 49, 317–334. [Google Scholar] [CrossRef]
- Münstedt, H. Extensional Rheology and Processing of Polymeric Materials. Int. Polym. Process. 2018, 33, 594–618. [Google Scholar] [CrossRef]
- Sarver, J.A.; Sumey, J.L.; Williams, M.L.; Bishop, J.P.; Dean, D.M.; Kiran, E. Foaming of poly(ethylene-co-vinyl acetate) and poly(ethylene-co-vinyl acetate-co-carbon monoxide) and their blends with carbon dioxide. J. Appl. Polym. Sci. 2018, 135, 45841. [Google Scholar] [CrossRef]
- Weingart, N.; Raps, D.; Lu, M.; Endner, L.; Altstädt, V. Comparison of the Foamability of Linear and Long-Chain Branched Polypropylene—The Legend of Strain-Hardening as a Requirement for Good Foamability. Polymers 2020, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-Y.; Dae Han, C. Measurement of the Viscosities of Mixtures of Thermoplastic Resin and Fluorocarbon Blowing Agent. J. Cell. Plast. 1982, 18, 361–370. [Google Scholar] [CrossRef]
- Pasquali, I.; Comi, L.; Pucciarelli, F.; Bettini, R. Swelling, melting point reduction and solubility of PEG 1500 in supercritical CO2. Int. J. Pharm. 2008, 356, 76–81. [Google Scholar] [CrossRef]
- Takahashi, S.; Hassler, J.C.; Kiran, E. Melting behavior of biodegradable polyesters in carbon dioxide at high pressures. J. Supercrit. Fluids 2012, 72, 278–287. [Google Scholar] [CrossRef]
- Doroudiani, S.; Park, C.B.; Kortschot, M.T. Effect of the crystallinity and morphology on the microcellular foam structure of semicrystalline polymers. Polym. Eng. Sci. 1996, 36, 2645–2662. [Google Scholar] [CrossRef]
- Tang, L.; Zhai, W.; Zheng, W. Autoclave preparation of expanded polypropylene/poly(lactic acid) blend bead foams with a batch foaming process. J. Cell. Plast. 2011, 47, 429–446. [Google Scholar] [CrossRef]
- Mouliné, P.; Simha, R. Statistical Thermodynamics of Gas Solubility in Polymers. In Foam Extrusion; Lee, S.-T., Park, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9780429184703. [Google Scholar]
- Zhang, Q.; Xanthos, M. Material Properties Affecting Extrusion Foaming. In Polymeric Foams: Mechanisms and Materials; Lee, S.-T., Ramesh, N.S., Eds.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0-8493-1728-2. [Google Scholar]
- Mangs, S. Insulation Materials in District Heating Pipes. Ph.D. Thesis, Chalmers University of Technology, Goteborg, Sweden, 2005. [Google Scholar]
- Zauner, C.; Bettermann, M. Flexible Foam with Improved Insulation Properties. European Patent EP3372631A1, 12 September 2018. [Google Scholar]
- Kurańska, M.; Prociak, A. Bio-Based Polyurethane Foams for Heat-Insulating Applications. In Nano and Biotech Based Materials for Energy Building Efficiency; Pacheco Torgal, F., Buratti, C., Kalaiselvam, S., Granqvist, C.-G., Ivanov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 357–373. ISBN 978-3-319-27503-1. [Google Scholar]
- Collishaw, P.G.; Evans, J.R.G. An assessment of expressions for the apparent thermal conductivity of cellular materials. J. Mater. Sci. 1994, 29, 2261–2273. [Google Scholar] [CrossRef]
- Isberg, J. The Thermal Conductivity of Polyurethane Foam. Ph.D. Thesis, Chalmers University of Technology, Goteborg, Sweden, 1988. [Google Scholar]
- Ramnäs, J. New Materials and Constructions for Improving the Quality and Livetime of District Heating Pipes Including Joints-Thermal, Mechanical and Environmental Performance: Annex VIII Project 1. Final Report. 2008. Available online: https://www.iea-dhc.org/the-research/annexes/2005-2008-annex-viii/annex-viii-project-01 (accessed on 1 September 2022).
- Peelman, N.; Ragaert, P.; Ragaert, K.; Meulenaer, B.d.; Devlieghere, F.; Cardon, L. Heat resistance of new biobased polymeric materials, focusing on starch, cellulose, PLA, and PHA. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Doyle, L.; Weidlich, I. Sustainable insulation for sustainable DHC. Energy Rep. 2021, 7, 150–157. [Google Scholar] [CrossRef]
- Antunes, M.; Velasco, J.I.; Realinho, V.; Solórzano, E. Study of the cellular structure heterogeneity and anisotropy of polypropylene and polypropylene nanocomposite foams. Polym. Eng. Sci. 2009, 49, 2400–2413. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, M.A. Crosslinked Polyolefin Foams: Production, Structure, Properties, and Applications. Crosslinking in Materials Science; Springer: Berlin/Heidelberg, Germany, 2005; pp. 97–126. ISBN 978-3-540-25831-5. [Google Scholar]
- Abe, S.; Yamaguchi, M. Study on the foaming of crosslinked polyethylene. J. Appl. Polym. Sci. 2001, 79, 2146–2155. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K.-I. Rheological properties and foam processability for blends of linear and crosslinked polyethylenes. J. Polym. Sci. B Polym. Phys. 2001, 39, 2159–2167. [Google Scholar] [CrossRef]
- Laguna-Gutierrez, E.; Pinto, J.; Kumar, V.; Rodriguez-Mendez, M.L.; Rodriguez-Perez, M.A. Improving the extensional rheological properties and foamability of high-density polyethylene by means of chemical crosslinking. J. Cell. Plast. 2018, 54, 333–357. [Google Scholar] [CrossRef]
- Feng, J.-M.; Wang, W.-K.; Yang, W.; Xie, B.-H.; Yang, M.-B. Structure and Properties of Radiation Cross-Linked Polypropylene Foam. Polym. Plast. Technol. Eng. 2011, 50, 1027–1034. [Google Scholar] [CrossRef]
- Danaei, M.; Sheikh, N.; Taromi, F.A. Radiation Cross-linked Polyethylene Foam: Preparation and Properties. J. Cell. Plast. 2005, 41, 551–562. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, E.; Salmazo, L.O.; Lopez-Gil, A.; Laguna-Gutierrez, E.; Rodriguez-Perez, M.A. Analysis of the foaming mechanisms of materials based on high-density polyethylene (HDPE) crosslinked with different irradiation doses. J. Appl. Polym. Sci. 2018, 135, 46276. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Rheology and extrusion foaming of chain-branched poly(lactic acid). Polym. Eng. Sci. 2010, 50, 629–642. [Google Scholar] [CrossRef]
- Khemani, K.C.; Mercer, J.W.; McConnel, R.L. Concentrates for Improving Polyester Compositions and Method of Making Same. U.S. Patent US5801206A, 9 September 1997. [Google Scholar]
- Tang, H.; Dai, W.; Chen, B. A new method for producing high melt strength polypropylene with reactive extrusion. Polym. Eng. Sci. 2008, 48, 1339–1344. [Google Scholar] [CrossRef]
- Frounchi, M. Studies on degradation of PET in mechanical recycling. Macromol. Symp. 1999, 144, 465–469. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Standau, T.; Nofar, M.; Dörr, D.; Ruckdäschel, H.; Altstädt, V. A Review on Multifunctional Epoxy-Based Joncryl® ADR Chain Extended Thermoplastics. Polym. Rev. 2021, 62, 1–55. [Google Scholar] [CrossRef]
- Zhong, W.; Ge, J.; Gu, Z.; Li, W.; Chen, X.; Zang, Y.; Yang, Y. Study on biodegradable polymer materials based on poly(lactic acid). I. Chain extending of low molecular weight poly(lactic acid) with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 1999, 74, 2546–2551. [Google Scholar] [CrossRef]
- Li, B.-H.; Yang, M.-C. Improvement of thermal and mechanical properties of poly(L-lactic acid) with 4,4-methylene diphenyl diisocyanate. Polym. Adv. Technol. 2006, 17, 439–443. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Q.-F.; Gu, S.-Y.; Zhang, N.-W.; Ren, T.-B. Chain-linked lactic acid polymers by benzene diisocyanate. J. Appl. Polym. Sci. 2006, 99, 1045–1049. [Google Scholar] [CrossRef]
- Gu, S.; Yang, M.; Yu, T.; Ren, T.; Ren, J. Synthesis and characterization of biodegradable lactic acid-based polymers by chain extension. Polym. Int. 2008, 57, 982–986. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Forsythe, J.S.; Cheah, K.; Nisbet, D.R.; Gupta, R.K.; Lau, A.; Donovan, A.R.; O′Shea, M.S.; Moad, G. Rheological properties of high melt strength poly(ethylene terephthalate) formed by reactive extrusion. J. Appl. Polym. Sci. 2006, 100, 3646–3652. [Google Scholar] [CrossRef]
- Wang, M.Y.; Guo, Z.Q.; Lei, B.Y.; Zhou, N.Q. Rheological and Thermal Behavior of Recycled PET Modified by PMDA. Adv. Mater. Res. 2011, 391–392, 688–691. [Google Scholar] [CrossRef]
- Madsen, M.T.; Skadhauge, L.R.; Nielsen, A.D.; Baelum, J.; Sherson, D.L. Pyromellitic dianhydride (PMDA) may cause occupational asthma. Occup. Environ. Med. 2019, 76, 175–177. [Google Scholar] [CrossRef]
- Naguib, H.E.; Park, C.B.; Panzer, U.; Reichelt, N. Strategies for achieving ultra low-density polypropylene foams. Polym. Eng. Sci. 2002, 42, 1481–1492. [Google Scholar] [CrossRef]
- Park, C.P.; Garcia, G.A. Development of Polypropylene Plank Foam Products. J. Cell. Plast. 2002, 38, 219–228. [Google Scholar] [CrossRef]
- Huovinen, P.; Harlin, A.; Jääskeläinen, P.; Kabasi, A.; Manner, N. High Melt Strength Polypropylene. U.S. Patent US6875826B1, 28 September 1998. [Google Scholar]
- Lugão, A.B.; Artel, B.; Yoshiga, A.; Lima, L.; Parra, D.F.; Bueno, J.R.; Liberman, S.; Farrah, M.; Terçariol, W.R.; Otaguro, H. Production of high melt strength polypropylene by gamma irradiation. Radiat. Phys. Chem. 2007, 76, 1691–1695. [Google Scholar] [CrossRef]
- Uzawa, T.; Goto, K.; Uozumi, T.; Sugano, T.; Zhu, W.; Mike Chung, T.C. Direct Synthesis of High-Melt-Strength Polypropylene Using the Fourth Generation Heterogeneous Ziegler–Natta Catalyst and Commercial Production Process. ACS Appl. Polym. Mater. 2020, 2, 1827–1838. [Google Scholar] [CrossRef]
- Weng, W.; Hu, W.; Dekmezian, A.H.; Ruff, C.J. Long Chain Branched Isotactic Polypropylene. Macromolecules 2002, 35, 3838–3843. [Google Scholar] [CrossRef]
- Langston, J.; Dong, J.Y.; Chung, T.C. One-Pot Process of Preparing Long Chain Branched Polypropylene Using C2-Symmetric Metallocene Complex and a “T” Reagent. Macromolecules 2005, 38, 5849–5853. [Google Scholar] [CrossRef]
- Kaminsky, W.; Laban, A. Metallocene catalysis. Appl. Catal. A Gen. 2001, 222, 47–61. [Google Scholar] [CrossRef]
- Razavi, A. Metallocene catalysts technology and environment. C. R. Acad. Sci. Ser. IIC. Chem. 2000, 3, 615–625. [Google Scholar] [CrossRef]
- Farthi, A. Mechanical Properties of Strand PET Foams at Different Length Scales. Ph.D. Thesis, University of Bayreuth, Bayreuth, Germany, 2018. [Google Scholar]
- Klempner, D.; Frisch, K.C. Handbook of Polymeric Foams and Foam Technology; Hanser: Munich, Germany, 1991; ISBN 3446150978. [Google Scholar]
- Van Krevelen, D.W.; Nijenhuis, K.t. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2009; ISBN 9780080915104. [Google Scholar]
- Park, C.B.; Behravesh, A.H.; Venter, R.D. Low density microcellular foam processing in extrusion using CO2. Polym. Eng. Sci. 1998, 38, 1812–1823. [Google Scholar] [CrossRef]
- Park, C.B.; Baldwin, D.F.; Suh, N.P. Effect of the pressure drop rate on cell nucleation in continuous processing of microcellular polymers. Polym. Eng. Sci. 1995, 35, 432–440. [Google Scholar] [CrossRef]
- Prociak, A.; Pielichowski, J.; Sterzynski, T. Thermal diffusivity of rigid polyurethane foams blown with different hydrocarbons. Polym. Test. 2000, 19, 705–712. [Google Scholar] [CrossRef]
- Meller, M.; Li, J.; Dolega, J. A foam Material with Very Low Thermal Conductivity and a Process for Manufacturing the Foam Material. European Patent EP2671911A1, 5 June 2012. [Google Scholar]
- Kang, M.J.; Kim, Y.H.; Park, G.P.; Han, M.S.; Kim, W.N.; Park, S.D. Liquid nucleating additives for improving thermal insulating properties and mechanical strength of polyisocyanurate foams. J. Mater. Sci. 2010, 45, 5412–5419. [Google Scholar] [CrossRef]
- Park, D.H.; Park, G.P.; Kim, S.H.; Kim, W.N. Effects of isocyanate index and environmentally-friendly blowing agents on the morphological, mechanical, and thermal insulating properties of polyisocyanurate-polyurethane foams. Macromol. Res. 2013, 21, 852–859. [Google Scholar] [CrossRef]
- Roux, G.A.; Balme, A.L. The Improvement of the Effective R-Value of Rigid Polyurethane Foams. J. Therm. Insul. 1990, 14, 98–106. [Google Scholar] [CrossRef]
- Buahom, P.; Wang, C.; Alshrah, M.; Wang, G.; Gong, P.; Tran, M.-P.; Park, C.B. Wrong expectation of superinsulation behavior from largely-expanded nanocellular foams. Nanoscale 2020, 12, 13064–13085. [Google Scholar] [CrossRef]
- Wang, G.; Wang, C.; Zhao, J.; Wang, G.; Park, C.B.; Zhao, G. Modelling of thermal transport through a nanocellular polymer foam: Toward the generation of a new superinsulating material. Nanoscale 2017, 9, 5996–6009. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, M. Kinetic Theory of Gases; Methuen, Co.: London, UK, 1934. [Google Scholar]
- Notario, B.; Pinto, J.; Solorzano, E.; Saja, J.A.d.; Dumon, M.; Rodríguez-Pérez, M.A. Experimental validation of the Knudsen effect in nanocellular polymeric foams. Polymer 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Martín-de León, J.; Bernardo, V.; Cimavilla-Román, P.; Pérez-Tamarit, S.; Rodríguez-Pérez, M.Á. Overcoming the Challenge of Producing Large and Flat Nanocellular Polymers: A Study with PMMA. Adv. Eng. Mater. 2019, 21, 1900148. [Google Scholar] [CrossRef]
- Martín-de León, J.; Bernardo, V.; Rodríguez-Pérez, M.Á. Nanocellular Polymers: The Challenge of Creating Cells in the Nanoscale. Materials 2019, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, V.; Martin-de Leon, J.; Pinto, J.; Verdejo, R.; Rodriguez-Perez, M.A. Modeling the heat transfer by conduction of nanocellular polymers with bimodal cellular structures. Polymer 2019, 160, 126–137. [Google Scholar] [CrossRef]
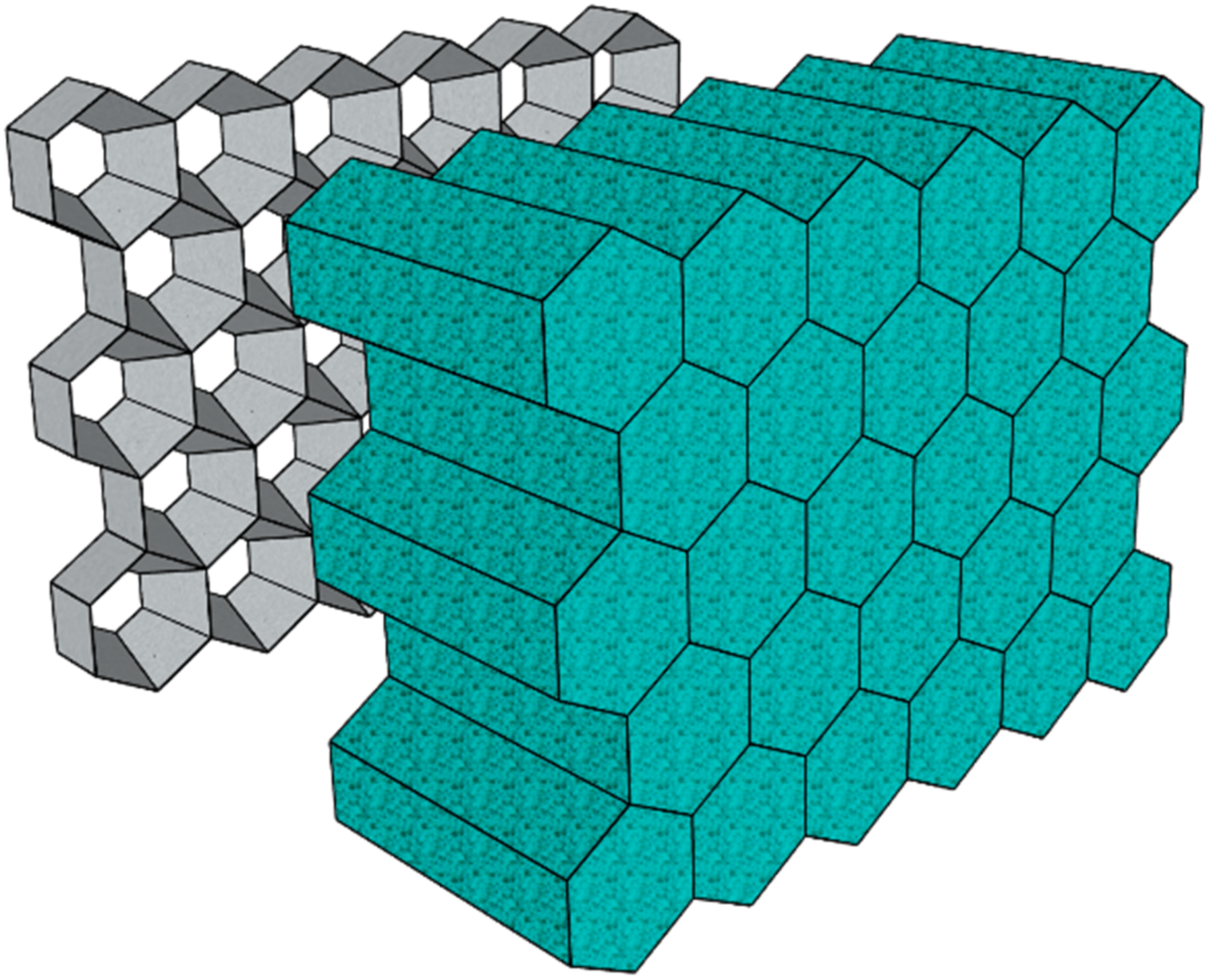
- Gibson, L.J.; Ashby, M. The mechanics of three-dimensional cellular materials. Proc. R. Soc. Lond. A 1982, 382, 43–59. [Google Scholar] [CrossRef]
- Zhu, H.X.; Knott, J.F.; Mills, N.J. Analysis of the elastic properties of open-cell foams with tetrakaidecahedral cells. J. Mech. Phys. Solids 1997, 45, 319–343. [Google Scholar] [CrossRef]
- Huber, A.T.; Gibson, L.J. Anisotropy of foams. J. Mater. Sci. 1988, 23, 3031–3040. [Google Scholar] [CrossRef]
- Sullivan, R.M.; Ghosn, L.J.; Lerch, B.A. A general tetrakaidecahedron model for open-celled foams. Int. J. Solids Struct. 2008, 45, 1754–1765. [Google Scholar] [CrossRef]
- Bureau, M.N.; Gendron, R. Mechanical-Morphology Relationship of PS Foams. J. Cell. Plast. 2003, 39, 353–367. [Google Scholar] [CrossRef]
- Chen, Y.; Das, R.; Battley, M. Effects of cell size and cell wall thickness variations on the stiffness of closed-cell foams. Int. J. Solids Struct. 2015, 52, 150–164. [Google Scholar] [CrossRef]
- Martini, J.E. The Production and Analysis of Microcellular Foam. Master′s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1981. [Google Scholar]
- Martini-Vvedensky, J.E.; Suh, N.P.; Waldman, F.A. Microcellular Closed Cell Foams and Their Method of Manufacture. U.S. Patent US4473665A, 25 September 1984. [Google Scholar]
- Kumar, V. Microcellular Polymers: Novel Materials for the 21st Century. Prog. Rubber Plast. Technol. 1993, 9, 54–70. [Google Scholar]
- Waldman, F.A. The Processing of Microcelluar Foam. Master′s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1982. [Google Scholar]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam: Process model and experimental results. Adv. Manuf. Process. 1986, 1, 341–364. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. Nucleation of microcellular foam: Theory and practice. Polym. Eng. Sci. 1987, 27, 500–503. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Doyle, L. A Circular Economy Approach to Multifunctional Sandwich Structures: Polymeric Foams for District Heating Pre-Insulated Pipes. Ph.D. Thesis, HafenCity University, Hamburg, Germany, 2022. [Google Scholar]
- Guo, Q.; Wang, J.; Park, C.B.; Ohshima, M. A Microcellular Foaming Simulation System with a High Pressure-Drop Rate. Ind. Eng. Chem. Res. 2006, 45, 6153–6161. [Google Scholar] [CrossRef]
- Tammaro, D.; Contaldi, V.; Carbone, M.P.; Di Maio, E.; Iannace, S. A novel lab-scale batch foaming equipment: The mini-batch. J. Cell. Plast. 2016, 52, 533–543. [Google Scholar] [CrossRef]
- Tammaro, D.; Astarita, A.; Di Maio, E.; Iannace, S. Polystyrene Foaming at High Pressure Drop Rates. Ind. Eng. Chem. Res. 2016, 55, 5696–5701. [Google Scholar] [CrossRef]
- Muratani, K.; Shimbo, M.; Miyano, Y. Correlation of Decompression Time and Foaming Temperature on the Cell Density of Foamed Polystyrene. Cell. Polym. 2005, 24, 15–27. [Google Scholar] [CrossRef]
- Chen, L.; Sheth, H.; Wang, X. Effects of Shear Stress and Pressure Drop Rate on Microcellular Foaming Process. J. Cell. Plast. 2001, 37, 353–363. [Google Scholar] [CrossRef]
- Wong, A.; Chu, R.K.; Leung, S.N.; Park, C.B.; Zong, J.H. A batch foaming visualization system with extensional stress-inducing ability. Chem. Eng. Sci. 2011, 66, 55–63. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, L.; Weidlich, I.; Di Maio, E. Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective. Materials 2022, 15, 6212. https://doi.org/10.3390/ma15186212
Doyle L, Weidlich I, Di Maio E. Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective. Materials. 2022; 15(18):6212. https://doi.org/10.3390/ma15186212
Chicago/Turabian StyleDoyle, Lucia, Ingo Weidlich, and Ernesto Di Maio. 2022. "Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective" Materials 15, no. 18: 6212. https://doi.org/10.3390/ma15186212
APA StyleDoyle, L., Weidlich, I., & Di Maio, E. (2022). Developing Insulating Polymeric Foams: Strategies and Research Needs from a Circular Economy Perspective. Materials, 15(18), 6212. https://doi.org/10.3390/ma15186212






