Mineral Content, Functional, Thermo-Pasting, and Microstructural Properties of Spontaneously Fermented Finger Millet Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Finger Millet Flour Production
2.3. Mineral Compositions of Fermented Finger Millet Flours
2.4. Functional Properties of Spontaneously Fermented Finger Millet Flours
2.4.1. Loose/Packed Bulk Density
2.4.2. Water/Oil Absorption Capacity
2.4.3. Swelling Capacity
2.4.4. Viscosity of Finger Millet Flours
2.5. Thermal Properties of Finger Millet Flours
2.6. Pasting Properties of Fermented Finger Millet Flours
2.7. Colour Profile of Spontaneously Fermented Finger Millet Flours
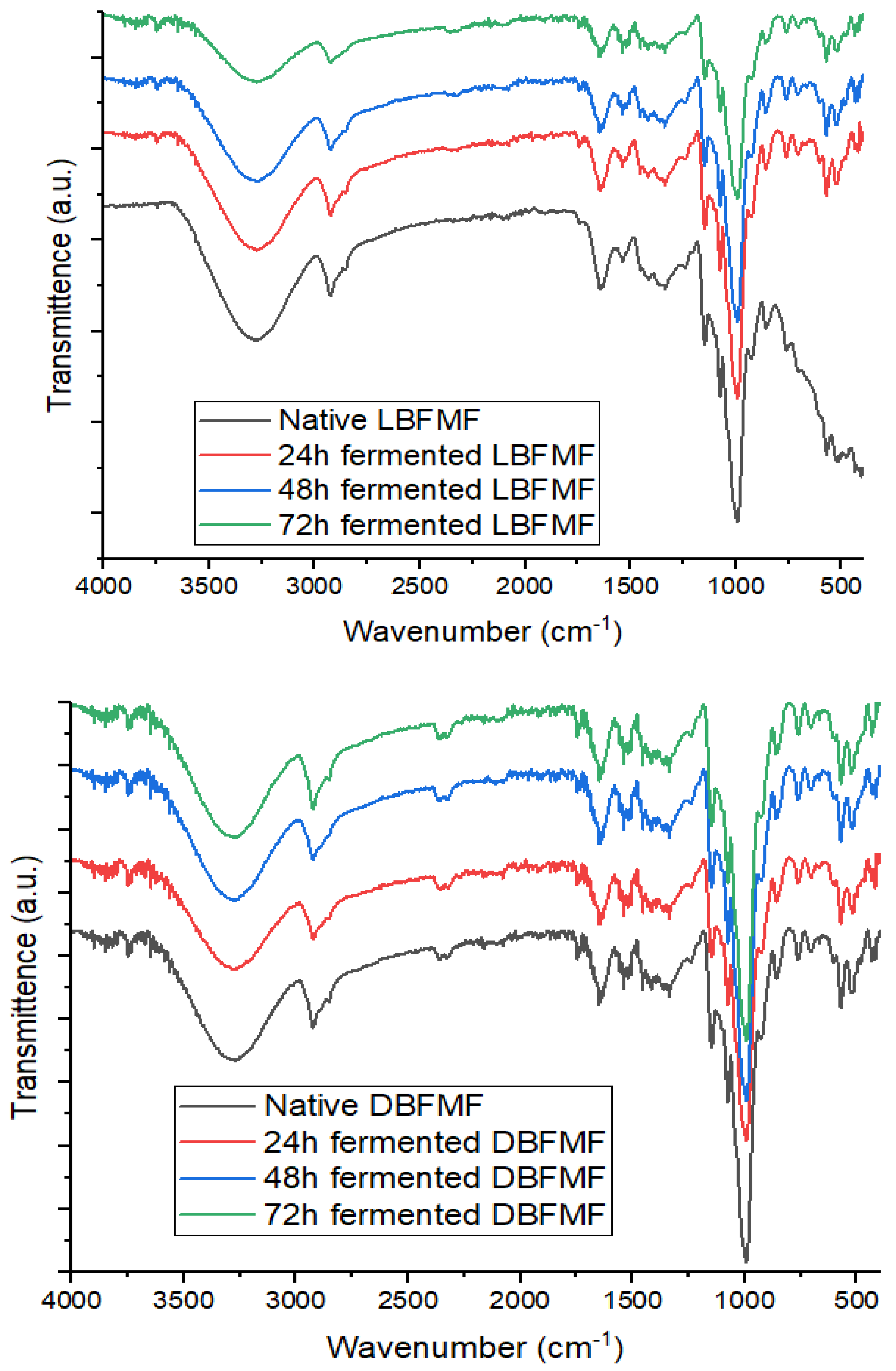
2.8. Fourier-Transform Infrared Spectra of Fermented Finger Millet Flours
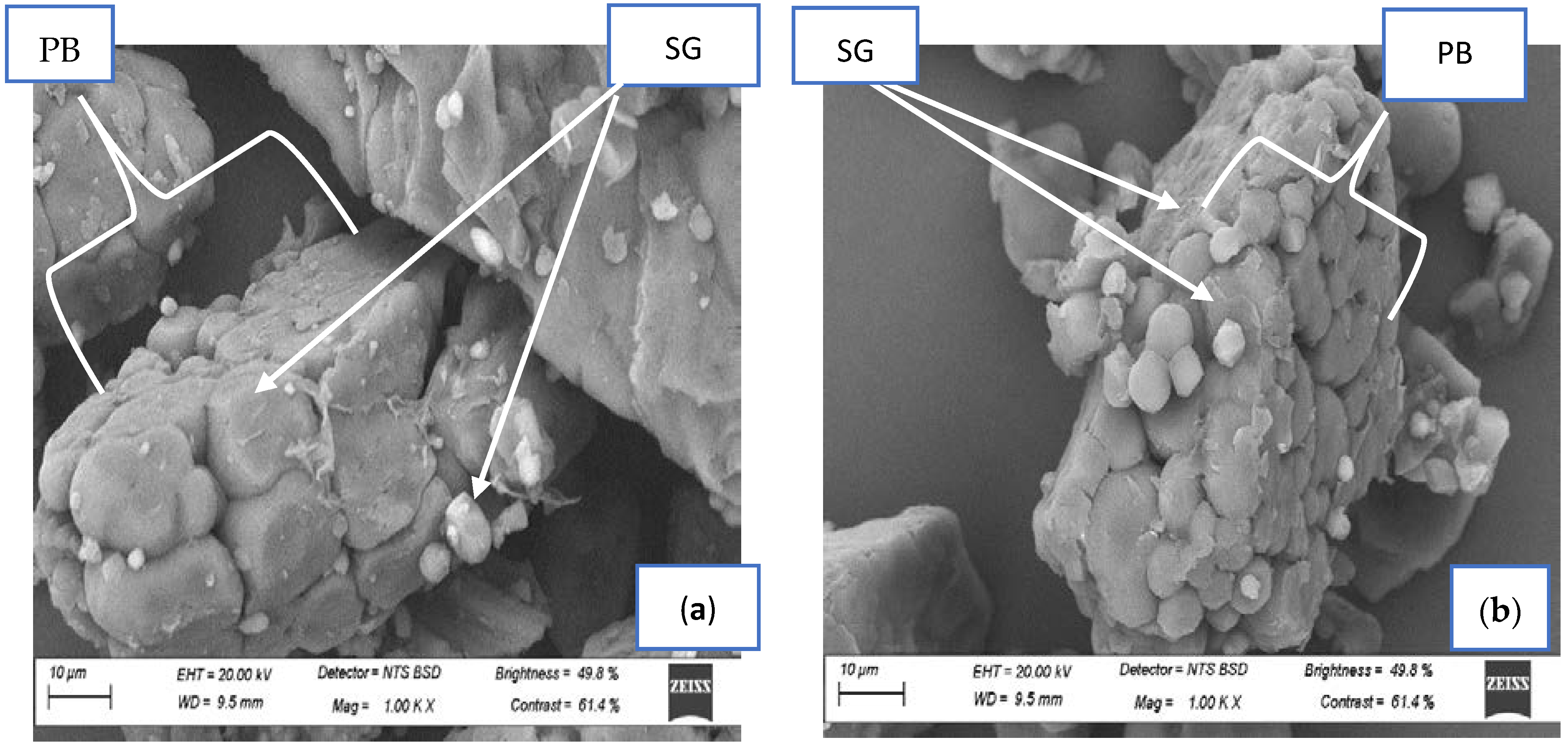
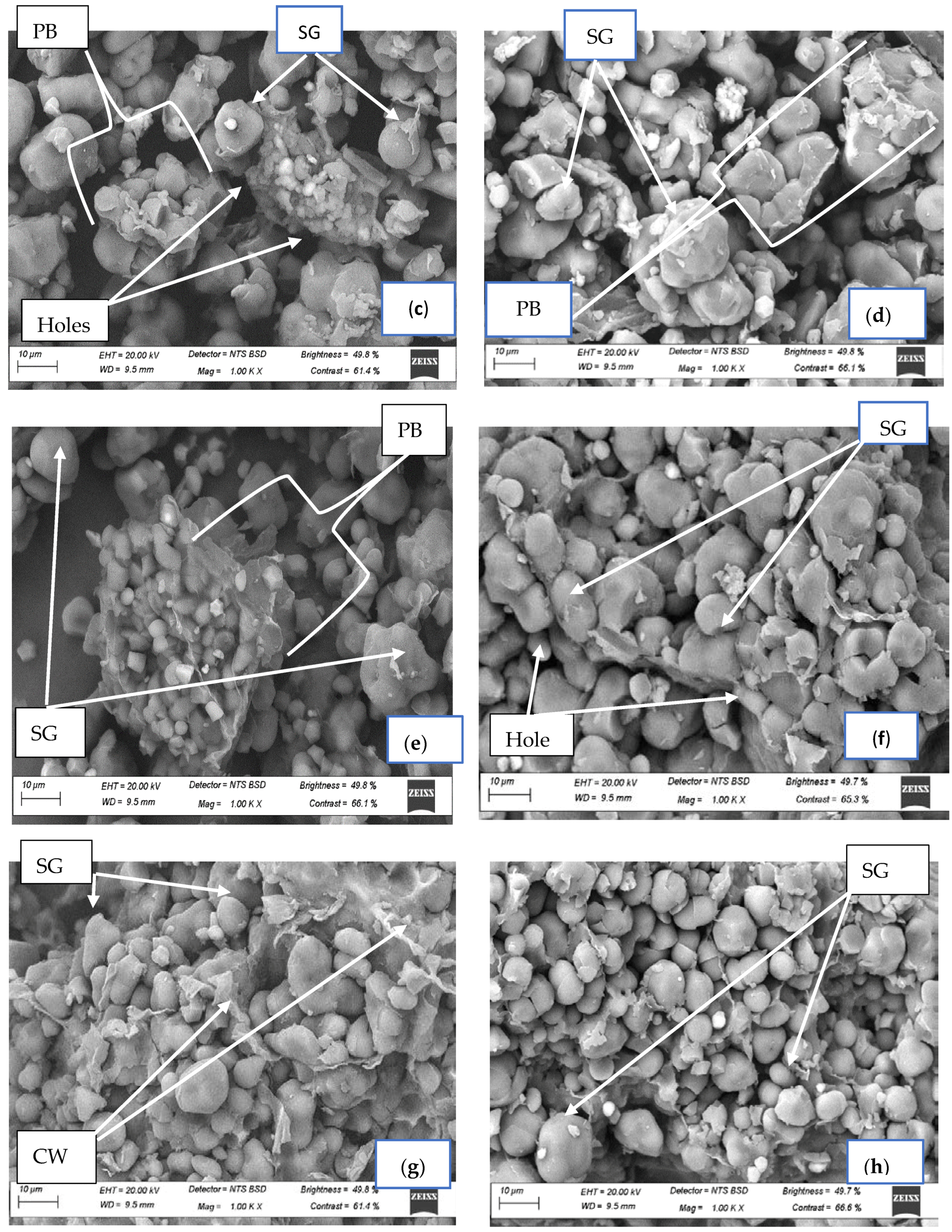
2.9. Microstructural Analysis of Fermented Finger Millet Flours Using Scanning Electron Microscopy (SEM)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Mineral Composition of Spontaneously Fermented FM Flours
3.2. Functional Properties of Fermented Finger Millet Flours
3.3. Viscosity of Spontaneously Fermented Finger Millet Flours
3.4. Thermal Properties of Spontaneously Fermented Finger Millet Flours
3.5. Pasting Properties of Spontaneously Fermented Finger Millet Flours
3.6. Colour Attributes of Finger Millet Flours
3.7. Fourier-Transform Infrared Spectra of Spontaneously Fermented Finger Millet Flours
3.8. Scanning Electron Microscopy of Spontaneously Fermented Finger Millet Flours
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narayanasamy, S. Efficacy of fermentation parameters on protein quality and microstructural properties of processed finger millet flour. J. Food Sci. Technol. 2021, 58, 3223–3234. [Google Scholar]
- Sandhya, T.S.; Prakash, N.B.; Nagaraja, A.; Reddy, N.Y.A. Effect of foliar silicic acid on growth, nutrient uptake and blast disease resistance of finger millet (Eleusine coracana (L.) Gaertn.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2111–2121. [Google Scholar] [CrossRef]
- Rathore, T.; Singh, R.; Kamble, D.B.; Upadhyay, A.; Thangalakshmi, S. Review on finger millet: Processing and value addition. J. Pharm. Innov. 2019, 8, 283–329. [Google Scholar]
- Opole, R. Opportunities for enhancing production, utilization and marketing of finger millet in Africa. Afr. J. Food Agric. Nutr. Dev. 2019, 19, 13863–13882. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2018, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ramashia, S.E.; Anyasi, T.A.; Gwata, E.T.; Meddows-Taylor, S.; Jideani, A.I.O. Processing, nutritional composition and health benefits of finger millet in sub-saharan Africa. Food Sci. Technol. 2019, 39, 253–266. [Google Scholar] [CrossRef]
- Kassa, M.K.; Emire, S.A. Evaluation of various properties of Amaranthus (Genus amaranthus L.) based composite flour blends for preparation of gluten-free biscuits. Croat. J. Food Sci. Technol. 2021, 13, 57–68. [Google Scholar]
- Azeez, S.O.; Chinma, C.E.; Bassey, S.O.; Eze, U.R.; Makinde, A.F.; Sakariyah, A.A.; Adebo, O.A. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT-Food Sci. Technol. 2022, 154, 112734. [Google Scholar] [CrossRef]
- Antony, U.; Sripriya, G.; Chandra, T. Effect of fermentation on the primary nutrients in finger millet (Eleusine coracana). J. Agric. Food Chem. 1996, 44, 2616–2618. [Google Scholar] [CrossRef]
- Chandra, D.; Chandra, S.; Sharma, A.K. Review of finger millet (Eleusine coracana (L.) Gaertn.): A powerhouse of health bene-fiting nutrients. Food Sci. Hum. Wellness 2016, 5, 149–155. [Google Scholar] [CrossRef]
- Omosebi, M.O.; Osundahunsi, O.F.; Fagbemi, T.N. Effect of extrusion on protein quality, antinutritional factors, and digestibility of complementary diet from quality protein maize and soybean protein concentrate. J. Food Biochem. 2018, 42, e12508. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, V.; Thakur, A. An overview of anti-nutritional factors in food. Int. J. Chem. Stud. 2019, 7, 2472–2479. [Google Scholar]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The nutritional use of millet grain for food and feed: A review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef]
- Budhwar, S.; Sethi, K.; Chakraborty, M. Efficacy of germination and probiotic fermentation on underutilized cereal and millet grains. J. Food Process. Preserv. 2020, 2, 12. [Google Scholar] [CrossRef]
- Davana, T.V.; Revanna, M.L.; Begum, S.S. Effect of malting on the nutritional composition, anti-nutrition factors and mineral composition on sorghum (Sorghum bicolor). Asian J. Dairy Food Res. 2021, 40, 451–455. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation, and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Amadou, I.; Gounga, M.E.; Shi, Y.-H.; Le, G.-W. Fermentation and heat-moisture treatment induced changes on the physicochemical properties of foxtail millet (Setaria italica) flour. Food Bioprod. Process. 2014, 92, 38–45. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Korhonen, J.; Palo-Oja, O.M.; El-Nezami, H.; Kolehmainen, M. Harnessing microbes for sustainable development: Food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Castro-Alba, V.; Granfeldt, Y. Quinoa fermentation and dry roasting to improve nutritional quality and sensory properties. In Biology & Biotechnology of Quinoa; Springer: Singapore, 2021; pp. 325–343. [Google Scholar]
- Inyang, C.; Zakari, U. Effect of germination and fermentation of pearl millet on proximate, chemical and sensory properties of instant “Fura”—A Nigerian cereal food. Pak. J. Nutr. 2008, 7, 9–12. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Mutshinyani, M.; Mashau, M.E.; Jideani, A.I.O. Bioactive compounds, antioxidant activity and consumer acceptability of porridges of finger millet (Eleusine coracana) flours: Effects of spontaneous fermentation. Int. J. Food Prop. 2020, 23, 1692–1710. [Google Scholar] [CrossRef]
- Srivastava, U.; Saini, P.; Singh, A. Effect of natural fermentation on antioxidant activity of pearl millet (Pennisetum glaucum). Curr. Nutr. Food Sci. 2020, 16, 306–313. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Echeta, C.K. The functional properties of foods and flours. Int. J. Adv. Acad. Res. 2019, 5, 139–160. [Google Scholar]
- Hasmadi, M.; Noorfarahzilah, M.; Noraidah, H.; Zainol, M.K.; Jahurul, M.H.A. Functional properties of composite flour: A review. Food Res. 2020, 4, 1820–1831. [Google Scholar]
- Shrestha, R.; Srivastava, S. Functional properties of finger millet and banyard millet flours and flour blends. Int. J. Sci. Res. 2017, 6, 775–780. [Google Scholar]
- Isibhakhomen, E.; Adeoti, O.V.; Taiwo, O.O.; Oluremi, F.E. Extending the use of an underutilised tuber I: Physicochemical and pasting properties of cocoyam (Xanthosoma sagittifolium) flour and its suitability for making biscuits. Afr. J. Food Sci. 2013, 7, 264–273. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Mulaba-Bafubiandi, A.; Adebo, O.A.; Kayitesi, E. Effect of fermentation and malting on the microstructure and selected physicochemical properties of pearl millet (Pennisetum glaucum) flour and biscuit. J. Cereal Sci. 2016, 70, 132–139. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef]
- Awolu, O.O.; Olarewaju, O.A.; Akinade, A.O. Effect of the addition of pearl millet flour subjected to different processing on the antioxidants, nutritional, pasting characteristics and cookies quality of rice-based composite flour. J. Nutri. Health Food Eng. 2017, 7, 00232. [Google Scholar] [CrossRef]
- Igbabul, B.; Hiikyaa, O.; Amove, J. Effect of fermentation on the proximate composition and functional properties of mahogany bean (Afzelia africana) flour. Curr. Res. Nutr. Food Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Onweluzo, J.C.; Nwabugwu, C.C. Fermentation of millet (Pennisetum americanum) and pigeon pea (Cajanus cajan) seeds for flour production: Effects on composition and selected functional properties. Pak. J. Nutr. 2009, 8, 737–744. [Google Scholar] [CrossRef]
- Ramashia, S.; Gwata, E.; Meddows-Taylor, S.; Anyasi, T.; Jideani, A. Nutritional composition of fortified finger millet (Eleusine coracana) flours fortified with vitamin B2 and zinc oxide. Food Res. 2021, 5, 456–467. [Google Scholar] [CrossRef]
- Amandikwa, C.; Iwe, M.O.; Uzomah, A.; Olawuni, A.I. Physico-chemical properties of wheat-yam flour composite bread. Niger. Food J. 2015, 33, 12–17. [Google Scholar] [CrossRef]
- Mudau, M.; Ramashia, S.E.; Mashau, M.E.; Silungwe, H. Physicochemical characteristics of bread partially substituted with finger millet (Eleusine corocana) flour. Braz. J. Food Technol. 2021, 24, e2020123. [Google Scholar] [CrossRef]
- Ngoma, K.; Mashau, M.E.; Silungwe, H. Physicochemical and functional properties of chemically pretreated Ndou sweet potato flour. Int. J. Food Sci. 2019, 2019, 4158213. [Google Scholar] [CrossRef]
- Siwatch, M.; Yadav, R.B.; Yadav, B.S. Thermal, pasting and rheological properties of processed buckwheat (Fagopyrum esculentum) flour. Asian J. Pharm. Clin. Res. 2017, 10, 134–137. [Google Scholar] [CrossRef][Green Version]
- Gull, A.; Prasad, K.; Kumar, P. Evaluation of functional, antinutritional, pasting and microstructural properties of Millet flours. J. Food Meas. Charact. 2015, 10, 96–102. [Google Scholar] [CrossRef]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of solid-state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black-eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Devi, P.B.; Rajendran, S. Impact of starter culture on nutraceutical and functional properties of underutilized millet-legume co-fermented Indian traditional product. LWT-Food Sci. Technol. 2021, 149, 111818. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Combination effect of germination and fermentation on functional properties of sorghum flour. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Adelakun, O.E.; Katawal, S.B. Physicochemical properties of fermented wheat-chickpea-rice weaning blend. Nutr. Food Sci. 2013, 43, 517–526. [Google Scholar] [CrossRef]
- Godswill, A.C. Proximate composition and functional properties of different grain flour composites for industrial applications. Int. J. Food Sci. 2019, 2, 43–64. [Google Scholar] [CrossRef]
- Akubor, P.I.; Badifu, G.I.O. Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. Int. J. Food Sci. Technol. 2004, 39, 223–229. [Google Scholar] [CrossRef]
- Adebowale, A.A.; Adegoke, M.T.; Sanni, S.A.; Adegunwa, M.O.; Fetuga, G. Functional properties and biscuit making potentials of sorghum-wheat flour composite. Am. J. Food Technol. 2012, 7, 372–379. [Google Scholar] [CrossRef]
- Tenagashaw, M.W.; Kenji, G.M.; Melaku, E.T.; Huyskens-Keil, S.; Kinyuru, J.N. Proximate composition and selected functional properties of complementary foods from teff fortified with soybean and orange-fleshed sweet potato. RUFORUM Work. Doc. Ser. 2016, 14, 953–965. [Google Scholar]
- Baranwal, D.; Sankhla, A. Physical and functional properties of malted composite flour for biscuit production. J. Pharmacogn. Phytochem. 2019, 8, 959–965. [Google Scholar]
- Sulieman, A.A.; Zhu, K.X.; Peng, W.; Hassan, H.A.; Obadi, M.; Siddeeg, A.; Zhou, H.M. Rheological and quality characteristics of composite gluten-free dough and biscuits supplemented with fermented and unfermented Agaricus bisporus polysaccharide flour. Food Chem. 2019, 271, 193–203. [Google Scholar] [CrossRef]
- Ayo-Omogie, H.N.; Ogunsakin, R. Assessment of chemical, rheological and sensory properties of fermented maize-cardaba banana complementary food. Food Nutr. Sci. 2013, 4, 844–850. [Google Scholar] [CrossRef]
- Adebowale, O.J.; Maliki, K. Effect of fermentation period on the chemical composition and functional properties of pigeon pea (Cajanus cajan) seed flour. Int. Food Res. J. 2011, 18, 1329–1333. [Google Scholar]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef] [PubMed]
- Uvere, P.O.; Ngoddy, P.O.; Nnanyelugo, D.O. Effect of amylase-rich flour (Arf) treatment on the viscosity of fermented complementary foods. Food Nutr. Bull. 2002, 23, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, A.; Nazni, P. Effect of processing techniques on nutritional, viscosity and osmolarity of barnyard millet based diarrheal replacement fluids. Curr. Res. Nutr. Food Sci. J. 2020, 8, 164–173. [Google Scholar] [CrossRef]
- Usman, M.A.; Bolade, M.K.; James, S. Functional properties of weaning food blends from selected sorghum (Sorghum bicolor (L.) Moench) varieties and soybean (Glycine max). Afr. J. Food Sci. 2016, 10, 112–121. [Google Scholar]
- Ramashia, S.E. Physical, Functional and Nutritional Properties of Flours from Finger Millet (Eleusine coracana) Varieties Fortified with Vitamin B2 and Zinc Oxide. Ph.D. Thesis, University of Venda, Thohoyandou, South Africa, 2018. [Google Scholar]
- Pawase, P.A.; Shingote, A.; Chavan, U.D. Studies on evaluation and determination of physical and functional properties of millets. (Ragi and Pearl Millet). Asian J. Dairy Food Res. 2019, 38, 203–212. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Hidayat, J.P. Functional and thermal properties of flour obtained from submerged fermentation of durian (Durio zibethinus Murr.) Seed chips using Lactobacillus plantarum. Potravin. Slovak J. Food Sci. 2018, 12, 607–614. [Google Scholar] [CrossRef]
- Olamiti, G.; Takalani, T.K.; Beswa, D.; Jideani, A.I.O. Effect of malting and fermentation on colour, thermal properties, functional groups and crystallinity level of flours from pearl millet (Pennisetum glaucum) and sorghum (Sorghum bicolor). Heliyon 2020, 6, e05467. [Google Scholar] [CrossRef]
- Nagaprabha, P.; Devisetti, R.; Bhattacharya, S. Physicochemical and microstructural characterisation of green gram and foxtail millet starch gels. J. Food Sci. Technol. 2017, 55, 782–791. [Google Scholar] [CrossRef]
- Waters, D.L.; Henry, R.J.; Reinke, R.F.; Fitzgerald, M.A. Gelatinization temperature of rice explained by poly-morphisms in starch synthase. Plant Biotechnol. J. 2006, 4, 115–122. [Google Scholar] [CrossRef]
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT-Food Sci. Technol. 2020, 140, 110749. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Xua, X.; Sulieman, A.A.; Mahdi, A.A.; Na, Y. Effects of fermentation time on rheological and phys-icochemical characteristics of koreeb (Dactyloctenium aegyptium) seed flour dough and kisra bread. J. Food Meas. Charact. 2019, 13, 2136–2146. [Google Scholar] [CrossRef]
- Bian, X.; Chen, J.-R.; Yang, Y.; Yu, D.-H.; Ma, Z.-Q.; Ren, L.-K.; Wu, N.; Chen, F.-L.; Liu, X.-F.; Wang, B.; et al. Effects of fermentation on the structure and physical properties of glutinous proso millet starch. Food Hydrocoll. 2021, 123, 107144. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, C.; Sethi, S.; Jambh, H. Effect of extruded finger millet on dough rheology and functional quality of pearl millet-based unleavened flatbread. Cereal Chem. 2020, 97, 991–998. [Google Scholar] [CrossRef]
- Said, H.N.; Kusnadi, J. Influence of natural fermentation on the morphology and physicochemical properties of Indonesian rice flour and their effect on rice paper. Int. J. ChemTech Res. 2015, 7, 1951–1959. [Google Scholar]
- Dasa, F.; Binh, L.N. Relation among proximate compositions, rheological properties, and injera making quality of millet varieties. Adv. Crop Sci. Technol. 2020, 8, 2. [Google Scholar]
- Adegunwa, M.O.; Adebowale, A.A.; Bakare, H.A.; Kalejaiye, K.K. Effects of treatments on the antinutritional factors and functional properties of Bambara groundnut (Voandzeia subterranea) flour. J. Food Process. Preserv. 2014, 38, 1875–1881. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N. Studies on functional, thermal, and pasting properties of flours from different chickpea (Cicer arietinum L.) cultivars. Food Chem. 2005, 91, 403–411. [Google Scholar] [CrossRef]
- Mudau, M.; Mashau, M.E.; Ramashia, S.E. Nutritional quality, antioxidant, microstructural and sensory proper-ties of spontaneously fermented gluten-free finger millet biscuits. Foods 2022, 11, 1265. [Google Scholar] [CrossRef]
- Geng, D.-H.; Liu, L.; Zhou, S.; Sun, X.; Wang, L.; Zhou, X.; Tong, L.-T. Effects of Lactobacillus plantarum inoculum on the fermentation rate and rice noodle quality. J. Oleo Sci. 2020, 69, 1031–1041. [Google Scholar] [CrossRef]
- Adebowale, Y.A.; Adeyemi, I.A.; Oshodi, A.A. Functional and physicochemical properties of flours of six Mucuna species. Afr. J. Biotechnol. 2005, 4, 1461–1468. [Google Scholar]
- Oloyede, O.O.; James, S.; Ocheme, O.B.; Chinma, C.E.; Akpa, V.E. Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour. Food Sci. Nutr. 2016, 4, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Farasara, R.; Hariyadi, P.; Fardiaz, D.; Dewanti-Hariyadi, R. Pasting properties of white corn flours of Anoman 1 and Pulut Harapan varieties as affected by fermentation process. Food Sci. Nutr. 2014, 5, 2038. [Google Scholar]
- Nazni, P.; Shobana, D.R. Effect of processing on the characteristic’s changes in barnyard and foxtail millet. J. Food Sci. Technol. 2016, 7, 1–9. [Google Scholar]
- Yuliana, N.; Nurdjanah, S.; Sugiharto, R.; Amethy, D. Effect of spontaneous lactic acid fermentation on physico-chemical properties of sweet potato flour. Microbiol. Indones. 2014, 8, 1. [Google Scholar] [CrossRef]
- Kiin-Kabari, D.B.; Eke-Ejiofor, J.; Giami, S.Y. Functional and pasting properties of wheat/plantain flours enriched with bambara groundnut protein concentrate. Int. J. Food Sci. Nutr. 2015, 5, 75–81. [Google Scholar]
- Oyeyinka, S.A.; Adeloye, A.A.; Olaomo, O.O.; Kayitesi, E. Effect of fermentation time on physicochemical properties of starch extracted from cassava root. Food Biosci. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Oladeji, B.S.; Irinkoyenikan, O.A.; Akanbi, C.T.; Gbadamosi, S.O. Effect of fermentation on the physicochemical properties, pasting profile and sensory scores of normal endosperm maize and quality protein maize flours. Int. Food Res. J. 2018, 25, 1100–1108. [Google Scholar]
- Eke-Ejiofor, J.; Oparaodu, F.O. Chemical, functional and pasting properties of flour from three millet varieties. J. Food Nutr. Res. 2019, 3, 15–21. [Google Scholar]
- Navyashree, N.; Sengar, A.S.; Sunil, C.K.; Venkatachalapathy, N. White Finger Millet (KMR-340): A comparative study to determine the effect of processing and their characterisation. Food Chem. 2021, 374, 131665. [Google Scholar] [CrossRef]
- Akinola, S.A.; Badejo, A.A.; Osundahunsi, O.F.; Edema, M.O. Effect of preprocessing techniques on pearl millet flour and changes in technological properties. Int. J. Food Sci. Technol. 2017, 52, 992–999. [Google Scholar] [CrossRef]
- Siroha, A.K.; Sandhu, K.S.; Kaur, M. Physicochemical, functional and antioxidant properties of flour from pearl millet varieties grown in India. J. Food Meas. Charact. 2016, 10, 311–318. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Isa, N.L.M.; Kormin, F.; Iwansyah, A.C.; Desnilasari, D.; Hesan, A. Physicochemical properties and characterization of fermented cassava flour by lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2021, 736, 012023. [Google Scholar] [CrossRef]
- Arslan, F.N.; Akin, G.; Karuk Elmas, S.N.; Üner, B.; Yilmaz, I.; Janssen, H.-G.; Kenar, A. FT-IR spectroscopy with chemometrics for rapid detection of wheat flour adulteration with barley flour. J. Consum. Prot. Food Saf. 2020, 15, 245–261. [Google Scholar] [CrossRef]
- Salmenkallio-Marttila, M.; Katina, K.; Autio, K. Effects of bran fermentation on quality and microstructure of high-fiber Wheat Bread. Cereal Chem. 2001, 78, 429–435. [Google Scholar] [CrossRef]
- Nainggolan, E.A.; Yudianto, D.; Sayekti, A. Effect of fermentation on physicochemical properties of fermented cassava flour. J. Phys. Conf. Ser. 2019, 1367, 012083. [Google Scholar] [CrossRef]
- Zhao, H.M.; Guo, X.N.; Zhu, K.X. Impact of solid-state fermentation on nutritional, physical and flavour properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Li, Z.L.; Kang, L.N.; Jiang, Y.Y.; Liu, X.Y.; Wang, J.H. Effects of Bacillus fermentation on the protein microstructure and anti-nutritional factors of soybean meal. Lett. Appl. Microbiol. 2017, 65, 520–526. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional quality improvement of soybean meal by Bacillus velezensis and Lactobacillus plantarum during two-stage solid-state fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef]
- Khoza, M.; Kayitesi, E.; Dlamini, B.C. Physicochemical characteristics, microstructure and health promoting properties of green bananaf. Foods 2021, 10, 2894. [Google Scholar] [CrossRef]
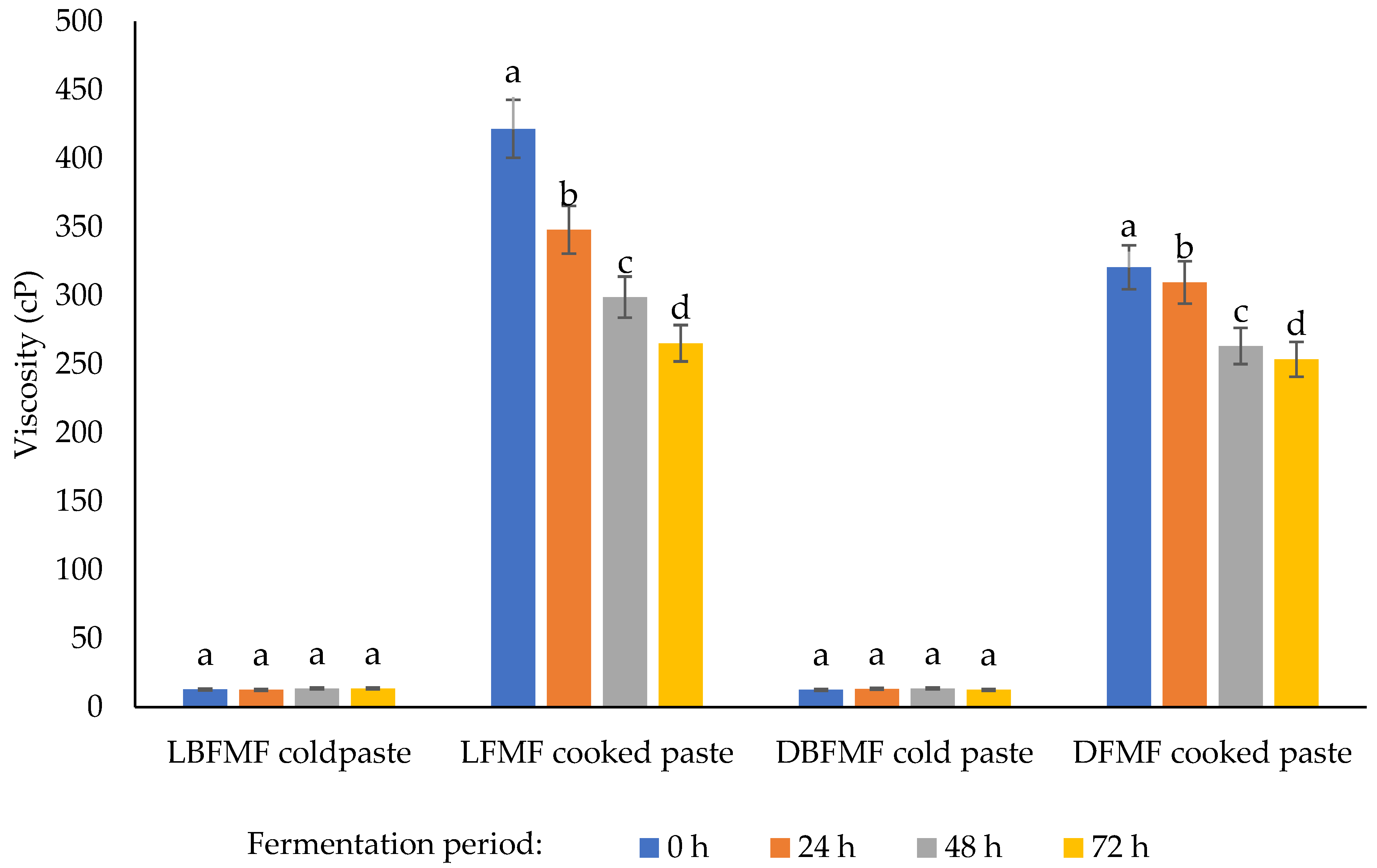
| Fermentation Period (h) | ||||
|---|---|---|---|---|
| FM Cultivars | 0 | 24 | 48 | 72 |
| LBFMF | ||||
| Macro-minerals | ||||
| Ca | 373.68 ± 1.67 a | 382.22 ± 1.34 b | 389.57 ± 1.95 c | 394.24 ± 1.48 d |
| P | 271.34 ± 1.61 a | 283.15 ± 1.61 b | 286.11 ± 0.53 c | 290.72 ± 1.17 d |
| K | 416.20 ± 1.91 a | 426.72 ± 1.36 b | 433.72 ± 0.73 c | 444.09 ± 1.58 d |
| Mg | 124.33 ± 1.27 a | 131.60 ± 1.89 b | 136.30 ± 0.89 c | 141.99 ± 2.16 d |
| Na | 9.88 ± 1.49 a | 14.47 ± 0.99 b | 16.83 ± 0.87 c | 18.43 ± 0.78 d |
| Micro-minerals | ||||
| Cu | 0.46 ± 0.12 a | 0.48 ± 0.26 b | 0.60± 0.07 b | 0.84 ± 0.09 c |
| Zn | 2.12 ± 0.12 a | 2.98 ± 0.27 b | 3.63 ± 0.10 c | 3.75 ± 0.22 d |
| Fe | 4.26 ± 1.07 a | 6.21 ± 1.33 b | 6.67 ± 0.54 b | 7.81 ± 0.29 c |
| Mn | 6.21 ± 0.52 a | 5.93 ± 1.12 a | 5.90 ± 0.74 a | 5.95 ± 0.54 a |
| DBFMF | ||||
| Macro-minerals | ||||
| Ca | 308.66 ± 3.06 a | 320.64 ± 2.35 b | 325.85 ± 1.70 c | 331.98 ± 1.36 d |
| P | 315.77 ± 0.52 a | 325.55 ± 0.77 b | 338.57 ± 0.70 c | 350.58 ± 0.87 d |
| K | 360.92 ± 0.84 a | 379.64 ± 0.44 b | 392.68 ± 0.74 c | 401.90 ± 1.55 d |
| Mg | 144.31 ± 1.27 a | 153.07 ± 0.17 b | 164.61 ± 0.82 c | 171.11 ± 1.89 d |
| Na | 5.66 ± 1.73 a | 10.94 ± 0.68 b | 13.36 ± 1.66 c | 14.83 ± 1.12 c |
| Micro-minerals | ||||
| Cu | 0.44 ± 0.11 a | 0.44 ± 0.05 a | 0.67 ± 0.10 b | 1.17 ± 0.08 c |
| Zn | 1.64 ± 0.05 b | 1.45 ± 0.18 a | 1.45 ± 0.28 a | 1.44 ± 0.11 a |
| Fe | 5.07 ± 0.35 a | 5.69 ± 0.59 a | 5.28 ± 1.01 a | 7.98 ± 0.33 d |
| Mn | 16.62 ± 1.15 b | 16.49 ± 0.32 b | 13.60 ± 0.70 a | 13.34 ± 1.02 a |
| Fermentation Period (h) | LBD (g/g) | PBD (g/g) | WAC (g/g) | OAC (g/g) | SC (mL) |
|---|---|---|---|---|---|
| LBFMF | |||||
| 0 | 0.55 ± 0.07 a | 0.79 ± 0.04 a | 1.96 ± 0.10 a | 1.20 ± 0.28 a | 14.33 ± 0.58 a |
| 24 | 0.56 ± 0.00 a | 0.76 ± 0.01 ab | 2.05 ± 0.06 ab | 1.28 ± 0.09 b | 14.00 ± 1.00 a |
| 48 | 0.56 ± 0.03 a | 0.75 ± 0.02 ab | 2.07 ± 0.01 ab | 1.36 ± 0.15 b | 14.00 ± 1.00 a |
| 72 | 0.52 ± 0.02 a | 0.73 ± 0.03 b | 2.10 ± 0.03 b | 1.44 ± 0.17 c | 13.33 ± 0.58 b |
| DBFMF | |||||
| 0 | 0.47 ± 0.04 a | 0.77 ± 0.00 b | 2.05 ± 0.08 a | 1.24 ± 0.15 a | 14.00 ± 0.00 a |
| 24 | 0.50 ± 0.03 a | 0.74 ± 0.03 ab | 2.06 ± 0.01 a | 1.30 ± 0.10 b | 13.67 ± 0.58 a |
| 48 | 0.52 ± 0.02 a | 0.74 ± 0.02 ab | 2.08 ± 0.01 a | 1.35 ± 0.20 c | 13.67 ± 0.57 a |
| 72 | 0.49 ± 0.04 a | 0.71 ± 0.00 a | 2.14 ± 0.05 b | 1.39 ± 0.11 d | 13.33 ± 0.58 b |
| Fermentation Period (h) | To (°C) | Tp (°C) | Tc (°C) | Tr (°C) | ∆H (J/g) |
|---|---|---|---|---|---|
| LBFMF | |||||
| 0 | 76.64 ± 1.44 a | 78.41 ± 1.16 a | 79.99 ± 1.50 a | 3.35 ± 1.60 a | 5.07 ± 0.79 ab |
| 24 | 79.24 ± 0.81 b | 80.97 ± 0.82 b | 82.25 ± 1.48 b | 3.01 ± 0.67 a | 4.30 ± 0.54 b |
| 48 | 81.12 ± 1.25 bc | 82.06 ± 0.97 c | 85.01 ± 1.29 c | 3.88 ± 1.22 a | 4.28 ± 1.20 ab |
| 72 | 82.47± 0.89 c | 84.48 ± 4.58 d | 86.39 ± 0.56 d | 3.92 ± 1.43 a | 2.99 ± 0.22 a |
| DBFMF | |||||
| 0 | 69.42 ± 1.00 a | 70.98 ± 0.93 a | 78.24 ± 2.95 a | 8.82 ± 1.96 a | 4.87 ± 1.92 c |
| 24 | 70.10 ± 0.25 b | 72.21 ± 1.04 ab | 78.89 ± 1.67 a | 8.79 ± 1.43 a | 4.50 ± 0.84 b |
| 48 | 71.03 ± 0.60 c | 73.62 ± 1.87 bc | 79.95 ± 1.20 b | 8.92 ± 0.66 a | 4.38 ± 1.06 ab |
| 72 | 72.04 ± 1.05 d | 75.67 ± 0.68 c | 81.63 ± 0.80 c | 9.59 ± 0.61 a | 3.85 ± 0.85 a |
| Fermentation Period (h) | Peak Viscosity (cP) | Trough Viscosity (cP) | Breakdown Viscosity (cP) | Final Viscosity (cP) | Setback Viscosity (cP) | Peak Time | PT (°C) |
|---|---|---|---|---|---|---|---|
| LBFMF | |||||||
| 0 | 2410.67 ± 48.23 a | 2308.67 ± 53.59 a | 102.00 ± 7.94 c | 2414.00 ± 66.84 a | 105.33 ± 18.01d | 5.11 ± 0.75 c | 74.82 ± 0.45 a |
| 24 | 2111.67 ± 24.01 c | 1974.33 ± 35.59 b | 137.33 ± 12.34 b | 2181.00 ± 15.72 b | 206.67 ± 20.60 c | 5.24 ± 0.10 c | 75.05 ± 0.05 b |
| 48 | 1709.67 ± 7.23d | 1349.67 ± 12.05d | 360.00 ± 18.73 a | 1616.33 ± 7.51 c | 266.67 ± 7.37 b | 6.00 ± 0.13 b | 75.13 ± 0.32 b |
| 72 | 2246.67 ± 12.58 b | 1889.67 ± 4.73 c | 357.00 ± 17.35 a | 2239.00 ± 11.36 b | 349.33 ± 16.07 a | 6.69 ± 0.08 a | 75.25 ± 0.43 b |
| DBFMF | |||||||
| 0 | 2876.67 ± 30.99 a | 2739.67 ± 32.01 a | 137.00 ± 2.73 d | 2959.00 ± 34.66 a | 219.33 ± 4.26 b | 6.07 ± 0.07 c | 75.02 ± 0.02 a |
| 24 | 2776.00 ± 25.51 b | 2652.33 ± 33.08 b | 123.67 ± 14.19 c | 2871.33 ± 32.96 b | 219.00 ± 7.81 b | 6.13 ± 0.07 c | 75.28 ± 0.23 b |
| 48 | 2762.00 ± 15.72 b | 2578.67 ± 18.58 c | 183.33 ± 33.62 b | 2874.67 ± 27.15 b | 296.00 ± 45.43 a | 6.40 ± 0.00 b | 75.42 ± 0.52 c |
| 72 | 2695.67 ± 11.59 c | 2480.33 ± 10.69 d | 215.33 ± 2.88 a | 2754.67 ± 10.97 c | 274.33 ± 7.57 a | 6.80 ± 0.07 a | 75.73 ± 0.43 d |
| Fermentation Period (h) | L* | a* | b* | Chroma | Hue Angle | ΔE |
|---|---|---|---|---|---|---|
| Light-brown FM flours | ||||||
| 0 | 75.05 ± 0.21 a | 4.28 ± 0.21 c | 7.32 ± 0.05 d | 8.48 ± 0.03 b | 59.67 ± 0.25 a | 0.00 ± 0.00 a |
| 24 | 78.04 ± 0.34 b | 3.32 ± 0.34 b | 6.72 ± 0.09 a | 7.50 ± 0.09 a | 63.70 ± 0.38 b | 3.19 ± 0.35 b |
| 48 | 78.75 ± 0.27 c | 3.26 ± 0.27 b | 6.85 ± 0.07 b | 7.58 ± 0.06 a | 64.58 ± 0.38 c | 3.86 ± 0.28 c |
| 72 | 83.24 ± 0.09 d | 2.62 ± 0.09 a | 7.00 ± 0.03 c | 7.48 ± 0.07 a | 69.46 ± 0.03 d | 8.35 ± 0.08 d |
| Dark-brown FM flours | ||||||
| 0 | 72.07 ± 0.25 a | 3.54 ± 0.03 c | 8.36 ± 0.04 d | 9.07 ± 0.02 d | 67.03 ± 0.26 a | 0.00 ± 0.00 a |
| 24 | 74.56 ± 0.26 b | 2.99 ± 0.06 b | 7.95 ± 0.07 c | 8.49 ± 0.08 c | 69.36 ± 0.33 b | 2.59 ± 0.27 b |
| 48 | 74.83 ± 0.35 b | 3.04 ± 0.01 b | 8.10 ± 0.05 b | 8.65 ± 0.04 b | 69.48 ± 0.14 b | 2.82 ± 0.34 b |
| 72 | 76.08 ± 0.42 c | 2.79 ± 0.04 a | 7.82 ± 0.06 a | 8.30 ± 0.06 a | 70.34 ± 0.41 c | 4.11 ± 0.40 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudau, M.; Ramashia, S.E.; Mashau, M.E. Mineral Content, Functional, Thermo-Pasting, and Microstructural Properties of Spontaneously Fermented Finger Millet Flours. Foods 2022, 11, 2474. https://doi.org/10.3390/foods11162474
Mudau M, Ramashia SE, Mashau ME. Mineral Content, Functional, Thermo-Pasting, and Microstructural Properties of Spontaneously Fermented Finger Millet Flours. Foods. 2022; 11(16):2474. https://doi.org/10.3390/foods11162474
Chicago/Turabian StyleMudau, Masala, Shonisani Eugenia Ramashia, and Mpho Edward Mashau. 2022. "Mineral Content, Functional, Thermo-Pasting, and Microstructural Properties of Spontaneously Fermented Finger Millet Flours" Foods 11, no. 16: 2474. https://doi.org/10.3390/foods11162474
APA StyleMudau, M., Ramashia, S. E., & Mashau, M. E. (2022). Mineral Content, Functional, Thermo-Pasting, and Microstructural Properties of Spontaneously Fermented Finger Millet Flours. Foods, 11(16), 2474. https://doi.org/10.3390/foods11162474






