The Impact of Atmospheric Plasma/UV Laser Treatment on the Chemical and Physical Properties of Cotton and Polyester Fabrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrics and Preparations
2.2. Atmospheric Plasma/UV Laser Treatment
2.3. Morphological Characterisation
2.4. Free Radical Identification
2.4.1. UV–Visible Spectrophotometric Analysis Using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) as a Radical Scavenger
2.4.2. Electron Paramagnetic Resonance (EPR) Analysis
2.5. Chemical Surface Changes
2.5.1. X-ray Photoelectron Spectroscopy (XPS)
2.5.2. Methylene Blue Dye Solution as Diagnostic Dye for Surface Chemical Changes
2.6. Thermal Behaviour
2.7. Wettability (Water Drop Test)
3. Results
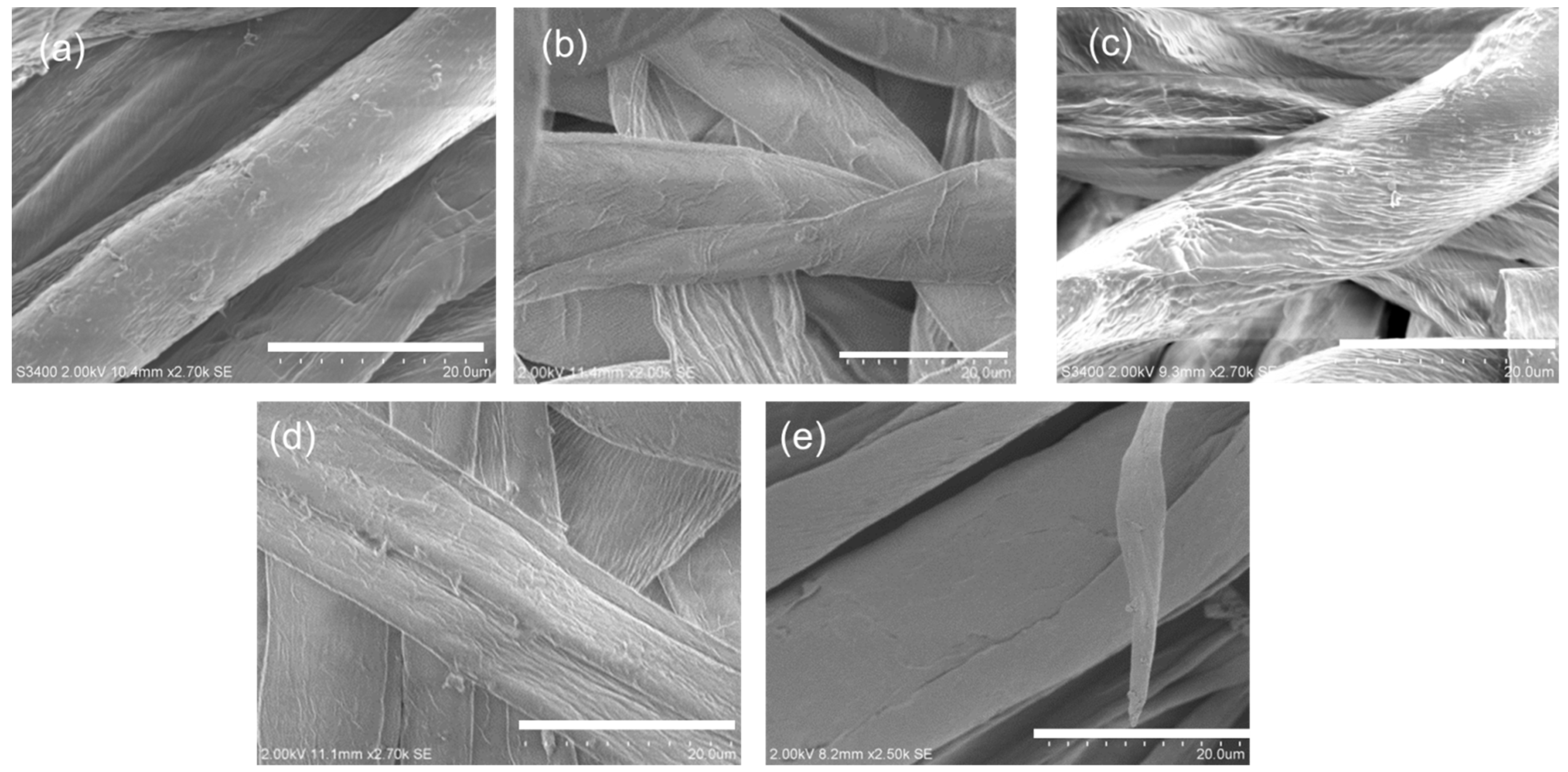
3.1. Impact of Plasma/UV Treatment on Surface Morphology
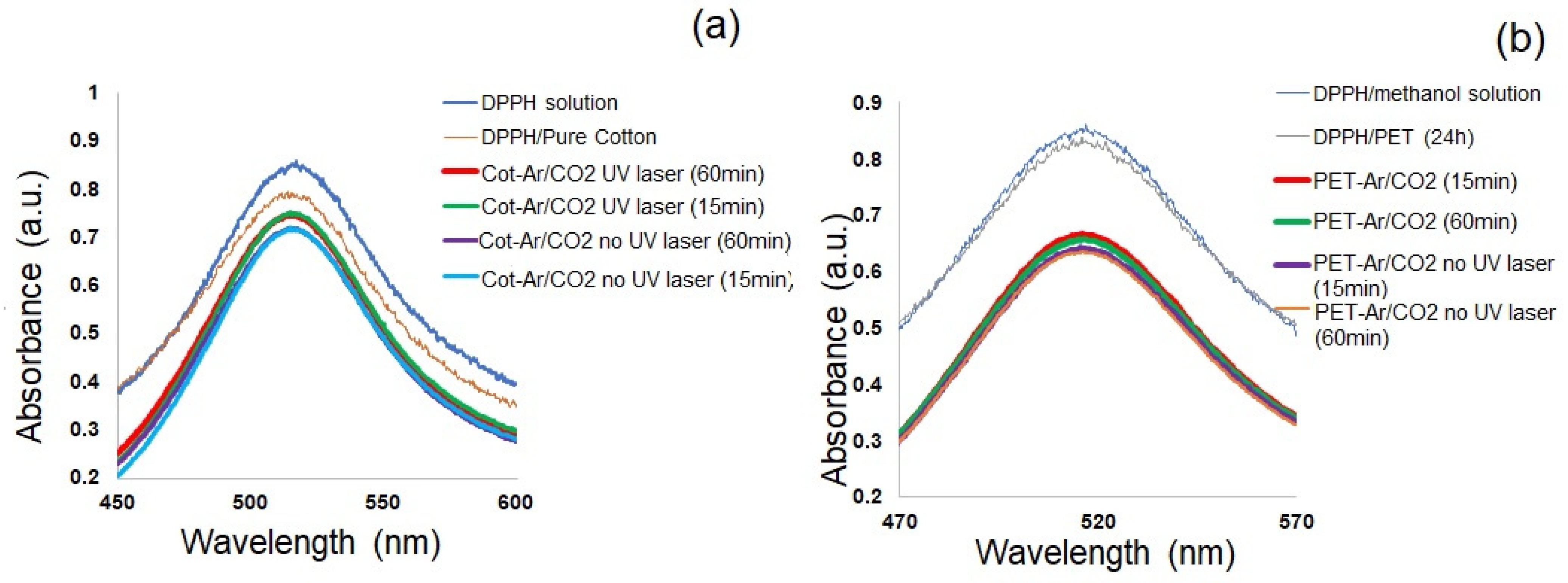
3.2. Impact of Plasma/UV Treatment on Free Radical Formation
3.2.1. Use of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) As a Radical Scavenger
3.2.2. EPR Analysis
3.3. Impact of Plasma/UV Treatment on Functional Groups Formation on the Surface
3.3.1. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.3.2. Determination of Carboxyl Groups by Methylene Blue Dye Uptake
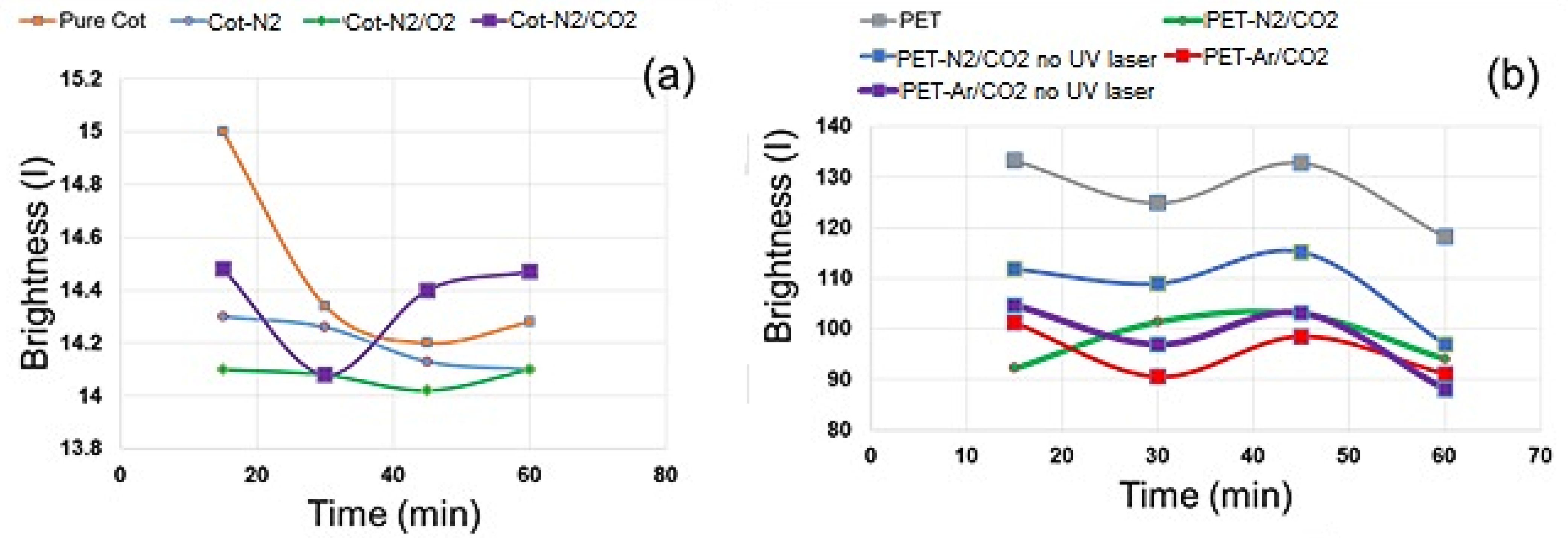
3.3.3. Wettability
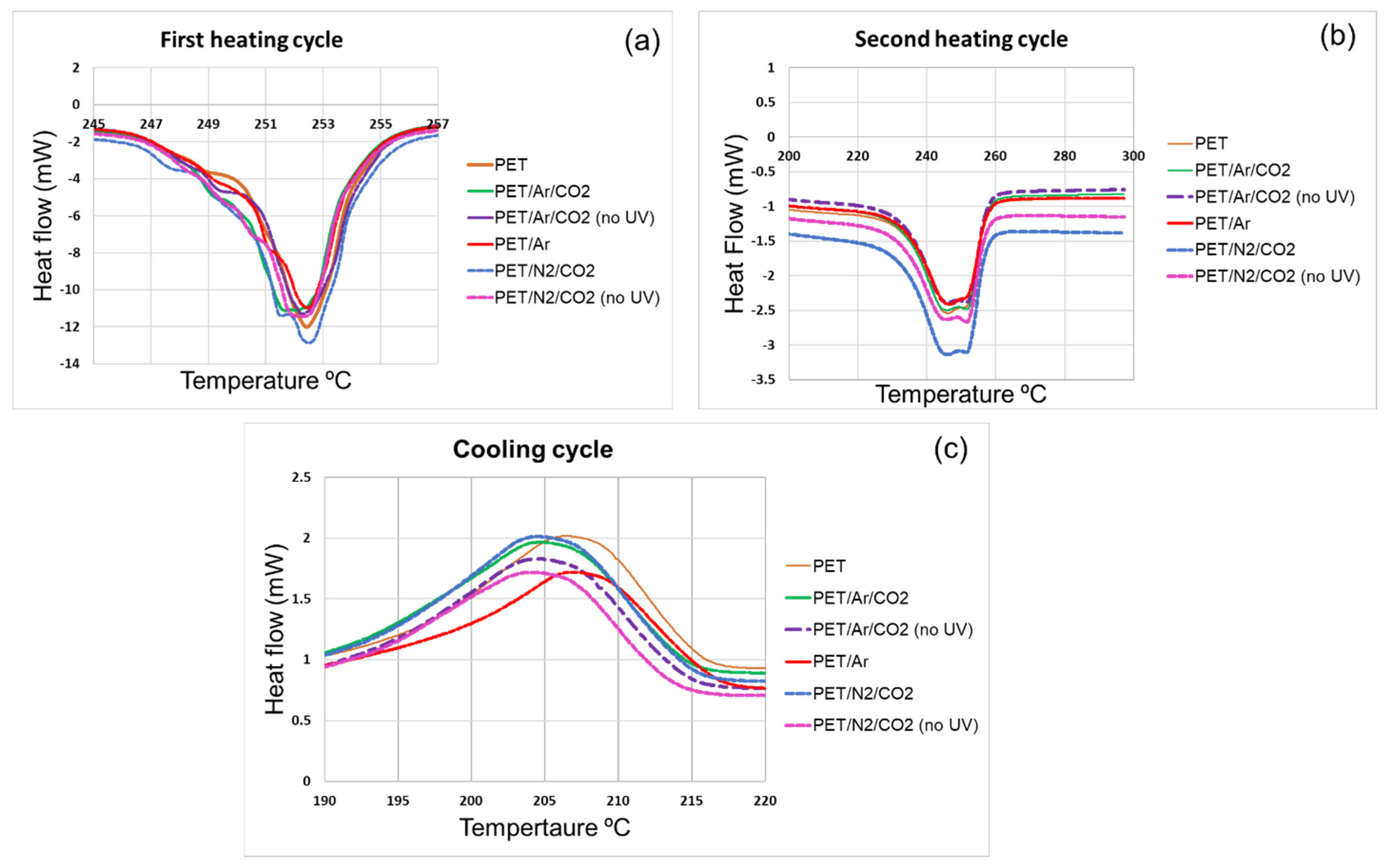
3.4. Impact of Plasma/UV Treatment on the Thermal Behaviour of Component Fibres
3.4.1. Thermal Analysis (TGA/DTG)
3.4.2. Thermophysical and Mechanical Studies (DSC and DMA Analysis) of Polyester Samples
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peran, J.; Ražić, S.E. Application of atmospheric pressure plasma technology for textile surface modification. Text. Res. J. 2020, 90, 1174–1197. [Google Scholar] [CrossRef]
- McCoustra, M.R.S.; Mather, R.R. Plasma modification of textiles: Understanding the mechanisms involved. Text. Prog. 2019, 50, 185–229. [Google Scholar] [CrossRef]
- Zile, A.; Oliveiro, F.R.; Souto, A.P. Plasma treatment in the textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, K. Surface modification of polyester fabrics for inkjet printing with atmospheric-pressure air/Ar plasma. Surf. Coat. Technol. 2009, 203, 2058–2063. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shakerinasab, E.; Eshghabadi, M.; Ghasemi, M. Characterization and performance of coupled atmospheric pressure argon plasma jet with n-hexane electrospray for hydrophobic layer coatings on cotton textile. Diam. Relat. Mater. 2019, 91, 34–45. [Google Scholar] [CrossRef]
- Kale, K.H.; Palaskar, S. Atmospheric pressure plasma polymerization of hexamethyldisiloxane for imparting water repellency to cotton fabric. Text. Res. J. 2010, 81, 608–620. [Google Scholar] [CrossRef]
- Ji, Y.-Y.; Hong, Y.-C.; Lee, S.-H.; Kim, S.-D.; Kim, S.-S. Formation of super-hydrophobic and water-repellency surface with hexamethyldisiloxane (HMDSO) coating on polyethyleneteraphtalate fiber by atmospheric pressure plasma polymerization. Surf. Coat. Technol. 2008, 202, 5663–5667. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Nazaré, S.; Masood, R.; Kandola, B.; Price, D. Surface modification of fabrics for improved flash-fire resistance using atmospheric pressure plasma in the presence of a functionalized clay and polysiloxane. Polym. Adv. Technol. 2011, 22, 22–29. [Google Scholar] [CrossRef]
- Lam, Y.; Kan, C.; Yuen, C. Effect of oxygen plasma pretreatment and titanium dioxide overlay coating on flame retardant finished cotton fabrics. BioResources 2011, 6, 1454–1474. [Google Scholar]
- Johansson, K. Plasma modification of natural cellulosic fibres. In Plasma Technologies for Textiles; Shishoo, R., Ed.; Woodhead Publishing: Cambridge, UK, 2007; pp. 247–281. [Google Scholar]
- Kan, C.-W.; Lam, C.-F. Atmospheric pressure plasma treatment for grey cotton knitted fabric. Polymers 2018, 10, 53. [Google Scholar] [CrossRef]
- Kan, C.-W.; Man, W.-S. Parametric study of effects of atmospheric pressure plasma treatment on the wettability of cotton fabric. Polymers 2018, 10, 233. [Google Scholar] [CrossRef]
- Karahan, H.A.; Özdoğan, E.; Demir, A.; Ayhan, H.; Seventekin, N. Effects of atmospheric pressure plasma treatments on certain properties of cotton fabrics. Fibres Text. East. Eur. 2009, 17, 19–22. [Google Scholar]
- Karahan, H.A.; Özdoğan, E. Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fibers Polym. 2008, 9, 21–26. [Google Scholar]
- Tian, L.; Nie, H.; Chatterton, N.P.; Branford-White, C.J.; Qiu, Y.; Zhu, L. Helium/oxygen atmospheric pressure plasma jet treatment for hydrophilicity improvement of grey cotton knitted fabric. Appl. Surf. Sci. 2011, 257, 7113–7118. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Ebrahimi, I. Atmospheric air-plasma treatment of polyester fiber to improve the performance of nanoemulsion silicone. Appl. Surf. Sci. 2011, 257, 4062–4068. [Google Scholar] [CrossRef]
- Leroux, F.; Perwuelz, A.; Campagne, C.; Behary, N. Atmospheric air-plasma treatments of polyester textile Structures. J. Adhes. Sci. Technol. 2006, 20, 939–957. [Google Scholar]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Atmospheric air plasma treatment of polyester textile materials. Textile structure influence on surface oxidation and silicon resin adhesion. Surf. Coat. Technol. 2009, 203, 3178–3183. [Google Scholar] [CrossRef]
- Wang, C.X.; Lv, J.C.; Ren, Y.; Zhi, T.; Chen, J.Y.; Zhou, Q.Q.; Lu, Z.Q.; Gao, D.W.; Jin, L.M. Surface modification of polyester fabric with plasma pretreatment and carbon nanotube coating for antistatic property improvement. Appl. Surf. Sci. 2015, 359, 196–203. [Google Scholar] [CrossRef]
- Rezaei, F.; Dickey, M.D.; Bourhamc, M.; Hauser, P.J. Surface modification of PET film via a large area atmospheric pressure plasma: An optical analysis of the plasma and surface characterization of the polymer film. Surf. Coat. Technol. 2017, 309, 371–381. [Google Scholar] [CrossRef]
- Mistry, P.; Turchan, J. Method and Apparatus for Surface Treatment of Materials Utilizing Multiple Combined Energy Sources. US Patent 9,309,619 B2, 12 April 2016. [Google Scholar]
- Horrocks, A.R.; Eivazi, S.; Ayesh, M.; Kandola, B. Environmentally sustainable flame retardant surface treatments for textiles: The potential of a novel atmospheric plasma/UV laser technology. Fibers 2018, 6, 31. [Google Scholar] [CrossRef]
- Weclawski, B.T.; Horrocks, A.R.; Ebdon, J.R.; Mosurkal, R.; Kandola, B.K. Combined atmospheric pressure plasma and UV surface functionalisation and diagnostics of nylon 6.6 fabrics. Appl. Surf. Sci. 2021, 562, 150090. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Al-Haiqi, A. Determination of DPPH free radical scavenging activity: Application of artificial neural networks. Food Chem. 2016, 194, 705–711. [Google Scholar] [CrossRef]
- Foti, M.C. Use and abuse of the DPPH(•) radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, B.; Lee, J.H.; Min, D.B. Effects of light, oxygen, and pH on the absorbance of 2,2-Diphenyl-1-picrylhydrazyl. J. Food Sci. 2003, 68, 487–490. [Google Scholar] [CrossRef]
- Scheider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Collins, T. ImageJ for microscopy. BioTechniques 2007, 43, 25–30. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester-cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Badia, J.D.; Stromberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate) (PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Rahimi, M.; Parvinzadeh, M.; Yousefpour, M.; Ahmadi, S. Thermal characterization and flammability of polyester fiber coated with nonionic and cationic softeners. J. Surf. Deterg. 2011, 14, 595–603. [Google Scholar] [CrossRef]
- Stanik, R.; Lucas, P.; Langkamp, A.; Modler, N.; Gude, M.; Pilawka, R. Influence of heat pretreatment on cross-linking behavior and thermal properties of thermoset semi-finished products with powder resin systems. Compos. Theory Pract. 2017, 17, 114–118. [Google Scholar]
- Sung, Y.T.; Kum, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Determining the degree of crosslinking of ethylene vinyl acetate photovoltaic module encapsulants—A comparative study. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Morton, W.E.; Hearle, J.W.S. Physical Properties of Textile Fibres, 3rd ed.; The Textile Institute: Manchester, UK, 1993; pp. 1–73. [Google Scholar]
- Dave, H.; Ledwani, L.; Chandwani, N.; Chauhan, N.; Nema, S.K. The removal of impurities from gray cotton fabric by atmospheric pressure plasma treatment and its characterization using ATR-FTIR spectroscopy. J. Text. Inst. 2014, 105, 586–596. [Google Scholar] [CrossRef]
- Goodman, I.; Nesbitt, B.F. The structures and reversible polymerization of cyclic oligomers from poly(ethylene terephthalate). J. Polym. Sci. 1960, 1, 384–396. [Google Scholar]
- Omerogullari, Z.; Kut, D. Application of low-frequency oxygen plasma treatment to polyester fabric to reduce the amount of flame retardant agent. J. Text. Res. 2012, 82, 613–621. [Google Scholar] [CrossRef]
- Ko, T.J.; Oh, K.H.; Moon, M.W. Plasma-induced hetero-nanostructures on a polymer with selective metal co-deposition. Adv. Mater. Interfaces 2015, 2, 1400431. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.D. Surface modification and preparation techniques for textile materials. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing Series in Textile Technology: Cambridge, UK, 2009; pp. 1–25. [Google Scholar]
- Raslan, W.M.; Rashed, U.S.; El-Sayad, H.; El-Halwagy, A.A. Ultraviolet protection, flame retardancy and antibacterial properties of treated polyester fabric using plasma-nano technology. Mater. Sci. Appl. 2011, 2, 1432–1442. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar] [CrossRef]
- Sabharwal, H.S.; Denes, F.; Nielsen, L.; Young, R.A. Free-radical formation in jute from argon plasma treatment. J. Agric. Food Chem. 1993, 41, 2202–2207. [Google Scholar] [CrossRef]
- Makarov, I.; Vinogradov, M.; Mironova, M.; Shandryuk, G.; Golubev, Y.; Berkovich, A. The Thermal Behavior of Lyocell Fibers Containing Bis(trimethylsilyl)acetylene. Polymers 2021, 13, 537. [Google Scholar] [CrossRef]
- Kan, C.-W.; Man, S.-W. Surface characterisation of atmospheric pressure plasma treated cotton fabric—Effect of operation parameters. Polymers 2018, 10, 250. [Google Scholar] [CrossRef]
- Pranslip, P.; Pruettiphap, M.; Bhanthumnavin, W.; Paosawatyanyong, B.; Kiatkamjornwong, S. Surface modification of cotton fabrics by gas plasmas for colorstrength and adhesion by inkjet ink printing. Appl. Polym. Sci. 2016, 264, 208–220. [Google Scholar]
- Ly, B.; Thielemans, W.; Dufresne, A.; Chaussy, D.; Belgacem, M.N. Surface functionalization of cellulose fibres and their incorporation in renewable polymeric matrices. Compos. Sci. Tech. 2008, 68, 3193–3201. [Google Scholar] [CrossRef]
- Peters, R.H. Textile Chemistry; Elsevier: Amsterdam, The Netherlands, 1967; Volume 2. [Google Scholar]
- Zhou, J.; Cai, D.; Xu, Q.; Zhang, Y.; Fu, F.; Diao, H.; Liu, X. Excellent binding effect of L-methionine for immobilizing silver nanoparticles onto cotton fabrics to improve the antibacterial durability against washing. RSC Adv. 2018, 8, 24458–24463. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.V.; Sarma, B.; Sarma, A. Plasma treatment on cotton fabrics to enhance the adhesion of reduced graphene oxide for electro-conductive properties. Diam. Relat. Mater. 2018, 84, 77–85. [Google Scholar] [CrossRef]
- Shahidi, S. Novel method for ultraviolet protection and flame retardancy of cotton fabrics by low-temperature plasma. Cellulose 2014, 21, 757–768. [Google Scholar] [CrossRef]
- Silva, S.S.; Luna, S.M.; Gomes, M.E.; Benesch, J.; Pashkuleva, I.; Mano, J.F.; Reis, R.L. Plasma surface modification of chitosan membranes: Characterization and preliminary cell response studies. Macromol. Biosci. 2008, 8, 568–576. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.C.L.; Alves, C.; Nascimento, J.H.; Neves, J.R.; Teixeira, V. Surface modification of polyester fabric by non-thermal plasma treatment. J. Phys. Conf. Ser. 2012, 406, 012017. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, C. Surface physical–morphological and chemical changes leading to performance enhancement of atmospheric pressure plasma treated polyester fabrics for inkjet printing. Appl. Surf. Sci. 2009, 255, 7561–7567. [Google Scholar] [CrossRef]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan on to polyethyleneterephthalate (PET) surfaces. Int. J. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Nourbakhsh, S. Antimicrobial performance of plasma corona modified cotton treated with silver nitrate. Russ. J. Appl. Chem. 2018, 91, 1338–1344. [Google Scholar] [CrossRef]
- Mirjalili, M.; Karimi, L. The impact of nitrogen low temperature plasma treatment upon the physical–chemical properties of polyester fabric. J. Text. Inst. 2013, 104, 98–107. [Google Scholar] [CrossRef]
- Abiad, M.G.; Campanella, O.H.; Carvajal, M.T. Assessment of thermal transitions by dynamic mechanical analysis (DMA) using a novel disposable powder holder. Pharmaceutics 2010, 2, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Li, R. Time-temperature superposition method for glass transition temperature of plastic materials. Mater. Sci. Eng. A 2000, 278, 36–45. [Google Scholar] [CrossRef]
- Steinmann, W.; Walter, S.; Beckers, M.; Seide, G.; Gries, T. Thermal analysis of phase transitions and crystallization in polymeric fibers. In Applications of Calorimetry in a Wide Context-Differential Scanning Calorimetry, Isothermal Titration Calorimetry and Microcalorimetry; Elkordy, A.A., Ed.; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/books/2519 (accessed on 24 July 2022).
- Falahat, A.; Ganjovi, A.; Taraz, M.; Rostami Ravari, M.N.; Shahedi, A. Optical characteristics of a RF DBD plasma jet in various Ar/O2 mixtures. Pramana-J. Phys. 2018, 90, 27. [Google Scholar] [CrossRef]
- Phillips, G.O.; Arthur, J.C. Chemical effects of light on cotton cellulose and related compounds: Part II: Photodegradation of cotton cellulose. Text. Res. J. 1964, 34, 572–580. [Google Scholar] [CrossRef]
- Kujirai, C. Studies on the photodegradation of cellulose (VII). Mechanism and quantum yield of photodegradation of cellulose. Sen’i Gakkaishi 1966, 22, 84–89. [Google Scholar] [CrossRef]
- Yurugi, T.; Iwata, H. Photodegradation of cellulosic materials. Nippon Kagaku Kaishi 1977, 4, 544–548. [Google Scholar] [CrossRef]
- Schnabel, W. Photoreactions in biopolymers. In Polymers and Light: Fundamentals and Technical Applications; Wiley-Verlag: Weinheim, Germany, 2007; Chapter 8. [Google Scholar] [CrossRef]
- Day, M.; Wiles, D.M. Photochemical degradation of polyethylene terephthalate. Part III: Decomposition products and reaction mechanism. J. Appl. Polym. Sci. 1972, 16, 203–215. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M. Fundamentals of Polymer Degradation and Stabilisation; Elsevier Applied Science: London, UK; New York, NY, USA, 1992; pp. 80–82. [Google Scholar]
- Bolland, J.L.; Gee, G. Kinetic studies in the chemistry of rubber and related materials. II. The kinetics of oxidation of unconjugated olefins. Trans. Faraday Soc. 1946, 42, 236–243. [Google Scholar] [CrossRef]
- Loucks, L.F.; Cvetanović, R.J. Photolysis of carbon dioxide at 1633 Å. J. Chem. Phys. 1972, 56, 321–329. [Google Scholar] [CrossRef]
- Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Show, P.L. Application of microwave plasma technology to convert carbon dioxide (CO2) into high value products: A review. J. Clean. Prod. 2022, 336, 130447. [Google Scholar] [CrossRef]




| Plasma Gas | Plasma Power (kW)/Number of Cycles | Power Dosage (W/m2·min) | UV Laser (228 W) |
|---|---|---|---|
| - | 0 | 0 | - |
| N2100% | 1/2 | 200 | √ |
| N2100% | 3.5 */1 | 350 | √ |
| N2100% | 1/5 | 500 | √ |
| N2100% | 1/10 | 1000 | √ |
| N280%/O220% | 1/2 | 200 | √ |
| N280%/CO220% | 1/2 | 200 | √ |
| N280%/CO220% | 1/2 | 200 | - |
| Ar100% | 1/2 | 200 | √ |
| Ar80%/O220% | 1 **/2 | 200 | √ |
| Ar80%/CO220% | 1/2 | 200 | √ |
| Ar80%/CO220% | 1/2 | 200 | - |
| Sample Code | UV Laser (228 W) | RSA, % | |
|---|---|---|---|
| 15 min | 60 min | ||
| DPPH solution | - | 0 | 0 |
| Pure cotton | - | 9.0 | 9.0 |
| DPPH/Cot-Ar/CO2 | √ | 13.0 | 13.0 |
| DPPH/Cot-Ar/CO2 | - | 17.0 | 17.0 |
| DPPH/Cot-N2/CO2 | √ | 17.0 | 18.0 |
| DPPH/Cot-N2/CO2 | - | 15.0 | 15.0 |
| Sample Code | UV Laser (228 W) | RSA, % | |
|---|---|---|---|
| 15 min | 60 min | ||
| DPPH solution | - | 0 | 0 |
| DPPH/PET | - | - | 2.3 (24 h) |
| DPPH/PET-Ar/CO2 | √ | 29.2 | 30.8 |
| DPPH/PET-Ar/CO2 | - | 35.7 | 34.1 |
| DPPH/PET-N2/CO2 | √ | 22.1 | 23.1 |
| DPPH/PET-N2/CO2 | - | 13.7 | 12.1 |
| DPPH/PET-Ar/O2 | √ | 3.5 | 8.9 |
| Sample Code | Carbon (1s) | Oxygen (1s) | Nitrogen (1s) | O/C Ratio |
|---|---|---|---|---|
| Pure cotton | 68.0 ± 0.6 | 31.0 ± 0.5 | 1.0 ± 0.2 | 0.45 |
| COT/N2-CO2 | 65.0 ± 1 | 30.0 ± 1 | 0.7 ± 0.1 | 0.46 |
| COT/N2-CO2 (No UV Laser) | 66.0 ± 1 | 33.0 ± 0.6 | 1.0 ± 0.5 | 0.50 |
| COT/Ar-CO2 | 67.0 ± 0.3 | 31.0 ± 1.0 | 1.0 ± 0.3 | 0.46 |
| COT/Ar-CO2 (No UV laser) | 68.0 ± 0.1 | 31.0 ± 0.2 | 0.7 ± 0.3 | 0.46 |
| Sample Code | Carbon (1s) | Oxygen (1s) | Silicon (2p) | O/C Ratio |
|---|---|---|---|---|
| PET | 80.0 ± 0.4 | 19.0 ± 0.5 | 0.6 ± 0.0 | 0.24 |
| PET-N2/CO2 | 77.0 ± 1.0 | 22.0 ± 1.0 | 0.6 ± 0.0 | 0.30 |
| PET-N2/CO2 (No UV Laser) | 74.0 ± 1.0 | 23.0 ± 1.0 | 4.0 ± 1.0 | 0.32 |
| PET-Ar/CO2 | 75.0 ± 1.0 | 23.0 ± 1.0 | 3.0 ± 1.0 | 0.31 |
| PET-Ar/CO2 (No UV Laser) | 69.0 ± 1.0 | 24.0 ± 1.0 | 7.0 ± 1.0 | 0.35 |
| Sample Code | Concentration (%) of C1s | % of All C-O Bonds | |||
|---|---|---|---|---|---|
| C-C, C-H | C-O-C | C=O | O-C=O | ||
| PET | 68.0 | 27.0 | 5.0 | - | 32 |
| PET-N2/CO2 | 52.0 | 39.0 | 9.0 | 1.0 | 49 |
| PET-N2/CO2 (No UV Laser) | 63.0 | 26.0 | 11.0 | - | 37 |
| PET-Ar/CO2 | 57.0 | 32.0 | 10.0 | - | 42 |
| PET-Ar/CO2 (No UV Laser) | 46.0 | 42.0 | 11.0 | 1.0 | 54 |
| Sample Code | UV Laser (MJ) | Water Drop Diameter after 2 min (mm) |
|---|---|---|
| Pure cotton | - | 15.0 ± 0.6 |
| COT-N2/CO2 | 650 | 15.0 ± 0.6 |
| COT-N2/CO2 | - | 20.0 ± 1.0 |
| COT-Ar/CO2 | 650 | 16.0 ± 1.0 |
| PET | 1.0 ± 1.0 | |
| PET-Ar | 650 | 5.0 ± 1.0 |
| PET-Ar/CO2 | 650 | 20.0 ± 1.0 |
| PET-Ar/CO2 | - | 25.0 ± 1.0 |
| PET-N2/CO2 | 650 | 25.0 ± 1.0 |
| PET-N2/CO2 | - | 35.0 ± 1.0 |
| Sample | Tg (°C) (DSC) | Tg’ (°C) (DMA) | Tm1 (°C) | Tc (°C) | Tm2 (°C) | ΔHf (J/g) | ΔHc (J/g) | ΔHf2 (J/g) | Crystallinity % First Heating Cycle | Cross-Linking % from the First Heating Cycle |
|---|---|---|---|---|---|---|---|---|---|---|
| PET | 79 | 125 | 252 | 207 | 246 | 56.0 | 38.0 | 32.0 | 40.0 ± 0.1 | - |
| PET-Ar | 79 | 131 | 252 | 208 | 245 | 56.0 | 37.0 | 33.0 | 40.0 ± 0.8 | - |
| PET-Ar/CO2 | 81 | 130 | 252 | 205 | 245 | 55.0 | 39.0 | 31.0 | 39.0 ± 0.8 | - |
| PET-Ar/CO2 (no UV) | 81 | 133 | 252 | 204 | 246 | 57.0 | 40.0 | 34.0 | 41.0 ± 0.1 | 0.7 |
| PET-N2/CO2 | 81 | 133 | 253 | 205 | 247 | 62.0 | 41.0 | 38.0 | 44.0 ± 0.6 | 10.0 |
| PET-N2/CO2 (no UV) | 82 | 133 | 252 | 204 | 245 | 67.0 | 44.0 | 39.0 | 48.0 ± 0.3 | 19.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayesh, M.; Horrocks, A.R.; Kandola, B.K. The Impact of Atmospheric Plasma/UV Laser Treatment on the Chemical and Physical Properties of Cotton and Polyester Fabrics. Fibers 2022, 10, 66. https://doi.org/10.3390/fib10080066
Ayesh M, Horrocks AR, Kandola BK. The Impact of Atmospheric Plasma/UV Laser Treatment on the Chemical and Physical Properties of Cotton and Polyester Fabrics. Fibers. 2022; 10(8):66. https://doi.org/10.3390/fib10080066
Chicago/Turabian StyleAyesh, Maram, A. Richard Horrocks, and Baljinder K. Kandola. 2022. "The Impact of Atmospheric Plasma/UV Laser Treatment on the Chemical and Physical Properties of Cotton and Polyester Fabrics" Fibers 10, no. 8: 66. https://doi.org/10.3390/fib10080066
APA StyleAyesh, M., Horrocks, A. R., & Kandola, B. K. (2022). The Impact of Atmospheric Plasma/UV Laser Treatment on the Chemical and Physical Properties of Cotton and Polyester Fabrics. Fibers, 10(8), 66. https://doi.org/10.3390/fib10080066







