KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Method
2.1. Search Strategy
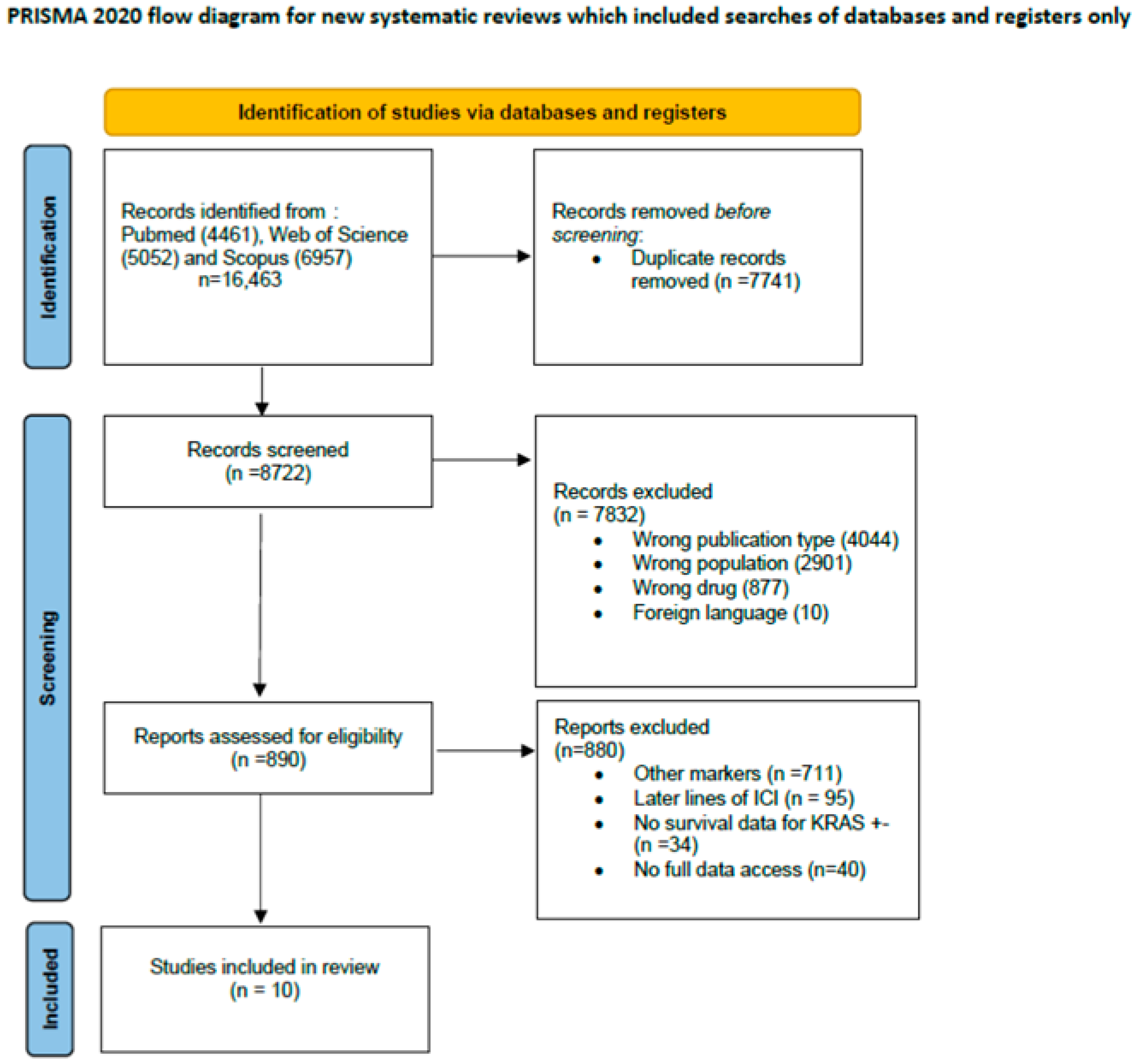
2.2. Article Screening and Selection
2.3. Data Abstraction and Quality Assessment
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
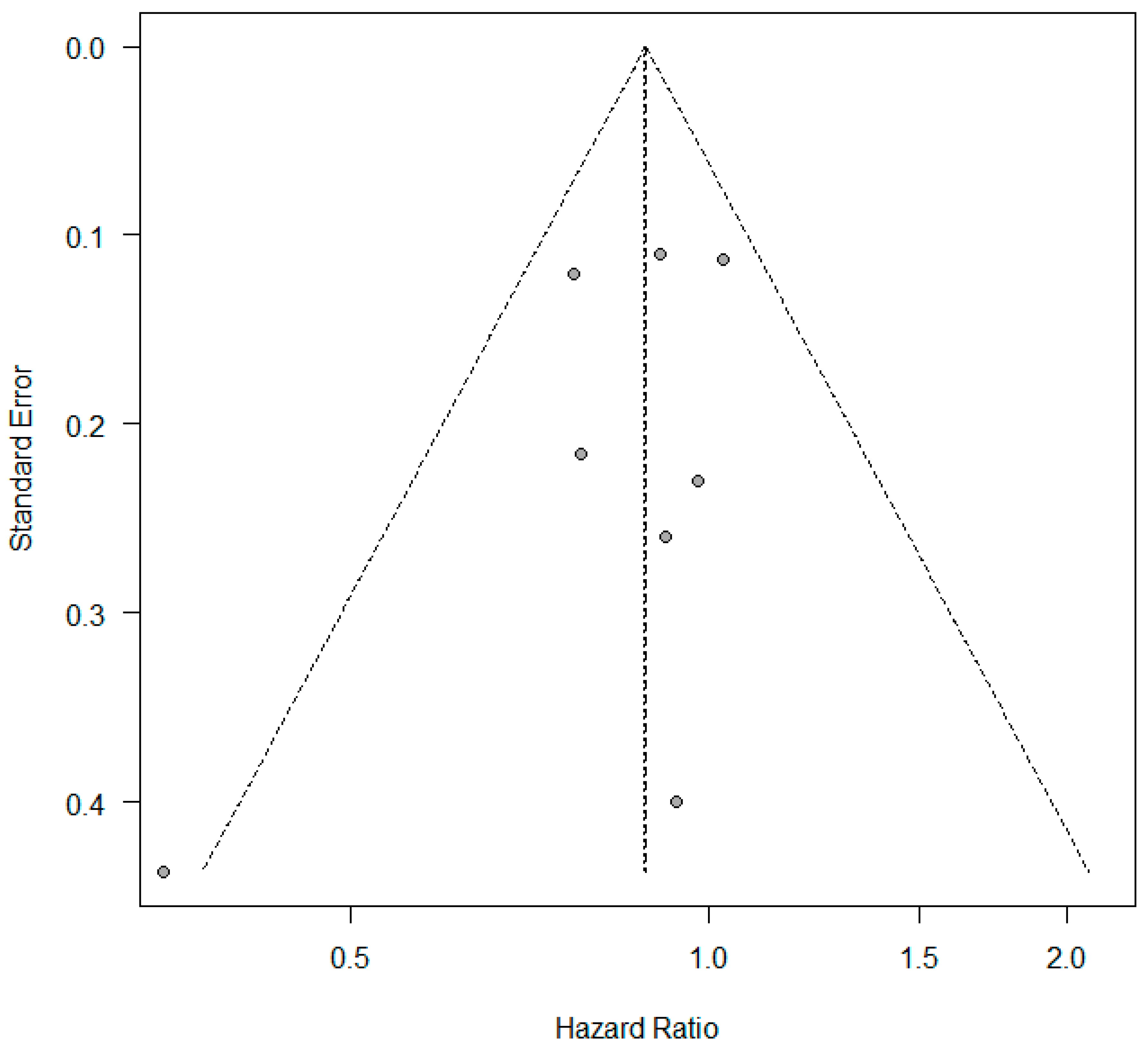
3.1. KRAS +/− Status and Overall Survival
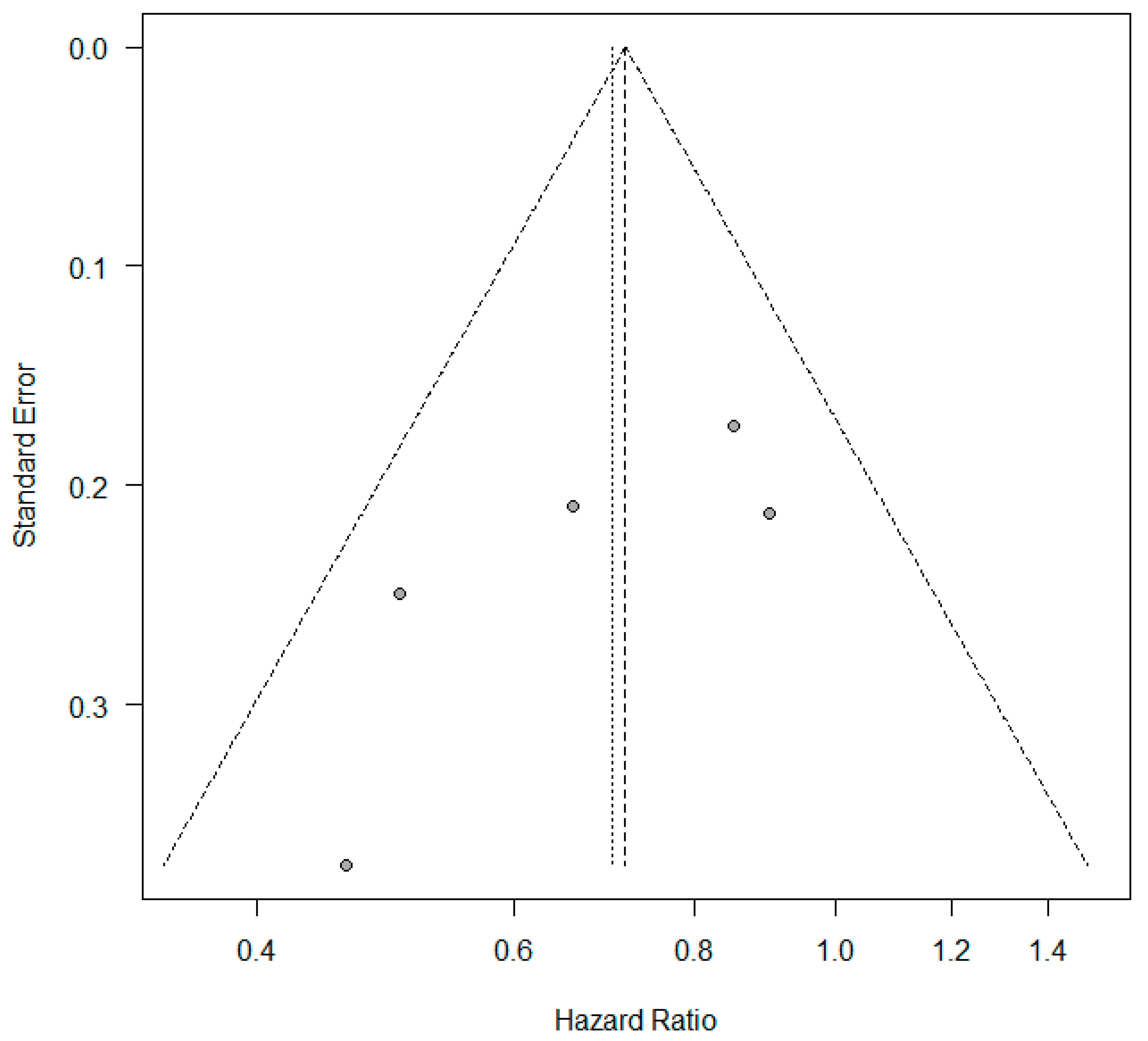
3.2. KRAS +/− Status and Progression-Free Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivares-Hernández, A.; González Del Portillo, E.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments-a systematic review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovács, S.A.; Fekete, J.T.; Győrffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol. Sin. 2023, 44, 1879–1889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liguori, L.; Salomone, F.; Viggiano, A.; Sabbatino, F.; Pepe, S.; Formisano, L.; Bianco, R.; Servetto, A. KRAS mutations in advanced non-small cell lung cancer: From biology to novel therapeutic strategies. Crit. Rev. Oncol./Hematol. 2025, 205, 104554. [Google Scholar] [CrossRef]
- Gu, X.; Si, J.; Guan, Y.; Xu, Y.; Shao, L.; Zhang, Y.; Xu, C.; Pan, W.; Lu, Y.; Song, Z.; et al. Efficacy of immune checkpoint inhibitors in patients with KRAS-mutant advanced non-small cell lung cancer: A retrospective analysis. Open Med. 2023, 18, 20230653. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, X.; Wen, T.; Qu, X. Unveiling the role of KRAS in tumor immune microenvironment. Biomed. Pharmacother. 2024, 171, 116058. [Google Scholar] [CrossRef]
- He, Q.; Liu, X.; Jiang, L.; Liu, P.; Xuan, W.; Wang, Y.; Meng, R.; Feng, H.; Lv, S.; Miao, Q.; et al. First-line treatments for KRAS-mutant non-small cell lung cancer: Current state and future perspectives. Cancer Biol. Ther. 2025, 26, 2441499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, H.; Li, Y.; Wang, Y.; Zou, B.; Chen, Y.; Zhang, Y.; Husain, H.; Forest, F.; Qian, F.; Zhang, L.; et al. Efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer patients with rare KRAS mutations: A real-world retrospective study. Transl. Lung Cancer Res. 2024, 13, 1672–1684. [Google Scholar] [CrossRef]
- Zhao, R.; Shu, Y.; Xu, W.; Jiang, F.; Ran, P.; Pan, L.; Wang, J.; Wang, W.; Zhao, J.; Wang, Y.; et al. The efficacy of immunotherapy in non-small cell lung cancer with KRAS mutation: A systematic review and meta-analysis. Cancer Cell Int. 2024, 24, 361. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. Available online: https://pubmed.ncbi.nlm.nih.gov/36872130/ (accessed on 2 July 2024). [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. Available online: https://pubmed.ncbi.nlm.nih.gov/36669645/ (accessed on 3 July 2024). [CrossRef]
- Cascetta, P.; Marinello, A.; Lazzari, C.; Gregorc, V.; Planchard, D.; Bianco, R.; Normanno, N.; Morabito, A. KRAS in NSCLC: State of the Art and Future Perspectives. Cancers 2022, 14, 5430. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, H.S.; Kim, B.J. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: A meta-analysis and review. Oncotarget 2017, 8, 48248–48252. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA; Chichester, UK, 2019. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analysis; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 2 July 2024).
- Lauko, A.; Kotecha, R.; Barnett, A.; Li, H.; Tatineni, V.; Ali, A.; Patil, P.; Mohammadi, A.M.; Chao, S.T.; Murphy, E.S.; et al. Impact of KRAS mutation status on the efficacy of immunotherapy in lung cancer brain metastases. Sci. Rep. 2021, 11, 18174. [Google Scholar] [CrossRef]
- Velcheti, V.; Hu, X.; Li, Y.; El-Osta, H.; Pietanza, M.C.; Burke, T. Real-World Time on Treatment with First-Line Pembrolizumab Monotherapy for Advanced NSCLC with PD-L1 Expression ≥ 50%: 3-Year Follow-Up Data. Cancers 2022, 14, 1041. [Google Scholar] [CrossRef]
- Cramond, F.; O’Mara-Eves, A.; Doran-Constant, L.; Rice, A.S.; Macleod, M.; Thomas, J. The development and evaluation of an online application to assist in the extraction of data from graphs for use in systematic reviews. Wellcome Open Res. 2019, 3, 157. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Jiménez-Aguilar, E.; Hong, F.; Wei, Z.; Nishino, M.; Plodkowski, A.J.; Sawan, P.; Luo, J.; Rizvi, H.; et al. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non-small cell lung cancer and a poor performance status. J. Immunother. Cancer 2020, 8, e001007. [Google Scholar] [CrossRef]
- Cefalì, M.; Epistolio, S.; Ramelli, G.; Mangan, D.; Molinari, F.; Martin, V.; Freguia, S.; Mazzucchelli, L.; Froesch, P.; Frattini, M.; et al. Correlation of KRAS G12C Mutation and High PD-L1 Expression with Clinical Outcome in NSCLC Patients Treated with Anti-PD1 Immunotherapy. J. Clin. Med. 2022, 11, 1627. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.; Kollmeier, J.; Vollbrecht, C.; Grah, C.; Matthes, B.; Pultermann, D.; von Laffert, M.; Lüders, H.; Olive, E.; Raspe, M.; et al. KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Liu, Y.; Dimou, A.; Patil, T.; Aisner, D.L.; Dong, Z.; Jiang, T.; Su, C.; Wu, C.; Ren, S.; et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 2019, 125, 1038–1049. [Google Scholar] [CrossRef]
- Noordhof, A.L.; Damhuis, R.A.M.; Hendriks, L.E.L.; de Langen, A.J.; Timens, W.; Venmans, B.J.W.; van Geffen, W.H. Prognostic impact of KRAS mutation status for patients with stage IV adenocarcinoma of the lung treated with first-line pembrolizumab monotherapy. Lung Cancer 2021, 155, 163–169. [Google Scholar] [CrossRef]
- Stares, M.; Ding, T.E.; Stratton, C.; Thomson, F.; Baxter, M.; Cagney, H.; Cumming, K.; Swan, A.; Ross, F.; Barrie, C.; et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 2022, 7, 100445. [Google Scholar] [CrossRef]
- Eklund, E.A.; Wiel, C.; Fagman, H.; Akyürek, L.M.; Raghavan, S.; Nyman, J.; Hallqvist, A.; Sayin, V.I. KRAS Mutations Impact Clinical Outcome in Metastatic Non-Small Cell Lung Cancer. Cancers 2022, 14, 2063. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hsu, M.; Cohen, R.B.; Langer, C.J.; Mamtani, R.; Aggarwal, C. Association Between KRAS Variant Status and Outcomes With First-line Immune Checkpoint Inhibitor-Based Therapy in Patients With Advanced Non-Small-Cell Lung Cancer. JAMA Oncol. 2021, 7, 937–939. [Google Scholar] [CrossRef]
- Ceddia, S.; Landi, L.; Cappuzzo, F. KRAS-Mutant Non-Small-Cell Lung Cancer: From Past Efforts to Future Challenges. Int. J. Mol. Sci. 2022, 23, 9391. [Google Scholar] [CrossRef]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; Wu, M.; Zhang, L.; Ji, M. Current status of KRAS G12C inhibitors in NSCLC and the potential for com-bination with anti-PD-(L)1 therapy: A systematic review. Front. Immunol. 2025, 16, 1509173. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, S.; Sun, Y.; Husain, H.; Zhou, H.; Lu, S.; Li, Z. The relationship between different subtypes of KRAS and PD-L1 & tumor mutation burden (TMB) based on next-generation sequencing (NGS) detection in Chinese lung cancer patients. Transl. Lung Cancer Res. 2022, 11, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Sun, T.; Feng, X.; Chen, Y.; Liu, Q.; Tang, H. PD-1/L1 immune checkpoint inhibitors for KRAS-mutant non-small cell lung cancer: A multicenter retrospective real-world study. BMC Cancer 2025, 25, 444. [Google Scholar] [CrossRef]
- Landre, T.; Justeau, G.; Assié, J.B.; Chouahnia, K.; Davoine, C.; Taleb, C.; Chouaïd, C.; Duchemann, B. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: A meta-analysis of randomized-controlled trials. Cancer Immunol. Immunother. 2022, 71, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Jeanson, A.; Tomasini, P.; Souquet-Bressand, M.; Brandone, N.; Boucekine, M.; Grangeon, M.; Chaleat, S.; Khobta, N.; Milia, J.; Mhanna, L.; et al. Efficacy of Immune checkpoint inhibitors in kras-mutant non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2019, 14, 1095–1101. [Google Scholar] [CrossRef]
- Zhao, D.; Li, H.; Mambetsariev, I.; Mirzapoiazova, T.; Chen, C.; Fricke, J.; Kulkarni, P.; Villaflor, V.; Arvanitis, L.; Hamilton, S.; et al. Clinical and Molecular Features of KRAS-Mutated Lung Cancer Patients Treated with Immune Checkpoint Inhibitors. Cancers 2022, 14, 4933. [Google Scholar] [CrossRef]
- Sun, L.; Handorf, E.A.; Zhou, Y.; Borghaei, H.; Aggarwal, C.; Bauman, J. Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels. Lung Cancer 2024, 190, 107510. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Aref, A.R.; Skoulidis, F.; Herter-Sprie, G.S.; Buczkowski, K.A.; Liu, Y.; Awad, M.M.; Denning, W.L.; et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res. 2016, 76, 999–1008. [Google Scholar] [CrossRef]
- Galan-Cobo, A.; Sitthideatphaiboon, P.; Qu, X.; Poteete, A.; Pisegna, M.A.; Tong, P.; Chen, P.H.; Boroughs, L.K.; Rodriguez, M.L.; Zhang, W.; et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res. 2019, 79, 3251–3267. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Arbour, K.C.; Lin, J.J.; Vajdi, A.; Vokes, N.; Hong, L.; Zhang, J.; Tolstorukov, M.Y.; Li, Y.Y.; Spurr, L.F.; et al. Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status. J. Thorac. Oncol. 2022, 17, 399–410. [Google Scholar] [CrossRef] [PubMed]
| Reference | Year | Checkpoint Inhibitor | Stage | Median Follow-Up | Marker | Method | Endpoints | KRAS+ (n) | KRAS− (n) | Total (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Alessi JV [22] | 2020 | Pembrolizumab | IV | 14.8 | KRAS | NR | OS, PFS | 81 | 153 | 234 |
| Cefalì M [23] | 2022 | Pembrolizumab | IIIB/C, IV | NR | KRAS G12C | NGS | PFS | 11 | 33 | 44 |
| Eklund E.A. [28] | 2021 | Pembrolizumab | IV | 7 months | KRAS | FISH | OS | 20 | 17 | 37 |
| Frost N [24] | 2021 | Pembrolizumab | III, IV | 26.4 | KRAS G12C | NGS | OS, PFS | 62 | 57 | 119 |
| Lauko A. [17] | 2021 | Nivolumab, Pembrolizumab | IV | NR | KRAS | NR | OS | 23 | 16 | 39 |
| Ng T. L. [25] | 2019 | Nivolumab, Pembrolizumab, Atezolizumab | III, IV | 7.1 months | KRAS | NGS, FISH | PFS | 77 | 112 | 189 |
| Noordhof A. L [26] | 2021 | Pembrolizumab | IV | 19.1 months | KRAS | NR | OS | 338 | 257 | 595 |
| Stares M [27] | 2022 | Pembrolizumab | IV | 20 months | KRAS | NR | OS, PFS | NR | NR | 130 |
| Sun L. [29] | 2021 | Not reported | IV | NR | KRAS | NR | OS | 363 | 342 | 705 |
| Velcheti V [18] | 2022 | Pembrolizumab | IIIB/C, IV | 34 months | KRAS | NR | rwToT | 164 | 166 | 330 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, F.; Milin-Lazović, J.; Nikolić, N.; Golubović, A.; Stjepanović, M.; Kontić, M. KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis. Curr. Oncol. 2025, 32, 365. https://doi.org/10.3390/curroncol32060365
Marković F, Milin-Lazović J, Nikolić N, Golubović A, Stjepanović M, Kontić M. KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis. Current Oncology. 2025; 32(6):365. https://doi.org/10.3390/curroncol32060365
Chicago/Turabian StyleMarković, Filip, Jelena Milin-Lazović, Nikola Nikolić, Aleksa Golubović, Mihailo Stjepanović, and Milica Kontić. 2025. "KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis" Current Oncology 32, no. 6: 365. https://doi.org/10.3390/curroncol32060365
APA StyleMarković, F., Milin-Lazović, J., Nikolić, N., Golubović, A., Stjepanović, M., & Kontić, M. (2025). KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis. Current Oncology, 32(6), 365. https://doi.org/10.3390/curroncol32060365







