Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses
Simple Summary
Abstract
1. Introduction
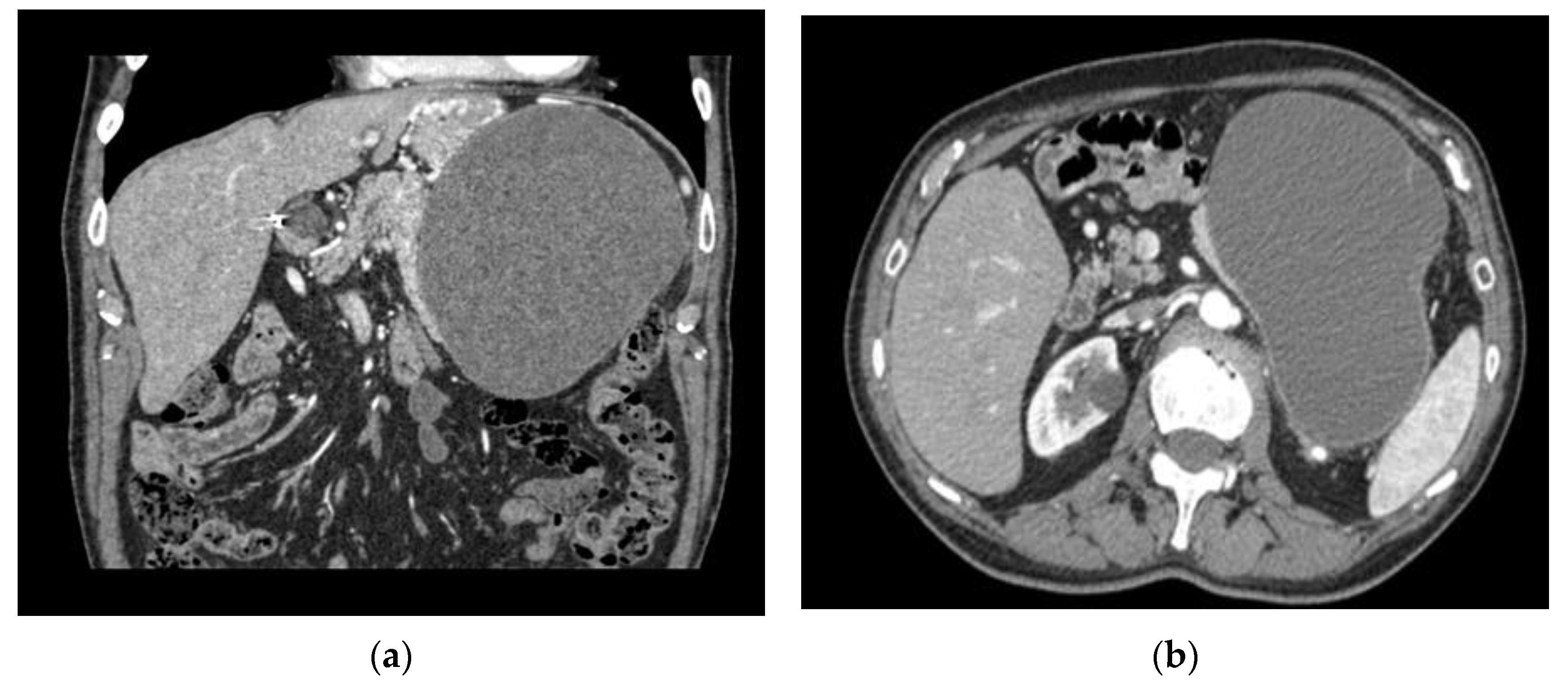
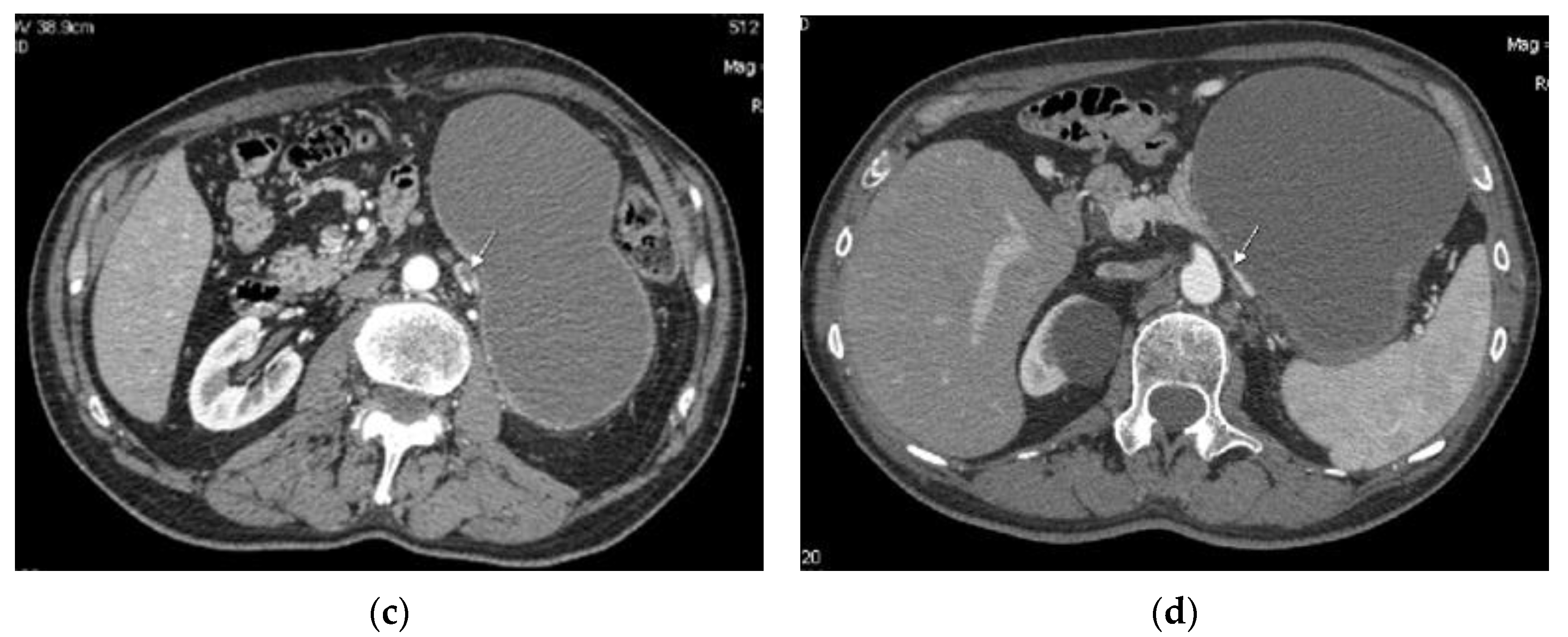
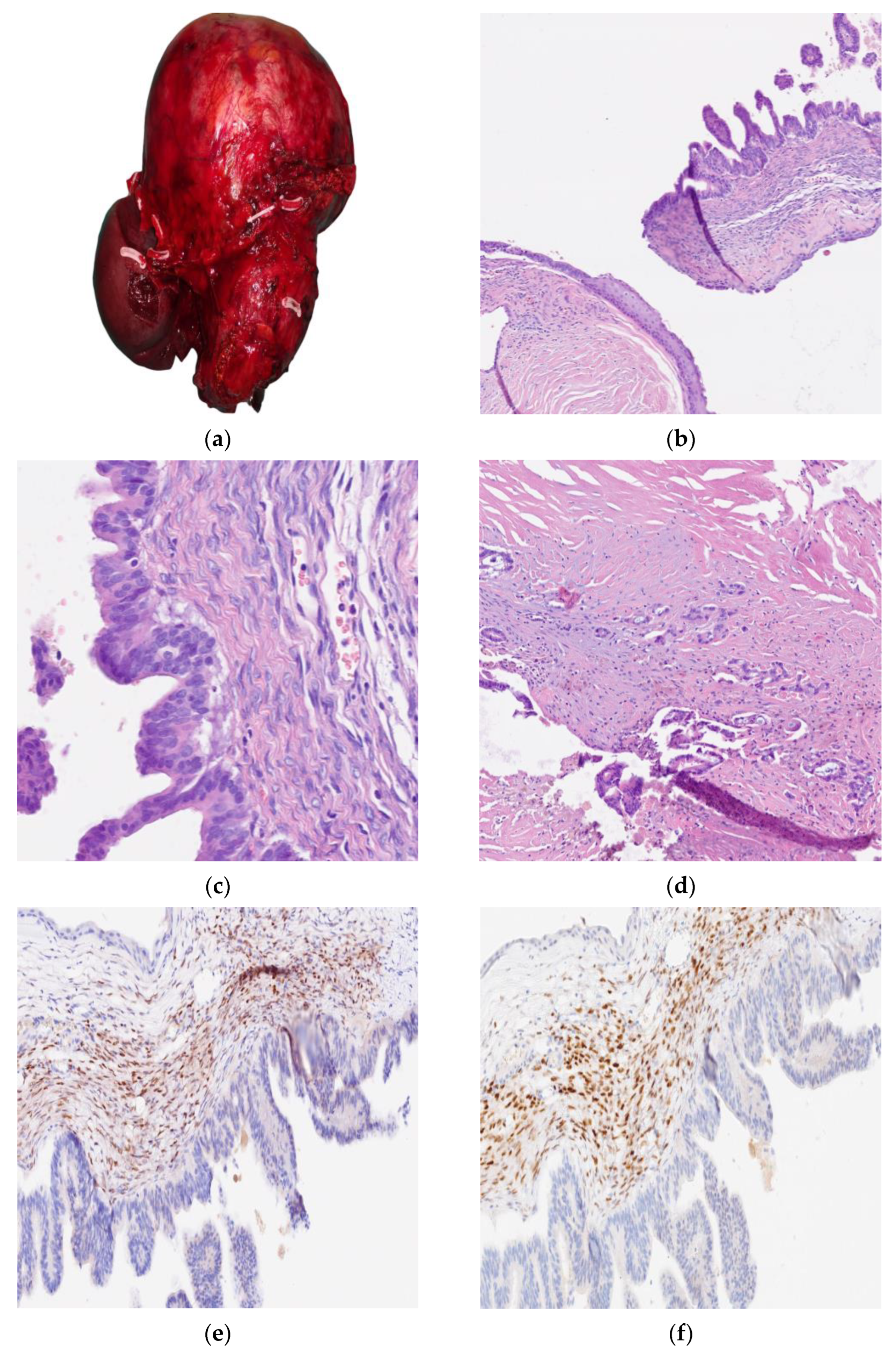
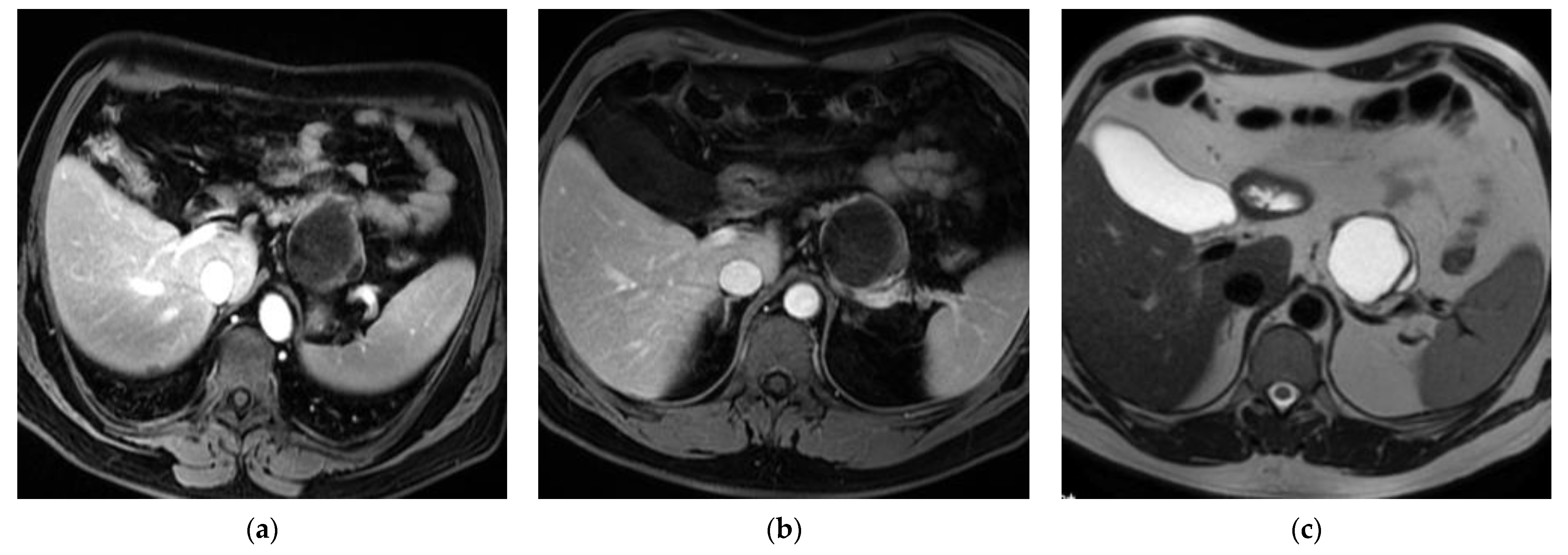
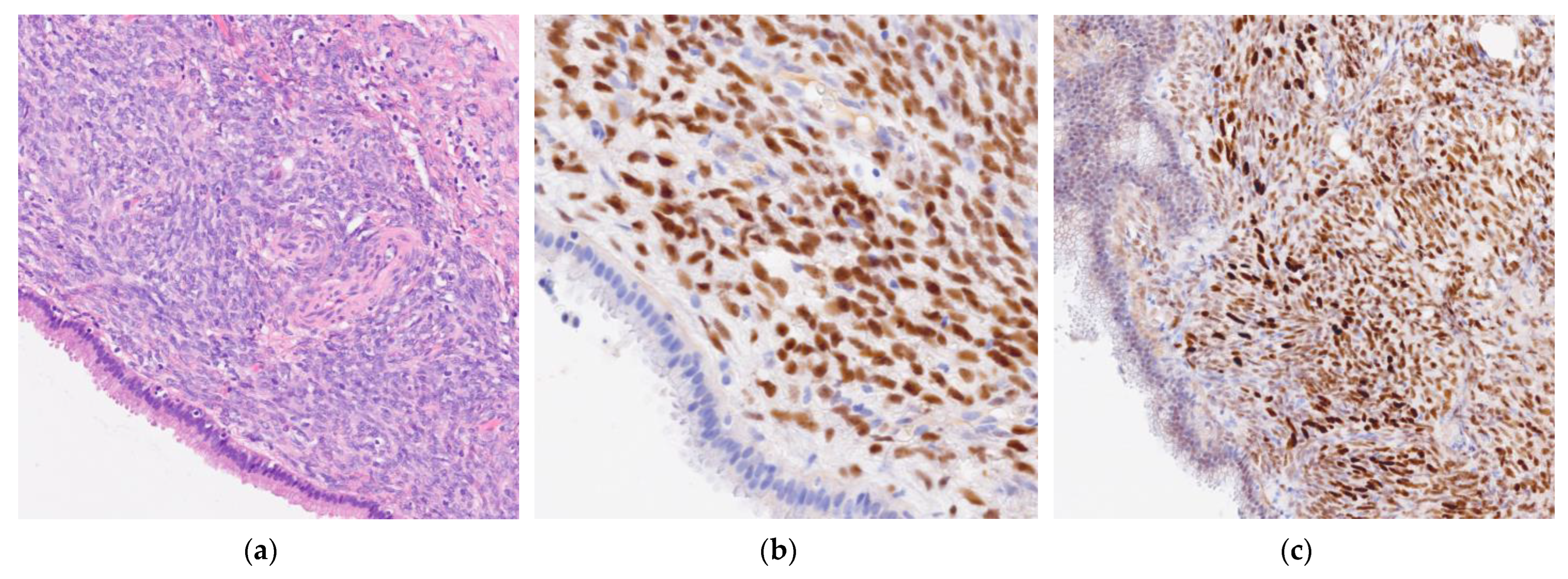
2. Cases
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCNs | Mucinous cystic neoplasms |
| IPMNs | Intraductal papillary mucinous neoplasms |
| ERs | Estrogen receptors |
| PRs | Progesterone receptors |
| CK7 | Cytokeratin 7 |
| CEA | Carcinoembryonic antigen |
| CK20 | Cytokeratin 20 |
| PDAC | Pancreatic ductal adenocarcinoma |
| MLE | Mucinous lining epithelium |
| NMLE | Non-mucinous lining epithelium |
| NGS | Next-generation sequencing |
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wouters, K.; Ectors, N.; Van Steenbergen, W.; Aerts, R.; Driessen, A.; Van Hoe, L.; Geboes, K. A pancreatic mucinous cystadenoma in a man with mesenchymal stroma, expressing oestrogen and progesterone receptors. Virchows Arch. 1998, 432, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Z.; Cui, H.; Xu, J.; Song, J. Clinical characteristics and survival prediction of surgical patients with invasive pancreatic cystic neoplasm: A large retrospective study over two decades. World J. Surg. Oncol. 2023, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.H.; An, J.; Choi, J.S. Comparison with surgically resected mucinous cystic neoplasm of pancreas and branch-duct type intraductal papillary mucinous neoplasm considering clinico-radiological high-risk features: A reassessment of current guidelines. Abdom. Imaging 2024, 49, 2746–2755. [Google Scholar] [CrossRef]
- Tamura, S.; Yamamoto, H.; Ushida, S.; Suzuki, K. Mucinous Cystic Neoplasms in Male Patients: Two Cases. Rare Tumors 2017, 9, 93–95. [Google Scholar] [CrossRef]
- Hu, F.; Hu, Y.; Wang, D.; Ma, X.; Yue, Y.; Tang, W.; Liu, W.; Wu, P.; Peng, W.; Tong, T. Cystic Neoplasms of the Pancreas: Differential Diagnosis and Radiology Correlation. Front. Oncol. 2022, 12, 860740. [Google Scholar] [CrossRef]
- Mamone, G.; Barresi, L.; Tropea, A.; Di Piazza, A.; Miraglia, R. MRI of mucinous pancreatic cystic lesions: A new updated morphological approach for the differential diagnosis. Updat. Surg. 2020, 72, 617–637. [Google Scholar] [CrossRef]
- Colak, M.A.; Freeman, A.J.; Heinzman, C.; Khan, M.A.; Mangray, S.; Potter, C.J.; Rasmussen, S.K.; Rees, M.A.; Nathan, J.D. A rare case of pancreatic mucinous cystic neoplasm in a pediatric patient. JPGN Rep. 2024, 6, 60–64. [Google Scholar] [CrossRef]
- Ethun, C.G.; Postlewait, L.M.; McInnis, M.R.; Merchant, N.; Parikh, A.; Idrees, K.; Isom, C.A.; Hawkins, W.; Fields, R.C.; Strand, M.; et al. The diagnosis of pancreatic mucinous cystic neoplasm and associated adenocarcinoma in males: An eight-institution study of 349 patients over 15 years. J. Surg. Oncol. 2017, 115, 784–787. [Google Scholar] [CrossRef]
- Sohrabi, C.; Mathew, G.; Maria, N.; Kerwan, A.; Franchi, T.; Agha, R.A. Collaborators The SCARE 2023 guideline: Updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2023, 109, 1136–1140. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Beal, E.W.; Pawlik, T.M.; Cloyd, J.; Dillhoff, M.E. Molecular Diagnosis of Cystic Neoplasms of the Pancreas: A Review. J. Gastrointest. Surg. 2020, 24, 1201–1214. [Google Scholar] [CrossRef]
- An, S.; Kim, M.; Kim, S.J.; Sung, Y.-N.; Kim, Y.W.; Song, K.B.; Hwang, D.W.; Kim, S.C.; Hruban, R.H.; Hong, S. Multiple KRAS mutations in the non-mucinous epithelial lining in the majority of mucinous cystic neoplasms of the pancreas. Histopathology 2019, 75, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.; Smyrk, T.; Zapiach, M.; Levy, M.; Pearson, R.; Clain, J.; Farnell, M.; Sarr, M.; Chari, S. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: Demographics, clinical features, and prevalence of cancer. Clin. Gastroenterol. Hepatol. 2004, 2, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Kitada, H.; Hifumi, M.; Takekuma, Y.; Kawaguchi, T.; Higuchi, D.; Yoshinaga, S.; Urata, T.; Yamane, T.; Yokomizo, H.; Fukuda, S. A case of mucinous carcinoma of the pancreas with marked calcification. Nihon Shokakibyo Gakkai Zasshi=Jpn. J. Gastro-Enterol. 2005, 102, 479–483. [Google Scholar]
- Suzuki, M.; Fujita, N.; Onodera, H.; Kayaba, Y.; Suzuki, S.; Kagaya, H.; Noguchi, T.; Kikuchi, T.; Mikuni, J.; Tateno, H. Mucinous cystic neoplasm in a young male patient. J. Gastroenterol. 2005, 40, 1070–1074. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Tan, Y.-M.; Kumarasinghe, M.P.; Ooi, L.L.P.J. Mucinous Cystic Tumor of the Pancreas with Ovarian-like Mesenchymal Stroma in a Male Patient. Dig. Dis. Sci. 2005, 50, 2170–2177. [Google Scholar] [CrossRef]
- Tokuyama, Y.; Osada, S.; Sanada, Y.; Takahashi, T.; Yamaguchi, K.; Yoshida, K. Mucinous cystic neoplasm of the pancreas in a male patient. Rare Tumors 2011, 3, 41–43. [Google Scholar] [CrossRef]
- Yamao, K.; Yanagisawa, A.; Takahashi, K.; Kimura, W.; Doi, R.; Fukushima, N.; Ohike, N.; Shimizu, M.; Hatori, T.; Nobukawa, B.; et al. Clinicopathological Features and Prognosis of Mucinous Cystic Neoplasm With Ovarian-Type Stroma. Pancreas 2011, 40, 67–71. [Google Scholar] [CrossRef]
- Casadei, R.; Pezzilli, R.; Calculli, L.; Santini, D.; Taffurelli, G.; Ricci, C.; D’ambra, M.; Minni, F. Pancreatic mucinous cystic neoplasm in a male patient. J. Pancreas 2012, 13, 687–689. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Zibari, G.B.; Wellman, G.; Abdehou, S.T.; Shokouh-Amiri, H. Mucinous cystic neoplasm of pancreas in a male patient: A case report and review of the literature. J. La State Med. Soc. 2014, 166, 67–69. [Google Scholar]
- Park, J.W.; Jang, J.-Y.; Kang, M.J.; Kwon, W.; Chang, Y.R.; Kim, S.-W. Mucinous cystic neoplasm of the pancreas: Is surgical resection recommended for all surgically fit patients? Pancreatology 2014, 14, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Tomishima, K.; Fujisawa, T.; Fukumura, Y.; Ushio, M.; Sato, S.; Amano, N.; Murata, A.; Tsuzura, H.; Sato, S.; Matsumoto, K.; et al. Mucinous Cystadenocarcinoma of the Pancreas with Cyst Infection in a Male Patient. Intern. Med. 2020, 59, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Pea, A.; Bevere, M.; Gkountakos, A.; Pasini, D.; Fiorini, D.; Mafficini, A.; Golovco, S.; Simbolo, M.; Pedron, S.; Sciammarella, C.; et al. Mucinous cystic neoplasms and simple mucinous cysts are two distinct precursors of pancreatic cancer: Clinicopathological, genomic, and transcriptomic characterization. J. Pathol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiao, Y.; Dal Molin, M.; Maitra, A.; de Wilde, R.F.; Wood, L.D.; Eshleman, J.R.; Goggins, M.G.; Wolfgang, C.L.; Canto, M.I.; et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 21188–21193. [Google Scholar] [CrossRef]
- Amato, E.; Molin, M.D.; Mafficini, A.; Yu, J.; Malleo, G.; Rusev, B.; Fassan, M.; Antonello, D.; Sadakari, Y.; Castelli, P.; et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 2014, 233, 217–227. [Google Scholar] [CrossRef]
- Wu, J.; Matthaei, H.; Maitra, A.; Dal Molin, M.; Wood, L.D.; Eshleman, J.R.; Goggins, M.; Canto, M.I.; Schulick, R.D.; Edil, B.H.; et al. Recurrent GNAS Mutations Define an Unexpected Pathway for Pancreatic Cyst Development. Sci. Transl. Med. 2011, 3, 92ra66. [Google Scholar] [CrossRef]
- Izeradjene, K.; Combs, C.; Best, M.; Gopinathan, A.; Wagner, A.; Grady, W.M.; Deng, C.-X.; Hruban, R.H.; Tuveson, D.A.; Hingorani, S.R. KrasG12D and Smad4/Dpc4 Haploinsufficiency Cooperate to Induce Mucinous Cystic Neoplasms and Invasive Adenocarcinoma of the Pancreas. Cancer Cell 2007, 11, 229–243. [Google Scholar] [CrossRef]
- Conner, J.R.; Mariño-Enríquez, A.; Mino-Kenudson, M.; Garcia, E.; Pitman, M.B.; Sholl, L.M.; Srivastava, A.; Doyle, L.A. Genomic Characterization of Low- and High-Grade Pancreatic Mucinous Cystic Neoplasms Reveals Recurrent KRAS Alterations in “High-Risk” Lesions. Pancreas 2017, 46, 665–671. [Google Scholar] [CrossRef]
- Fujikura, K.; Akita, M.; Abe-Suzuki, S.; Itoh, T.; Zen, Y. Mucinous cystic neoplasms of the liver and pancreas: Relationship between KRAS driver mutations and disease progression. Histopathology 2017, 71, 591–600. [Google Scholar] [CrossRef]
- Schofield, H.K.; Tandon, M.; Park, M.-J.; Halbrook, C.J.; Ramakrishnan, S.K.; Kim, E.C.; Shi, J.; Omary, M.B.; Shah, Y.M.; Esni, F.; et al. Pancreatic HIF2α Stabilization Leads to Chronic Pancreatitis and Predisposes to Mucinous Cystic Neoplasm. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 169–185.e2. [Google Scholar] [CrossRef] [PubMed]
- Fogliati, A.; Crippa, S.; Marchegiani, G.; Belfiori, G.; Pea, A.; Graham, R.P.; Fiorentini, G.; Tomasoni, G.; Aleotti, F.; Kendrick, M.L.; et al. Implications of pregnancy on MCN of the pancreas: A multicentric case-control study. Pancreatology 2024, 24, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Tsantoulis, P.; Tille, J.; Vitonis, A.; Doyle, L.; Hornick, J.L.; Kaya, G.; Barnes, L.; Cramer, D.W.; Puppa, G.; et al. Primordial germ cells as a potential shared cell of origin for mucinous cystic neoplasms of the pancreas and mucinous ovarian tumors. J. Pathol. 2018, 246, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Dhamor, D.; Irrinki, S.; Naik, A.; Kurdia, K.C.; Rastogi, P.; Gupta, P.; Kapoor, V.K. Pregnancy-associated mucinous cystic neoplasms of the pancreas–A systematic review. Am. J. Surg. 2022, 225, 630–638. [Google Scholar] [CrossRef]
- Seyithanoglu, D.; Durak, G.; Keles, E.; Medetalibeyoglu, A.; Hong, Z.; Zhang, Z.; Taktak, Y.B.; Cebeci, T.; Tiwari, P.; Velichko, Y.S.; et al. Advances for Managing Pancreatic Cystic Lesions: Integrating Imaging and AI Innovations. Cancers 2024, 16, 4268. [Google Scholar] [CrossRef]
| Author, y | Size (mm) | Age (y) | M/Tot 1 | Site | Histology | Immunohistochemistry |
|---|---|---|---|---|---|---|
| Wouters, 1998 [2] | 25 | 43 | 1/1 | Tail | Adenoma | ER, PR, VIMENTINA, DESMINA, ACTML |
| Reddy, 2004 [14] | - | - | 1/56 | - | Adenoma | - |
| Kitada, 2005 [15] | 50 | 52 | 1/1 | Body–Tail | Adenoma | - |
| Suzuki, 2005 [16] | 50 | 25 | 1/1 | Tail | Adenoma | PAS, Alcian blu, ER, PR |
| Goh, 2005 [17] | 30 | 28 | 1/1 | Tail | Adenoma | VIMENTINA, ER, PR |
| Tokuyama, 2011 [18] | 65 | 39 | 1/1 | Body–Tail | Adenoma | ER, PR |
| Yamao, 2011 [19] | 15 | 26 36 72 | 3/156 | - | MIC 2 Adenoma Adenoma | ER, PR |
| Casadei, 2012 [20] | 40 | 65 | 1/1 | Body–Tail | Adenoma | ER, PR CALRETININA |
| Fallahzadeh, 2014 [21] | 47 | 48 | 1/1 | Tail | Adenoma | ER, PR |
| Park, 2014 [22] | 40 | 55 | 1/178 | Tail | Adenoma | ER, PR |
| Tamura, 2017 [5] | 51 25 | 50 73 | 2/2 | Tail Tail | Adenoma Adenoma | ER, PR ER |
| Tomishima, 2020 [23] | 40 | 59 | 1/1 | Tail | MIC 2 | ER, PR |
| Pea, 2025 [24] | 11.5 8 | 74 65 | 2/18 | Tail Body–Tail | Adenoma | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaspina, L.; Calomino, N.; Carbone, L.; Batsikosta, A.; Rossi, F.; Poto, G.E.; Visani, A.; Mundo, L.; Barbato, B.; Monteleone, I.; et al. Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses. Curr. Oncol. 2025, 32, 352. https://doi.org/10.3390/curroncol32060352
Malaspina L, Calomino N, Carbone L, Batsikosta A, Rossi F, Poto GE, Visani A, Mundo L, Barbato B, Monteleone I, et al. Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses. Current Oncology. 2025; 32(6):352. https://doi.org/10.3390/curroncol32060352
Chicago/Turabian StyleMalaspina, Lara, Natale Calomino, Ludovico Carbone, Anastasia Batsikosta, Fabiola Rossi, Gianmario Edoardo Poto, Aurora Visani, Lucia Mundo, Bina Barbato, Ilaria Monteleone, and et al. 2025. "Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses" Current Oncology 32, no. 6: 352. https://doi.org/10.3390/curroncol32060352
APA StyleMalaspina, L., Calomino, N., Carbone, L., Batsikosta, A., Rossi, F., Poto, G. E., Visani, A., Mundo, L., Barbato, B., Monteleone, I., Roviello, F., & Tripodi, S. A. (2025). Mucinous Cystic Neoplasms in Male Patients: Histopathological and Molecular Diagnoses. Current Oncology, 32(6), 352. https://doi.org/10.3390/curroncol32060352





