Renal Function Deterioration in Postoperative (Adjuvant) Chemotherapy for Colon Cancer—Real-Life Data †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyzed Data Set
2.2. Data Analysis and Study Endpoints
2.3. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Preterm Termination of Adjuvant CTH
3.3. Deterioration in Kidney Function During Three and 6-Month Adjuvant CTH
3.4. A Subanalysis of Patients Who Experience Kidney Function Deterioration After 3-Month CTH
3.5. A Subanalysis of Patients Who Did Not Experience Kidney Function Deterioration After 3-Month CTH
3.6. Factors Explaining Significant eGFR Decline During the 6-Month Regimen
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aCTH | Adjuvant chemotherapy |
| CTH | Chemotherapy |
| eGFR | Estimated glomerular filtration rates |
| 5-FU | 5-fluorouracil |
| DFS | Disease-free survival |
| OS | Overall survival |
| FOLFOX4 | Oxaliplatin/5-FU/folinic acid |
| CT | Computed tomography |
| CKD-EPI | Chronic Kidney Disease Epidemiology Collaboration |
| Scr | Serum creatinine |
| CKD | Chronic kidney disease |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| CrCl | Creatinine Clearance |
| NKF | National Kidney Foundation |
| CTCAE | Common Terminology Criteria for Adverse Effects |
References
- Cancer.net. Available online: https://www.cancer.net/ (accessed on 8 December 2024).
- Taieb, J.; Gallois, C. Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers 2020, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995, 345, 939–944.
- Sargent, D.J.; Goldberg, R.M.; Jacobson, S.D.; Macdonald, J.S.; Labianca, R.; Haller, D.G.; Shepherd, L.E.; Seitz, J.F.; Francini, G. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N. Engl. J. Med. 2001, 345, 1091–1097. [Google Scholar] [CrossRef]
- Twelves, C.J. Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial: Overview of efficacy, safety, and cost-effectiveness. Clin. Colorectal Cancer. 2006, 6, 278–287. [Google Scholar] [CrossRef]
- André, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Figer, A.; Hill, M.; Nowacki, M.P.; Lim, R.; Sirzén, F.; Cartwright, T.; Park, Y.-S.; Haller, D.G.; Schmoll, H.-J.; et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: A planned safety analysis in 1,864 patients. J. Clin. Oncol. 2007, 25, 102–109. [Google Scholar]
- Ohtsu, A.; Meyerhardt, J.A.; Grothey, A.; Shields, A.F.; Yoshino, T.; Sobrero, A.; Iveson, T.; Taieb, J.; for the IDEA Steering Committee; Souglakos, I.; et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) Collaboration: Prospective Combined Analysis of Phase III Trials Investigating Duration of Adjuvant Therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) Regimen for Patients with Stage III Colon Cancer: Trial Design and Current Status. Curr. Colorectal Cancer Rep. 2013, 9, 261–269. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration); et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Indridason, O.S.; Dubourg, L.; Jager, K.J.; Moranne, O.; Schaeffner, E.; Palsson, R.; Mariat, C.; Gambaro, G.; Glassock, R.J.; Melsom, T.; et al. CKD: A Call for an Age-Adapted Definition. J. Am. Soc. Nephrol. 2019, 30, 1785–1805. [Google Scholar]
- Ferguson, T.W.; Komenda, P.; Tangri, N. Change in estimated glomerular filtration rate and outcomes in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Puig, P.; Salazar, R.; Taieb, J.; Martinelli, E.; Tabernero, J.; Yoshino, T.; Hochhauser, D.; Arnold, D.; Iveson, T.; Labianca, R.; et al. ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 3, 1291–1305. [Google Scholar]
- Zhang, L.; Li, Q.; Hu, C.; Zhang, Z.; She, J.; Shi, F. Real-world analysis of survival benefit of surgery and adjuvant therapy in elderly patients with colorectal cancer. Sci. Rep. 2023, 13, 14866. [Google Scholar] [CrossRef] [PubMed]
- Raycraft, T.; Cheung, W.Y.; Yin, Y.; Speers, C.; Ko, J.J.; Mariano, C. Causes of mortality in older patients with stage 3 colon cancer. J. Geriatr. Oncol. 2019, 10, 138–142. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Rougier, P.; Marshall, J.; Garin, A.; Bajetta, E.; Schilsky, R.L.; McKendrick, J.; Rosso, R.; Maroun, J.; Hoff, P.; et al. Capecitabine Colorectal Cancer Study Group. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann. Oncol. 2002, 13, 566–575. [Google Scholar]
- Twelves, C.; Poole, C.; Harper, P.; Cassidy, J.; Banken, L.; Reigner, B.; Weidekamm, E.; Gardiner, J.; Monkhouse, J.; Johnston, P. Effect of renal impairment on the pharmacokinetics and tolerability of capecitabine (Xeloda) in cancer patients. Cancer Chemother. Pharmacol. 2002, 49, 225–234. [Google Scholar]
- Hamilton, A.; Ivy, P.; Egorin, M.; Mani, S.; Mulkerin, D.; Doroshow, J.H.; Graham, M.; Ramanathan, R.K.; Grem, J.L.; Goetz, A.; et al. Administration of oxaliplatin to patients with renal dysfunction: A preliminary report of the National Cancer Institute organ dysfunction working group. Semin. Oncol. 2003, 30 (Suppl. S15), 20–25. [Google Scholar]
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Kozlowski, L.; Bielawska, K.; Zhymaila, A.; Malyszko, J. Chronic Kidney Disease Prevalence in Patients with Colorectal Cancer Undergoing Surgery. Diagnostics 2022, 12, 2137. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Nozawa, H.; Kitayama, J.; Sunami, E.; Watanabe, T. Impact of chronic kidney disease on outcomes of surgical resection for primary colorectal cancer: A retrospective cohort review. Dis. Colon. Rectum. 2012, 55, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 9 June 2025).
- Gladys, A.; Kozak, S.; Owczarek, A.J.; Cedrych, E.; Niemir, Z.I.; Lacki-Zynzeling, S.; Chudek, A.; Mrochen-Domin, I.; Gisterek, I.; Chudek, J. 23P Renal impairment evaluation on postoperative (adjuvant) chemotherapy for colon cancer. Ann. Oncol. 2024, 35, S11. [Google Scholar] [CrossRef]

| All [N = 145] | Completed 6-Month Regimen [N = 114] | Non-Completed 6-Month Regimen [N = 31] | p | |
|---|---|---|---|---|
| Women [N (%)] | 76 (52.4) | 61 (53.5) | 15 (48.4) | 0.61 |
| Age [years] | 62 ± 11 | 62 ± 11 | 63 ± 10 | 0.54 |
| Age ≤50 years [N (%)] | 21 (14.5) | 18 (15.8) | 3 (9.7) | 0.57 |
| Age ≥ 70 years [N (%)] | 32 (22.1) | 25 (21.9) | 7 (22.6) | 0.94 |
| BMI [kg/m2] | 26.9 ± 4.3 | 27.0 ± 4.3 | 26.5 ± 4.6 | 0.52 |
| Obesity [N (%)] | 33 (23.1) | 26 (23.2) | 7 (22.6) | 0.94 |
| Clinical stage [N (%)] | ||||
| II | 32 (22.1) | 25 (21.9) | 7 (22.6) | 0.99 |
| III | 99 (68.3) | 78 (68.4) | 21 (67.7) | |
| IV | 14 (9.6) | 11 (9.7) | 2 (9.7) | |
| Chemotherapy regimen [N (%)] | ||||
| 5-FU (de Gramont) | 53 (36.6) | 42 (36.8) | 11 (35.5) | 0.31 |
| FOLFOX-4 | 55 (37.9) | 46 (40.4) | 9 (29.0) | |
| XELOX | 37 (25.5) | 26 (22.8) | 11 (35.5) | |
| Comorbidity [N (%)] | ||||
| Hypertension | 86 (59.3) | 72 (63.2) | 14 (45.2) | 0.07 |
| Coronary artery disease | 18 (12.4) | 13 (11.4) | 5 (16.1) | 0.48 |
| Atrial fibrillation | 6 (4.1) | 5 (4.4) | 1 (3.2) | 0.77 |
| Diabetes mellitus | 28 (19.3) | 20 (17.5) | 8 (25.8) | 0.30 |
| Chronic kidney disease | 2 (1.4) | 2 (1.8) | 0 | 1.00 |
| All [N = 145] | Completed 6-Month Regimen [N = 114] | Non-Completed 6-Month Regimen [N = 31] | p | |
|---|---|---|---|---|
| eGFR—estimated glomerular filtration rate | ||||
| Initial [mL/min/1.73 m2] | 93.5 ± 18.8 | 92.3 ± 19.5 | 90.6 ± 15.8 | 0.34 |
| After 3-month aCTH [mL/min/1.73 m2] | 91.1 ± 20.4 | 91.8 ± 20.8 | 88.8 ± 19.1 | 0.47 |
| After 6-month aCTH [mL/min/1.73 m2] | – | 91.2 ± 22.1 | – | – |
| eGFR changes | ||||
| During 3-month aCTH [mL/min/1.73 m2] | −2.3 ± 12.4 | −2.5 ± 12.7 | −1.9 ± 11.5 | 0.81 |
| After the third month of aCTH [%] | −2.2 ± 13.5 | −2.3 ± 13.3 | −2.0 ± 14.4 | 0.92 |
| During 6-month aCTH [mL/min/1.73 m2] | – | −3.1 ± 11.6 | – | – |
| During 6-month aCTH [%] | – | −3.3 ± 12.5 | – | – |
| eGFR decline | ||||
| ≥1.5 mL/min/1.73 m2 after 3-month aCTH [N (%)] | 77 (52.1) | 62 (54.4) | 15 (48.4) | 0.55 |
| ≥3.0 mL/min/1.73 m2 after 6-month aCTH [N (%)] | – | 54 (47.4) | – | – |
| ≥1.5 mL/min/1.73 m2 after the third month of aCTH [N (%)] | – | 47 (41.2) | – | – |
| >30% after 3-month aCTH [N (%)] | 5 (3.4) | 4 (3.5) | 1 (3.2) | 0.94 |
| >30% after 6-month aCTH [N (%)] | – | 3 (2.6) | – | – |
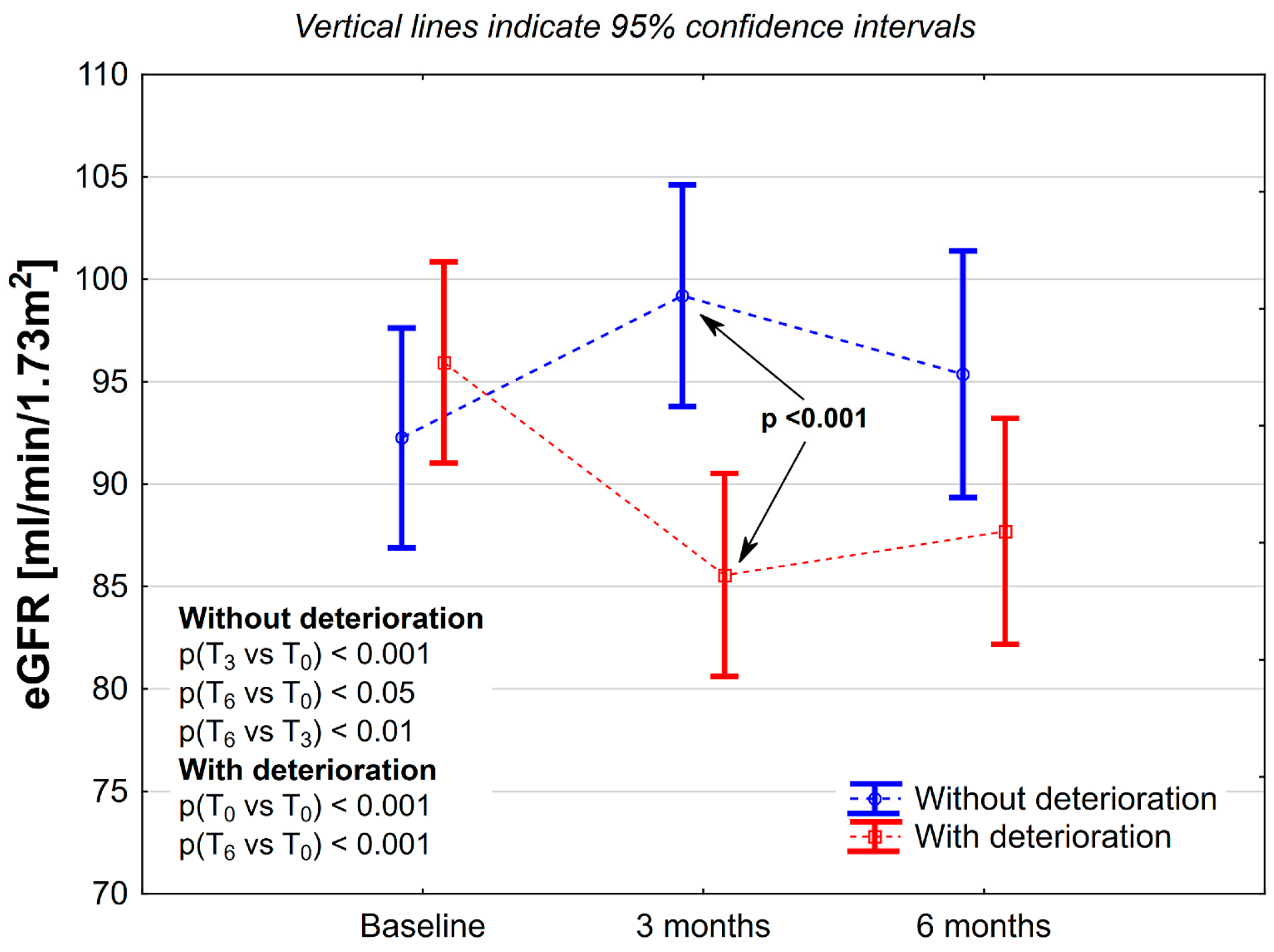
| Deterioration [N = 62] | No Deterioration [N = 52] | p | |
|---|---|---|---|
| Female [N (%)] | 33 (53.2) | 28 (53.8) | 0.95 |
| Age [years] | |||
| Age ≥ 70 [N (%)] | 19 (30.6) | 6 (11.5) | <0.05 |
| Obesity [N (%)] | 15 (24.6) | 11 (21.6) | 0.71 |
| Diabetes mellitus [N (%)] | 15 (24.2) | 5 (9.6) | <0.05 |
| Chemotherapy [N (%)] | |||
| 5-FU (de Gramont) | 27 (43.6) | 15 (28.8) | <0.01 |
| FOLFOX-4 | 7 (11.3) | 19 (36.5) | |
| XELOX | 28 (45.2) | 18 (34.6) | |
| Estimated glomerular filtration rate—eGFR | |||
| Initial [mL/min/1.73 m2] | 95.9 ± 21.1 | 92.3 ± 17.4 | 0.32 |
| After 3-month aCTH [mL/min/1.73 m2] | 86.6 ± 20.6 | 99.2 ± 18.5 | <0.001 |
| After 6-month aCTH [mL/min/1.73 m2] | 87.7 ± 23.2 | 95.3 ± 20.3 | 0.07 |
| eGFR change | |||
| During 3-month aCTH [mL/min/1.73 m2] | −10.4 ± 9.6 | 6.9 ± 8.7 | <0.001 |
| During 3-month aCTH [%] | −10.9 ± 9.4 | 7.9 ± 9.6 | <0.001 |
| During 6-month aCTH [mL/min/1.73 m2] | −8.2 ± 10.4 | 3.1 ± 10.0 | <0.001 |
| During 6-month aCTH [%] | −8.9 ± 10.4 | 3.4 ± 11.6 | <0.001 |
| eGFR decline | |||
| >30% after 3-month aCTH [N (%)] | 4 (6.4) | – | – |
| ≥1.5 mL/min/1.73 m2 after the third month aCTH [N (%)] | 20 (32.3) | 27 (51.9) | <0.05 |
| ≥3.0 mL/min/1.73 m2 after 6-month aCTH [N (%)] | 45 (72.6) | 9 (17.3) | <0.001 |
| >30% after 6-month aCTH [N (%)] | 3 (4.8) | – | – |
| Kidney dysfunction | |||
| After 3-month aCTH [N (%)] | 4 (6.4) | 1 (1.9) | 0.37 |
| After 6-month aCTH [N (%)] | 1 (1.6) | 0 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gładyś, A.; Kozak, S.; Owczarek, A.J.; Cedrych, E.; Niemir, Z.I.; Łącki-Zynzeling, S.; Chudek, A.; Mrochen-Domin, I.; Gisterek-Grocholska, I.; Chudek, J. Renal Function Deterioration in Postoperative (Adjuvant) Chemotherapy for Colon Cancer—Real-Life Data. Curr. Oncol. 2025, 32, 351. https://doi.org/10.3390/curroncol32060351
Gładyś A, Kozak S, Owczarek AJ, Cedrych E, Niemir ZI, Łącki-Zynzeling S, Chudek A, Mrochen-Domin I, Gisterek-Grocholska I, Chudek J. Renal Function Deterioration in Postoperative (Adjuvant) Chemotherapy for Colon Cancer—Real-Life Data. Current Oncology. 2025; 32(6):351. https://doi.org/10.3390/curroncol32060351
Chicago/Turabian StyleGładyś, Aleksandra, Sylwia Kozak, Aleksander Jerzy Owczarek, Ewa Cedrych, Zofia Irena Niemir, Stanisław Łącki-Zynzeling, Anna Chudek, Izolda Mrochen-Domin, Iwona Gisterek-Grocholska, and Jerzy Chudek. 2025. "Renal Function Deterioration in Postoperative (Adjuvant) Chemotherapy for Colon Cancer—Real-Life Data" Current Oncology 32, no. 6: 351. https://doi.org/10.3390/curroncol32060351
APA StyleGładyś, A., Kozak, S., Owczarek, A. J., Cedrych, E., Niemir, Z. I., Łącki-Zynzeling, S., Chudek, A., Mrochen-Domin, I., Gisterek-Grocholska, I., & Chudek, J. (2025). Renal Function Deterioration in Postoperative (Adjuvant) Chemotherapy for Colon Cancer—Real-Life Data. Current Oncology, 32(6), 351. https://doi.org/10.3390/curroncol32060351






