Therapeutic Advances in Initially Unresectable Locally Advanced Intrahepatic Cholangiocarcinoma: Emerging Treatments and the Role of Liver Transplantation
Abstract
1. Introduction
2. Pathogenesis, Presentation, and Diagnosis
3. Surgery as the Gold Standard for iCCA Treatment
4. Systemic Chemotherapy for Initially Unresectable iCCA
5. Use of Locoregional and Liver-Directed Therapies in Initially Unresectable iCCA
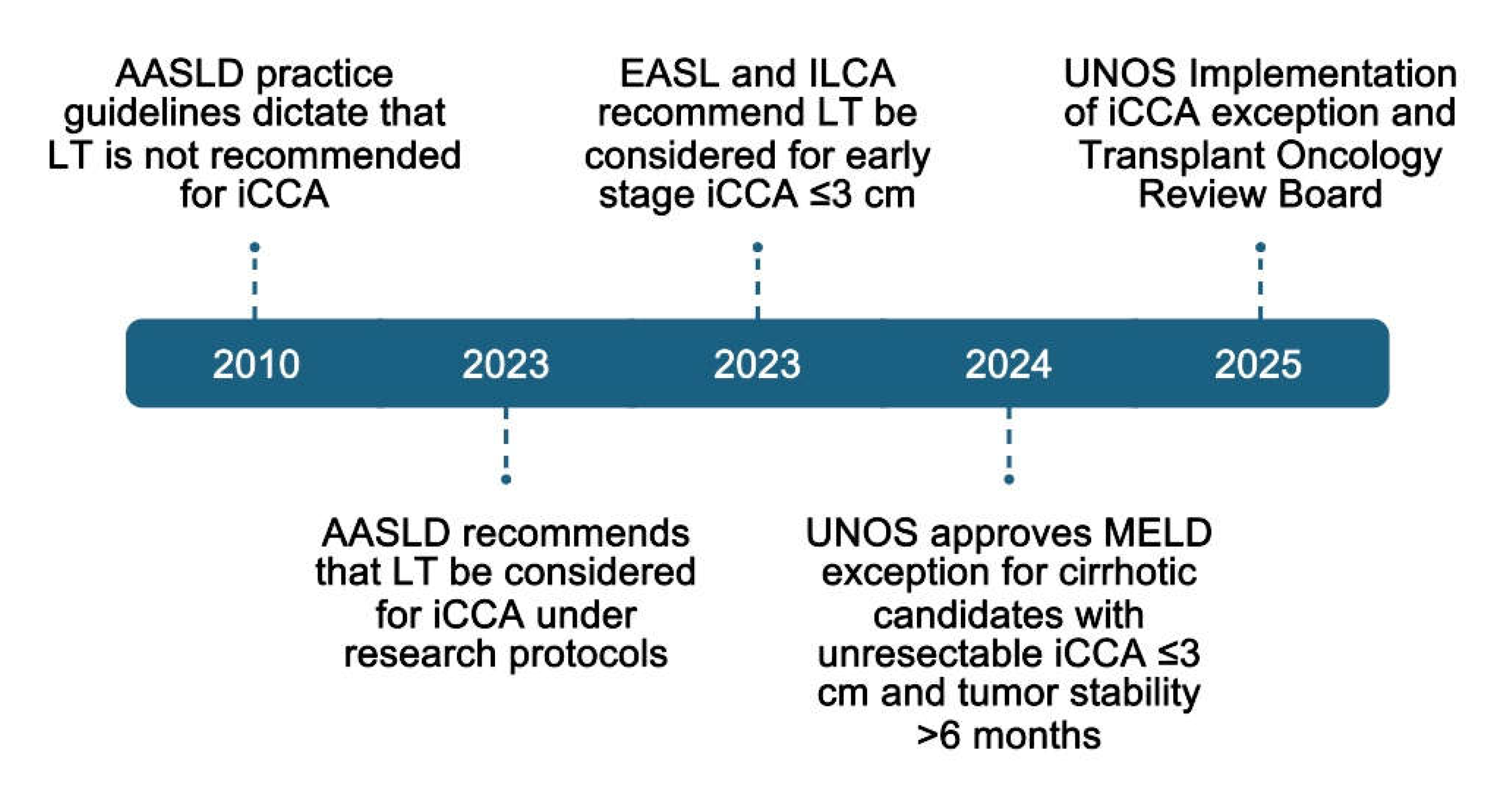
6. Liver Transplantation for Intrahepatic Cholangiocarcinoma
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Olaizola, P.; Paiva, N.A.; Olaizola, I.; Agirre-Lizaso, A.; Landa, A.; Bujanda, L.; Perugorria, M.J.; Banales, J.M. Pathogenesis of Cholangiocarcinoma. Annu. Rev. Pathol. 2021, 16, 433–463. [Google Scholar] [CrossRef] [PubMed]
- Panayotova, G.; Guerra, J.; Guarrera, J.V.; Lunsford, K.E. The Role of Surgical Resection and Liver Transplantation for the Treatment of Intrahepatic Cholangiocarcinoma. J. Clin. Med. 2021, 10, 2428. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Connor, A.A.; Kodali, S.; Abdelrahim, M.; Javle, M.M.; Brombosz, E.W.; Ghobrial, R.M. Intrahepatic cholangiocarcinoma: The role of liver transplantation, adjunctive treatments, and prognostic biomarkers. Front. Oncol. 2022, 12, 996710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alsaraf, Y.; Bandaru, S.S.; Lyons, S.; Reap, L.; Ngo, T.; Yu, Z.; Yu, Q. Epidemiology, survival and new treatment modalities for intrahepatic cholangiocarcinoma. J. Gastrointest. Oncol. 2024, 15, 1777–1788. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Cerrito, L.; Ainora, M.E.; Borriello, R.; Piccirilli, G.; Garcovich, M.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. Contrast-Enhanced Imaging in the Management of Intrahepatic Cholangiocarcinoma: State of Art and Future Perspectives. Cancers 2023, 15, 3393. [Google Scholar] [CrossRef]
- Weber, S.M.; Ribero, D.; O’Reilly, E.M.; Kokudo, N.; Miyazaki, M.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 669–680. [Google Scholar] [CrossRef]
- Orcutt, S.T.; Anaya, D.A. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control 2018, 25, 1073274817744621. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.C.; Nathan, H.; Sotiropoulos, G.C.; Paul, A.; Alexandrescu, S.; Marques, H.; Pulitano, C.; Barroso, E.; Clary, B.M.; Aldrighetti, L.; et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J. Clin. Oncol. 2011, 29, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qin, L.X.; Zhou, J.; Sun, H.C.; Qiu, S.J.; Ye, Q.H.; Wang, L.; Tang, Z.Y.; Fan, J. Staging, prognostic factors and adjuvant therapy of intrahepatic cholangiocarcinoma after curative resection. Liver Int. 2014, 34, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Ribero, D.; Pinna, A.D.; Guglielmi, A.; Ponti, A.; Nuzzo, G.; Giulini, S.M.; Aldrighetti, L.; Calise, F.; Gerunda, G.E.; Tomatis, M.; et al. Surgical Approach for Long-term Survival of Patients with Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch. Surg. 2012, 147, 1107–1113. [Google Scholar] [CrossRef]
- Farges, O.; Fuks, D.; Boleslawski, E.; Le Treut, Y.P.; Castaing, D.; Laurent, A.; Ducerf, C.; Rivoire, M.; Bachellier, P.; Chiche, L.; et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: A multicenter study by the AFC-IHCC-2009 study group. Ann. Surg. 2011, 254, 824–829, discussion 830. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Moore, M.J.; Cox, T.F.; Valle, J.W.; Palmer, D.H.; McDonald, A.C.; Carter, R.; Tebbutt, N.C.; Dervenis, C.; Smith, D.; et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA 2012, 308, 147–156. [Google Scholar] [CrossRef]
- Shroff, R.T.; Kennedy, E.B.; Bachini, M.; Bekaii-Saab, T.; Crane, C.; Edeline, J.; El-Khoueiry, A.; Feng, M.; Katz, M.H.G.; Primrose, J.; et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1015–1027. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Qin, S.; Chen, L.T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Shroff, R.T.; King, G.; Colby, S.; Scott, A.J.; Borad, M.J.; Goff, L.; Matin, K.; Mahipal, A.; Kalyan, A.; Javle, M.M.; et al. SWOG S1815: A Phase III Randomized Trial of Gemcitabine, Cisplatin, and Nab-Paclitaxel Versus Gemcitabine and Cisplatin in Newly Diagnosed, Advanced Biliary Tract Cancers. J. Clin. Oncol. 2025, 43, 536–544. [Google Scholar] [CrossRef]
- Yadav, S.; Xie, H.; Bin-Riaz, I.; Sharma, P.; Durani, U.; Goyal, G.; Borah, B.; Borad, M.J.; Smoot, R.L.; Roberts, L.R.; et al. Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur. J. Surg. Oncol. 2019, 45, 1432–1438. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Neoadjuvant therapy for cholangiocarcinoma: A comprehensive literature review. Cancer Treat. Res. Commun. 2021, 27, 100354. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, B.; Gelli, M.; Pittau, G.; Allard, M.A.; Pereira, B.; Serji, B.; Vibert, E.; Castaing, D.; Adam, R.; Cherqui, D.; et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br. J. Surg. 2018, 105, 839–847. [Google Scholar] [CrossRef]
- Maithel, S.K.; Keilson, J.M.; Cao, H.S.T.; Rupji, M.; Mahipal, A.; Lin, B.S.; Javle, M.M.; Cleary, S.P.; Akce, M.; Switchenko, J.M.; et al. NEO-GAP: A Single-Arm, Phase II Feasibility Trial of Neoadjuvant Gemcitabine, Cisplatin, and Nab-Paclitaxel for Resectable, High-Risk Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2023, 30, 6558–6566. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Gemcitabine, Cisplatin, and Nab-Paclitaxel Before Surgery in Patients with High-Risk Liver Bile Duct Cancer. Available online: https://clinicaltrials.gov/study/NCT03579771 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Durvalumab with Gemcitabine and Cisplatin for the Treatment of High-Risk Resectable Liver Cancer Before Surgery. Available online: https://clinicaltrials.gov/study/NCT06050252 (accessed on 2 May 2025).
- U.S. National Library of Medicine. A Single-Arm Study of Pembrolizumab with Gemcitabine and Cisplatin as Perioperative Therapy for Potentially Resectable Intrahepatic Cholangiocarcinoma. Available online: https://clinicaltrials.gov/study/NCT05967182 (accessed on 2 May 2025).
- U.S. National Library of Medicine. Gemcitabine/Cisplatin/Nab-Paclitaxel and Rilvegostomig in Resectable iCCA (NEOLANGIO). Available online: https://clinicaltrials.gov/study/NCT06569225 (accessed on 2 May 2025).
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients with Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef]
- Tao, R.; Krishnan, S.; Bhosale, P.R.; Javle, M.M.; Aloia, T.A.; Shroff, R.T.; Kaseb, A.O.; Bishop, A.J.; Swanick, C.W.; Koay, E.J.; et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients with Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J. Clin. Oncol. 2016, 34, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Urrunaga, N.H.; Siddiqui, O.; Wu, A.; Schliep, M.; Mossahebi, S.; Shetty, K.; Regine, W.F.; Molitoris, J.K.; Lominadze, Z. Proton beam stereotactic body radiotherapy and hypofractionated therapy with pencil beam scanning is safe and effective for advanced hepatocellular carcinoma and intrahepatic cholangiocarcinoma: A single center experience. J. Radiosurg. SBRT 2023, 9, 43–52. [Google Scholar]
- Bourien, H.; Pircher, C.C.; Guiu, B.; Lamarca, A.; Valle, J.W.; Niger, M.; Edeline, J. Locoregional Treatment in Intrahepatic Cholangiocarcinoma: Which Treatment for Which Patient? Cancers 2023, 15, 4217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.Y.; Zhou, G.H.; Zhang, Y.L.; Nie, C.H.; Zhu, T.Y.; Wang, H.L.; Chen, S.Q.; Wang, B.Q.; Yu, Z.N.; Wu, L.M.; et al. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J. Cancer 2020, 11, 4534–4541. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.H.; Yoon, H.J.; Lee, I.S.; Yoon, H.K.; Kim, K.P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef]
- Vogl, T.J.; Naguib, N.N.; Nour-Eldin, N.E.; Bechstein, W.O.; Zeuzem, S.; Trojan, J.; Gruber-Rouh, T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int. J. Cancer 2012, 131, 733–740. [Google Scholar] [CrossRef]
- Kiefer, M.V.; Albert, M.; McNally, M.; Robertson, M.; Sun, W.; Fraker, D.; Olthoff, K.; Christians, K.; Pappas, S.; Rilling, W.; et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: A 2-center study. Cancer 2011, 117, 1498–1505. [Google Scholar] [CrossRef]
- Martin, R.C.G., 2nd; Simo, K.A.; Hansen, P.; Rocha, F.; Philips, P.; McMasters, K.M.; Tatum, C.M.; Kelly, L.R.; Driscoll, M.; Sharma, V.R.; et al. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann. Surg. Oncol. 2022, 29, 5462–5473. [Google Scholar] [CrossRef] [PubMed]
- Edeline, J.; Touchefeu, Y.; Guiu, B.; Farge, O.; Tougeron, D.; Baumgaertner, I.; Ayav, A.; Campillo-Gimenez, B.; Beuzit, L.; Pracht, M.; et al. Radioembolization Plus Chemotherapy for First-line Treatment of Locally Advanced Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Schartz, D.A.; Porter, M.; Schartz, E.; Kallas, J.; Gupta, A.; Butani, D.; Cantos, A. Transarterial Yttrium-90 Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2022, 33, 679–686. [Google Scholar] [CrossRef]
- Chan, S.L.; Chotipanich, C.; Choo, S.P.; Kwang, S.W.; Mo, F.; Worakitsitisatorn, A.; Tai, D.; Sundar, R.; Ng, D.C.E.; Loke, K.S.H.; et al. Selective Internal Radiation Therapy with Yttrium-90 Resin Microspheres Followed by Gemcitabine plus Cisplatin for Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Single-Arm Multicenter Clinical Trial. Liver Cancer 2022, 11, 451–459. [Google Scholar] [CrossRef]
- Moris, D.; Palta, M.; Kim, C.; Allen, P.J.; Morse, M.A.; Lidsky, M.E. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J. Clin. 2023, 73, 198–222. [Google Scholar] [CrossRef] [PubMed]
- Massani, M.; Bonariol, L.; Stecca, T. Hepatic Arterial Infusion Chemotherapy for Unresectable Intrahepatic Cholangiocarcinoma, a Comprehensive Review. J. Clin. Med. 2021, 10, 2552. [Google Scholar] [CrossRef]
- Boehm, L.M.; Jayakrishnan, T.T.; Miura, J.T.; Zacharias, A.J.; Johnston, F.M.; Turaga, K.K.; Gamblin, T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015, 111, 213–220. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Groot Koerkamp, B.; Do, R.K.; Gönen, M.; Fong, Y.; Allen, P.J.; D’Angelica, M.I.; Kingham, T.P.; DeMatteo, R.P.; Klimstra, D.S.; et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016, 122, 758–765. [Google Scholar] [CrossRef]
- Cercek, A.; Boerner, T.; Tan, B.R.; Chou, J.F.; Gönen, M.; Boucher, T.M.; Hauser, H.F.; Do, R.K.G.; Lowery, M.A.; Harding, J.J.; et al. Assessment of Hepatic Arterial Infusion of Floxuridine in Combination with Systemic Gemcitabine and Oxaliplatin in Patients with Unresectable Intrahepatic Cholangiocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2020, 6, 60–67. [Google Scholar] [CrossRef]
- Zhao, R.; Zhou, J.; Miao, Z.; Xiong, X.; Wei, W.; Li, S.; Guo, R. Efficacy and safety of lenvatinib plus durvalumab combined with hepatic arterial infusion chemotherapy for unresectable intrahepatic cholangiocarcinoma. Front. Immunol. 2024, 15, 1397827. [Google Scholar]
- Mayo, S.C.; Patel, R.K.; Walker, B.S.; Eil, R.; Wong, M.; Fung, A.; Brody, J.R.; Anand, S.; Corless, C.L.; Hansen, L.; et al. A phase II trial of induction systemic mFOLFIRINOX followed by hepatic arterial infusion of floxuridine and dexamethasone given concurrently with systemic mFOLFIRI as a first-line therapy in patients with unresectable liver-dominant intrahepatic cholangiocarcinoma (HELIX-1). J. Clin. Oncol. 2024, 42, 511. [Google Scholar]
- Zhu, M.; Jin, M.; Zhao, X.; Shen, S.; Chen, Y.; Xiao, H.; Wei, G.; He, Q.; Li, B.; Peng, Z. Anti-PD-1 antibody in combination with radiotherapy as first-line therapy for unresectable intrahepatic cholangiocarcinoma. BMC Med. 2024, 22, 165. [Google Scholar] [CrossRef]
- Victory, J.H.; Smith, E.C.; Ryan, C.E.; Lambdin, J.; Sarvestani, A.L.; Friedman, L.R.; Eade, A.V.; Larrain, C.; Pu, T.; Luberice, K.; et al. Hepatic artery infusion pump (HAIP) therapy in combination with targeted delivery of IL-12 for patients with metastatic colorectal cancer or intrahepatic cholangiocarcinoma: A phase II trial protocol. J. Gastrointest. Oncol. 2024, 15, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Hendricks-Wenger, A.; Weber, P.; Simon, A.; Saunier, S.; Coutermarsh-Ott, S.; Grider, D.; Vidal-Jove, J.; Allen, I.C.; Luyimbazi, D.; Vlaisavljevich, E. Histotripsy for the Treatment of Cholangiocarcinoma Liver Tumors: In Vivo Feasibility and Ex Vivo Dosimetry Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 2953–2964. [Google Scholar] [CrossRef]
- Sun, D.; Lv, G.; Dong, J. Liver Transplantation for Intrahepatic Cholangiocarcinoma: What Are New Insights and What Should We Follow? Front. Oncol. 2021, 11, 841694. [Google Scholar] [CrossRef]
- Kodali, S.; Connor, A.A.; Thabet, S.; Brombosz, E.W.; Ghobrial, R.M. Liver transplantation as an alternative for the treatment of intrahepatic cholangiocarcinoma: Past, present, and future directions. Hepatobiliary Pancreat. Dis. Int. 2024, 23, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Penn, I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991, 110, 726–734, discussion 734–735. [Google Scholar]
- Borakati, A.; Froghi, F.; Bhogal, R.H.; Mavroeidis, V.K. Liver transplantation in the management of cholangiocarcinoma: Evolution and contemporary advances. World J. Gastroenterol. 2023, 29, 1969–1981. [Google Scholar] [CrossRef]
- Sapisochin, G.; de Lope, C.R.; Gastaca, M.; de Urbina, J.O.; Suarez, M.A.; Santoyo, J.; Castroagudín, J.F.; Varo, E.; López-Andujar, R.; Palacios, F.; et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: Should liver transplantation be reconsidered in these patients? Am. J. Transplant. 2014, 14, 660–667. [Google Scholar] [CrossRef]
- Sapisochin, G.; Facciuto, M.; Rubbia-Brandt, L.; Marti, J.; Mehta, N.; Yao, F.Y.; Vibert, E.; Cherqui, D.; Grant, D.R.; Hernandez-Alejandro, R.; et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016, 64, 1178–1188. [Google Scholar] [CrossRef]
- De Martin, E.; Rayar, M.; Golse, N.; Dupeux, M.; Gelli, M.; Gnemmi, V.; Allard, M.A.; Cherqui, D.; Sa Cunha, A.; Adam, R.; et al. Analysis of Liver Resection Versus Liver Transplantation on Outcome of Small Intrahepatic Cholangiocarcinoma and Combined Hepatocellular-Cholangiocarcinoma in the Setting of Cirrhosis. Liver Transpl. 2020, 26, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Sempoux, C.; Jibara, G.; Ward, S.C.; Fan, C.; Qin, L.; Roayaie, S.; Fiel, M.I.; Schwartz, M.; Thung, S.N. Intrahepatic cholangiocarcinoma: New insights in pathology. Semin. Liver Dis. 2011, 31, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.J.; Lu, C.D.; Dong, H.; Fu, X.H.; Zhang, H.W.; Yao, X.P. Hepatitis B virus-related combined hepatocellular-cholangiocarcinoma: Clinicopathological and prognostic analysis of 390 cases. Eur. J. Gastroenterol. Hepatol. 2014, 26, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Lunsford, K.E.; Court, C.; Lee, Y.S.; Lu, D.S.; Naini, B.V.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Propensity-Matched Analysis of Patients with Mixed Hepatocellular-Cholangiocarcinoma and Hepatocellular Carcinoma Undergoing Liver Transplantation. Liver Transpl. 2018, 24, 1384–1397. [Google Scholar] [CrossRef]
- Dageforde, L.A.; Vachharajani, N.; Tabrizian, P.; Agopian, V.; Halazun, K.; Maynard, E.; Croome, K.; Nagorney, D.; Hong, J.C.; Lee, D.; et al. Multi-Center Analysis of Liver Transplantation for Combined Hepatocellular Carcinoma-Cholangiocarcinoma Liver Tumors. J. Am. Coll. Surg. 2021, 232, 361–371. [Google Scholar] [CrossRef]
- Hong, J.C.; Jones, C.M.; Duffy, J.P.; Petrowsky, H.; Farmer, D.G.; French, S.; Finn, R.; Durazo, F.A.; Saab, S.; Tong, M.J.; et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: A 24-year experience in a single center. Arch. Surg. 2011, 146, 683–689. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Javle, M.; Heyne, K.; Shroff, R.T.; Abdel-Wahab, R.; Gupta, N.; Mobley, C.M.; Saharia, A.; Victor, D.W.; Nguyen, D.T.; et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: A prospective case-series. Lancet Gastroenterol. Hepatol. 2018, 3, 337–348. [Google Scholar] [CrossRef]
- McMillan, R.R.; Javle, M.; Kodali, S.; Saharia, A.; Mobley, C.; Heyne, K.; Hobeika, M.J.; Lunsford, K.E.; Victor, D.W., 3rd; Shetty, A.; et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transplant. 2022, 22, 823–832. [Google Scholar] [CrossRef]
- Semaan, S.; Connor, A.A.; Saharia, A.; Kodali, S.; Elaileh, A.; Patel, K.; Soliman, N.; Basra, T.; Victor, D.W., 3rd; Simon, C.J.; et al. Transplantation for Peri-Hilar and Intrahepatic Cholangiocarcinoma with mTOR Immunosuppression. Transplant. Proc. 2025, 57, 255–263. [Google Scholar] [CrossRef]
- Yaqub, S.; Busund, S.; Smedman, T.M.; Syversveen, T.; Khan, A.; Solheim, J.M.; Folseraas, T.; Wiencke, K.; Lassen, K.; Dueland, S.; et al. Liver transplantation for locally advanced non-resectable intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: Early results from the TESLA trial. Br. J. Surg. 2025, 112, znaf054. [Google Scholar] [CrossRef]
- Fernandes, E.S.M.; Mello, F.P.T.; Andrade, R.O.; Girão, C.L.; Cesar, C.; Pimentel, L.S.; Coelho, H.S.M.; Basto, S.T.; Siqueira, M.; Brito, A.; et al. Living donor liver transplant for intrahepatic cholangiocarcinoma. An initial brazilian experience. Arq. Bras. Cir. Dig. 2024, 37, e1839. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.M.; Dunne, R.F.; Melaragno, J.I.; Chávez-Villa, M.; Hezel, A.; Liao, X.; Ertreo, M.; Al-Judaibi, B.; Orloff, M.; Hernandez-Alejandro, R.; et al. Neoadjuvant pemigatinib as a bridge to living donor liver transplantation for intrahepatic cholangiocarcinoma with FGFR2 gene rearrangement. Am. J. Transplant. 2025, 25, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Butler, J.R.; Noguchi, D.; Ha, M.; Aziz, A.; Agopian, V.G.; DiNorcia, J., 3rd; Yersiz, H.; Farmer, D.G.; Busuttil, R.W.; et al. A 3-Decade, Single-Center Experience of Liver Transplantation for Cholangiocarcinoma: Impact of Era, Tumor Size, Location, and Neoadjuvant Therapy. Liver Transpl. 2022, 28, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Maspero, M.; Sposito, C.; Bongini, M.A.; Cascella, T.; Flores, M.; Maccauro, M.; Chiesa, C.; Niger, M.; Pietrantonio, F.; Leoncini, G.; et al. Liver Transplantation for Intrahepatic Cholangiocarcinoma After Chemotherapy and Radioembolization: An Intention-To-Treat Study. Transpl. Int. 2024, 37, 13641. [Google Scholar] [CrossRef]
- Teixeira, C.; Viamonte, B.; Graça, L.; Pinto Marques, H.; Rego, I.; Ribeiro, M.J. Liver Transplant After Neoadjuvant Treatment for Long-Term Survivors with Intrahepatic Cholangiocarcinoma: Does It Have a Role? Cureus 2024, 16, e75935. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Liver Transplantation for Early Intrahepatic Cholangiocarcinoma (LT for iCCA). Available online: https://clinicaltrials.gov/study/NCT02878473 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Liver Transplant for Stable, Advanced Intrahepatic Cholangiocarcinoma. Available online: https://clinicaltrials.gov/study/NCT04195503 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Liver Transplantation for Non-Resectable Intrahepatic Cholangiocarcinoma: A Prospective Exploratory Trial (TESLA Trial). Available online: https://clinicaltrials.gov/study/NCT04556214 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Liver Transplantation in Intrahepatic Cholangiocarcinoma. Available online: https://clinicaltrials.gov/study/NCT06140134 (accessed on 6 May 2025).
- U.S. National Library of Medicine. LIver Transplantation for Non-Resectable Intrahepatic CholAngiocarcinoma (LIRICA) (LIRICA). Available online: https://clinicaltrials.gov/study/NCT06098547 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Liver Transplantation for Unresectable Intrahepatic Colangiocarcinoma After Sustained Response to Neoadjuvant Treatments (iCOLA). Available online: https://clinicaltrials.gov/study/NCT06862934 (accessed on 6 May 2025).
- U.S. National Library of Medicine. Living Donor Liver Transplantation for Intrahepatic Cholangiocarcinoma (LIVINCA). Available online: https://clinicaltrials.gov/study/NCT06539377 (accessed on 6 May 2025).
- Bowlus, C.L.; Arrivé, L.; Bergquist, A.; Deneau, M.; Forman, L.; Ilyas, S.I.; Lunsford, K.E.; Martinez, M.; Sapisochin, G.; Shroff, R.; et al. AASLD practice guidance on primary sclerosing cholangitis and cholangiocarcinoma. Hepatology 2023, 77, 659–702. [Google Scholar] [CrossRef]
- EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [CrossRef]
- National Liver Review Board (NLRB). Updates Related to Transplant Oncology—Public Comment Proposal; Organ Procurement and Transplantation Network Liver & Intestinal Organ Transplantation Committee: Washington, DC, USA, 2024. [Google Scholar]
- Andraus, W.; Ochoa, G.; de Martino, R.B.; Pinheiro, R.S.N.; Santos, V.R.; Lopes, L.D.; Júnior, R.M.A.; Waisberg, D.R.; Santana, A.C.; Tustumi, F.; et al. The role of living donor liver transplantation in treating intrahepatic cholangiocarcinoma. Front. Oncol. 2024, 14, 1404683. [Google Scholar] [CrossRef]
| Reference | Intervention | Study | Status | Endpoint | Results |
|---|---|---|---|---|---|
| Maithel 2023 [28] | Gemcitabine/cisplatin/nab-paclitaxel + resection | Multi-institutional, phase II | Complete | Primary: completion of both preoperative chemotherapy + resection Secondary: AEs, radiologic response, RFS and OS | Primary: 30 completed preoperative chemotherapy and 22 were resected Secondary: 90% with disease control, 23% with partial response Median OS 24 months Median RFS 7.1 months |
| NCT03579771 [29] | Gemcitabine/cisplatin/nab-paclitaxel + FGFR2 inhibitor (for patients with FGFR2 fusion or rearrangement) + resection | Single-arm, phase II | Active, notrecruiting | Primary: completion of all preoperative + operative therapy, safety Secondary: radiological response, RFS, OS | - |
| NCT06050252 [30] | Gemcitabine/cisplatin/durvalumab + resection | Multi- institutional, phase II | Actively recruiting | Primary: completion rate of neoadjuvant treatment + resection Secondary: major pathologic response | - |
| NCT05967182 [31] | Gemcitabine/ cisplatin/ pembrolizumab + resection | Single-arm, phase II trial | Actively recruiting | Primary: RFS and major pathologic response | - |
| NCT06569225 [32] | Gemcitabine/ cisplatin/ nab-paclitaxel/ rilvegostomig + resection | Multi- institutional, phase II | Not yet recruiting | Primary: major pathologic response Secondary: radiologic objective response rate, AEs, rate of R0 | - |
| Reference | Intervention | Study | n | Median OS | Median PFS |
|---|---|---|---|---|---|
| Hong 2016 [37] | High-dose hypofractionated proton beam therapy | Phase II, multi-institutional single-arm study | 37 | 22.5 months (95% CI 12.4–49.7) | 8.4 months (95% CI 5.0–15.7) |
| Martin 2022 [45] | DEBIRI TACE + Gem/Cis | Phase II, multicenter randomized study | 48 | 33.7 months (95% CI 13.5–54.5) | 31.9 months (95% CI 8.5–75.3) |
| Edeline 2020 [46] | TARE + Gem/Cis | Phase II, multicenter clinical trial | 41 | 22 months (95% CI 14–52) | 14 months (95% CI 8–17 months) |
| Chan 2022 [48] | TARE + Gem/Cis | Phase II, multicenter single-arm clinical trial | 16 | 21.6 months (95% CI 7.3–25.2) | 9 months (95% CI 3.2–13.1) |
| Cercek 2020 [53] | HAI floxuridine + systemic Gem/Ox | Phase II, single-arm clinical trial | 38 | 25 months (95% CI, 20.60 not reached) | 11.7 months (1-sided 95% CI 11.1) |
| Reference | Study Design | Population | Intervention | Key Outcomes |
|---|---|---|---|---|
| Penn 1991 [61] | Retrospective | iCCA patients | LT | 2- and 5-yr OS: 30%, 17% Recurrence: 44% |
| Sapisochin 2014 [63] | Retrospective multicenter | 29 iCCA patients with cirrhosis (8 with “very early” iCCA ≤ 2 cm) | LT | In “very early” subgroup: Recurrence: 0% OS 1-, 3-, 5-yr: 100%, 73%, 73% |
| Sapisochin 2016 [64] | Retrospective multicenter | “Very early” iCCA (≤2 cm) vs. advanced iCCA (>2 cm or multifocal) | LT | Recurrence at 1, 3, and 5 yrs: 7%, 18%, 18% (“very early”) vs. 30%, 47%, 61%; OS: 93%, 84%, 65% vs. 79%, 50%, 45% |
| De Martin 2020 [65] | Retrospective multicenter | Cirrhotic patients with iCCA or combined HCC and cholangiocarcinoma ≤ 5 cm | LT vs. resection | 5-yr RFS: 75% (LT) vs. 36% (resection) For tumors 2–5 cm: recurrence 21% (LT) vs. 48% (resection); RFS 74% vs. 40% |
| Hong 2011 [70] | Retrospective | iCCA and pCCA patients | LT ± neoadjuvant/adjuvant therapy | 5-yr RFS: 47% (neoadjuvant + adjuvant) vs. 33% (adjuvant only) vs. 20% (none) |
| Lunsford 2018 [71] | Prospective case series | Unresectable iCCA, 6 patients | LT after >6 months disease stability | 1-, 3-, 5-yr OS: 100%, 83.3%, 83.3% RFS: 50% |
| McMillan 2022 [72] | Prospective case series follow-up | Unresectable iCCA, 18 patients | LT after >6 months disease stability | 1-, 3-, 5-yr OS: 100%, 71%, 57% 1-, 3-yr RFS: 72%, 52% |
| Semaan 2025 [73] | Retrospective single-center | Unresectable iCCA, 26 patients | Neoadjuvant treatment + LT | 1-, 3-yr OS: 96%, 82.7% 1-, 3-yr RFS: 70.8%, 56.3% |
| Yaqub 2025 [74] | Prospective single-center | Unresectable locally advanced iCCA with prior response to neoadjuvant therapy | LT | 5 patients underwent LT 2 had recurrence at 12 and 13 months |
| Teixeira 2024 [79] | Case report | Unresectable locally advanced iCCA | Y90 TARE + Gem/Cis + FOLFOX + LT | Recurrence-free at 16-month follow-up |
| Fernandes 2024 [75] | Case reports | Unresectable locally advanced iCCA | Gemcitabine/cisplatin OR gemcitabine/cisplatin/durvalumab + LT | Recurrence-free at 23-month and 6-month follow-up |
| Byrne 2025 [76] | Case report | Unresectable iCCA | Y90 TARE + Gem/Cis + Pemigatinib + LT | Recurrence-free at 1-year follow-up |
| Ito 2022 [77] | Retrospective | 30 iCCA patients | LT ± neoadjuvant/systemic + LRT | 1-, 3-, and 5-yr OS: 73%, 46%, 42% 1-, 3-, 5-yr OS (for patients transplanted 2008–2019): 100%, 86%, 69%100% 5-yr OS and RFS (for patients treated with systemic + LRT) |
| Maspero 2024 [78] | Prospective single-center | 13 iCCA patients, 4 patients transplanted | Gem/Cis + TARE + LT | 5-yr OS: 100% (LT) vs. 0% (no LT) |
| Reference | Location | Description | Neoadjuvant | Study Type | Status |
|---|---|---|---|---|---|
| NCT02878473 [80] | Toronto, Canada | 5-year overall survival; LT for pts with cirrhosis and unresectable iCCA ≤2 cm confirmed by biopsy | None or LRT | Multicenter clinical trial, not randomized, phase 2 | Terminated |
| NCT04195503 [81] | Toronto, Canada | 5-year overall survival; LDLT for locally advanced unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX Alone | Prospective single-center | Recruiting |
| NCT04556214 [82] | Oslo, Norway | 5-year overall survival; LT for locally advanced unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX or LRT | Prospective single-center | Recruiting |
| NCT06140134 [83] | New Jersey, USA | 5-year overall survival; LT for locally advanced unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX ± IO ± TARE | Multicenter clinical trial, not randomized, phase 2 | Recruiting |
| NCT06098547 [84] | Padova, Italy | 3-year overall survival; with matched retrospective comparison to CTX alone; LT for locally advanced unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX Alone | Prospective single-center | Recruiting |
| NCT06862934 [85] | Milan, Italy | 3-year overall survival; LT for locally advanced unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX+IO+TARE | Prospective single-center | Recruiting |
| NCT06539377 [86] | Jena, Germany | 5-year overall survival; LDLT for locally advanced unresectable G1/G2 iCCA or HCC/CCA unresectable iCCA with no distant mets, LN, or vascular invasion | >6 months stability CTX + TARE | Prospective single-center | Not yet recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopiano, S.; Guarrera, J.V.; Lunsford, K.E. Therapeutic Advances in Initially Unresectable Locally Advanced Intrahepatic Cholangiocarcinoma: Emerging Treatments and the Role of Liver Transplantation. Curr. Oncol. 2025, 32, 293. https://doi.org/10.3390/curroncol32060293
Lopiano S, Guarrera JV, Lunsford KE. Therapeutic Advances in Initially Unresectable Locally Advanced Intrahepatic Cholangiocarcinoma: Emerging Treatments and the Role of Liver Transplantation. Current Oncology. 2025; 32(6):293. https://doi.org/10.3390/curroncol32060293
Chicago/Turabian StyleLopiano, Sofia, James V. Guarrera, and Keri E. Lunsford. 2025. "Therapeutic Advances in Initially Unresectable Locally Advanced Intrahepatic Cholangiocarcinoma: Emerging Treatments and the Role of Liver Transplantation" Current Oncology 32, no. 6: 293. https://doi.org/10.3390/curroncol32060293
APA StyleLopiano, S., Guarrera, J. V., & Lunsford, K. E. (2025). Therapeutic Advances in Initially Unresectable Locally Advanced Intrahepatic Cholangiocarcinoma: Emerging Treatments and the Role of Liver Transplantation. Current Oncology, 32(6), 293. https://doi.org/10.3390/curroncol32060293