Exploring Cancer Patients’ and Caregivers’ Perspectives and Knowledge Regarding Biomarker Testing in Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
2.2. Survey Design and Development
2.3. Survey Administration
2.4. Data Storage
2.5. Data Analysis
2.6. Ethics Approval
3. Results
3.1. Quantitative Analysis
3.1.1. Demographics
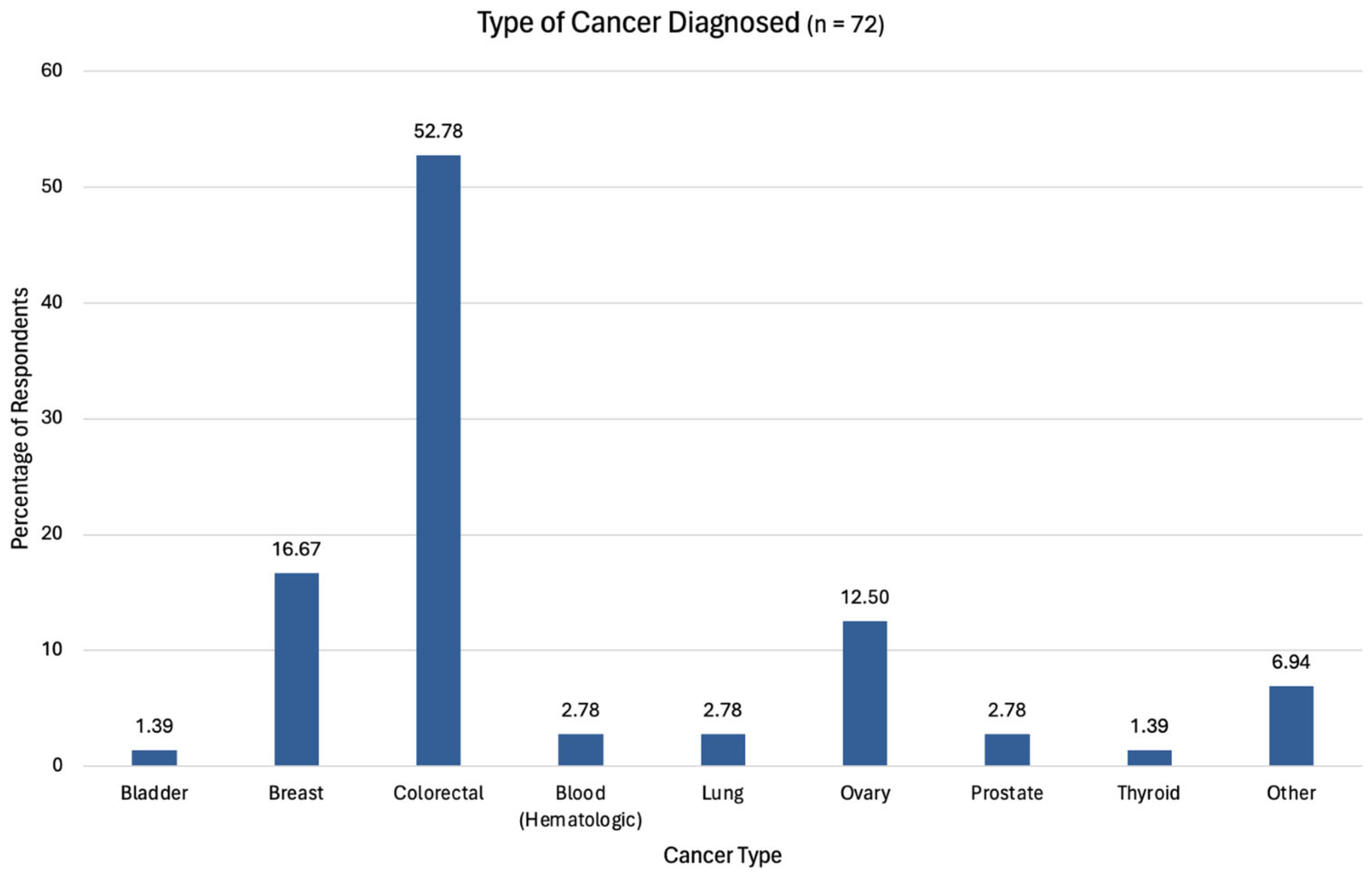
3.1.2. Cancer Connection and Status
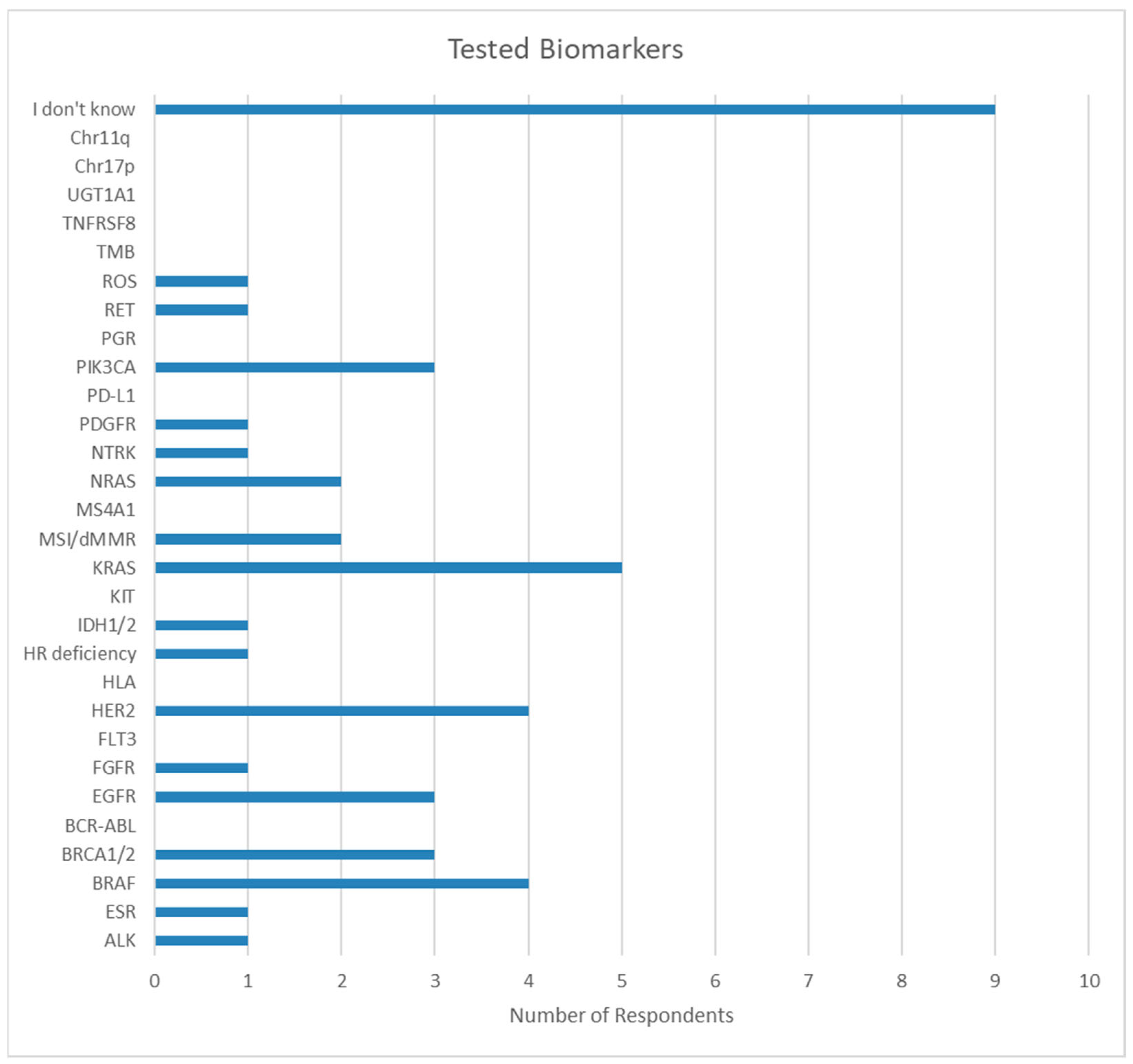
3.1.3. Biomarker Knowledge
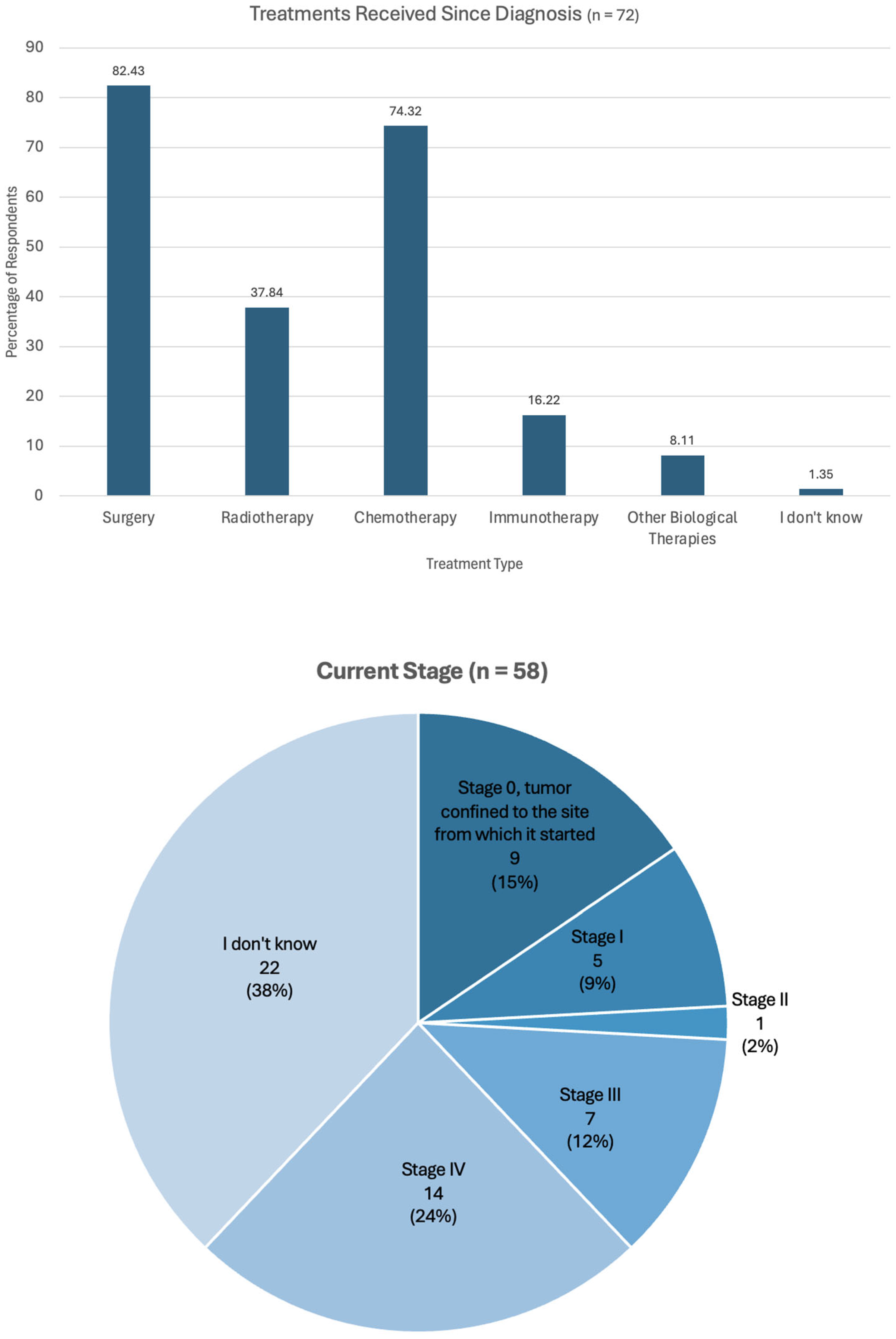
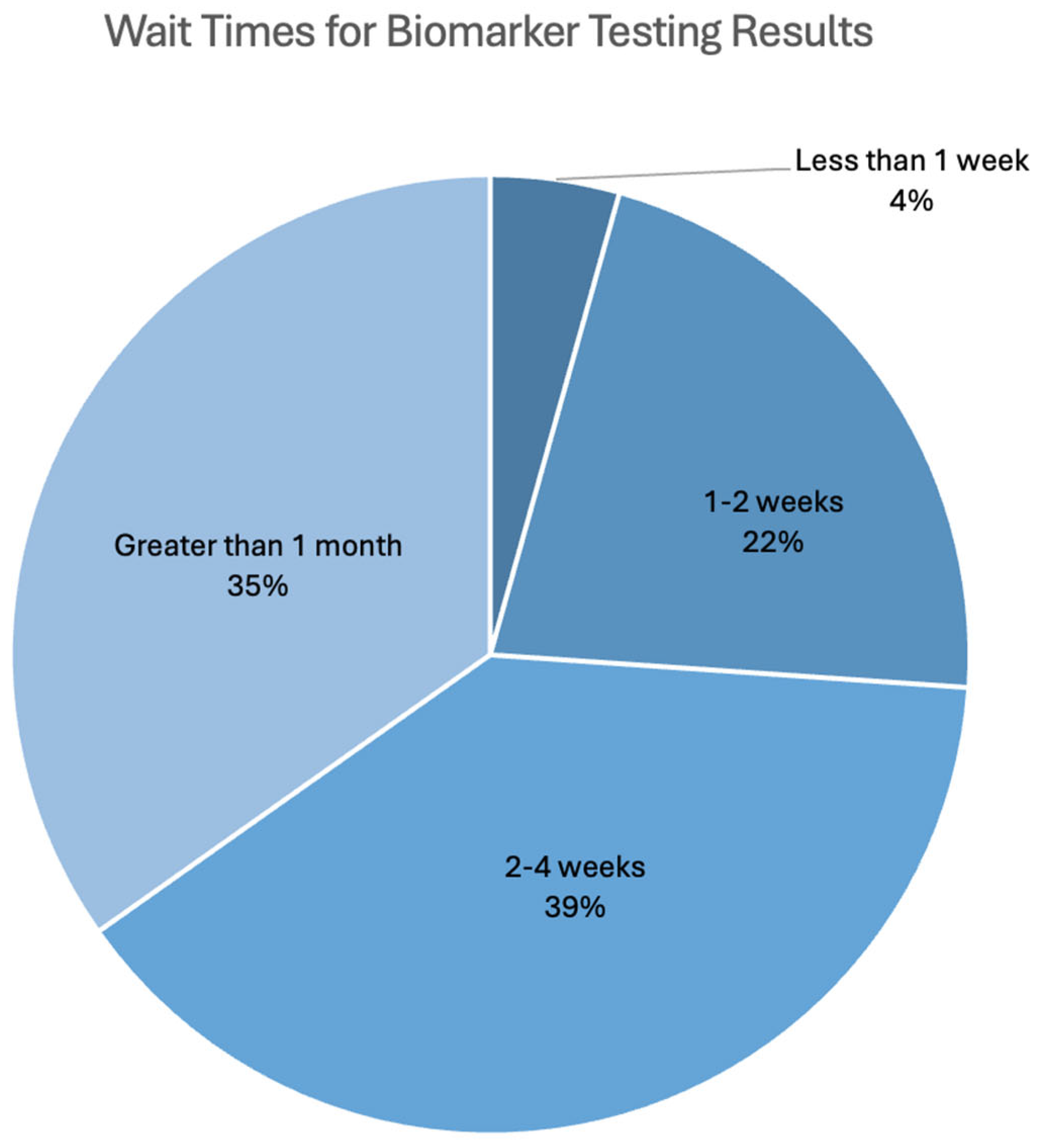
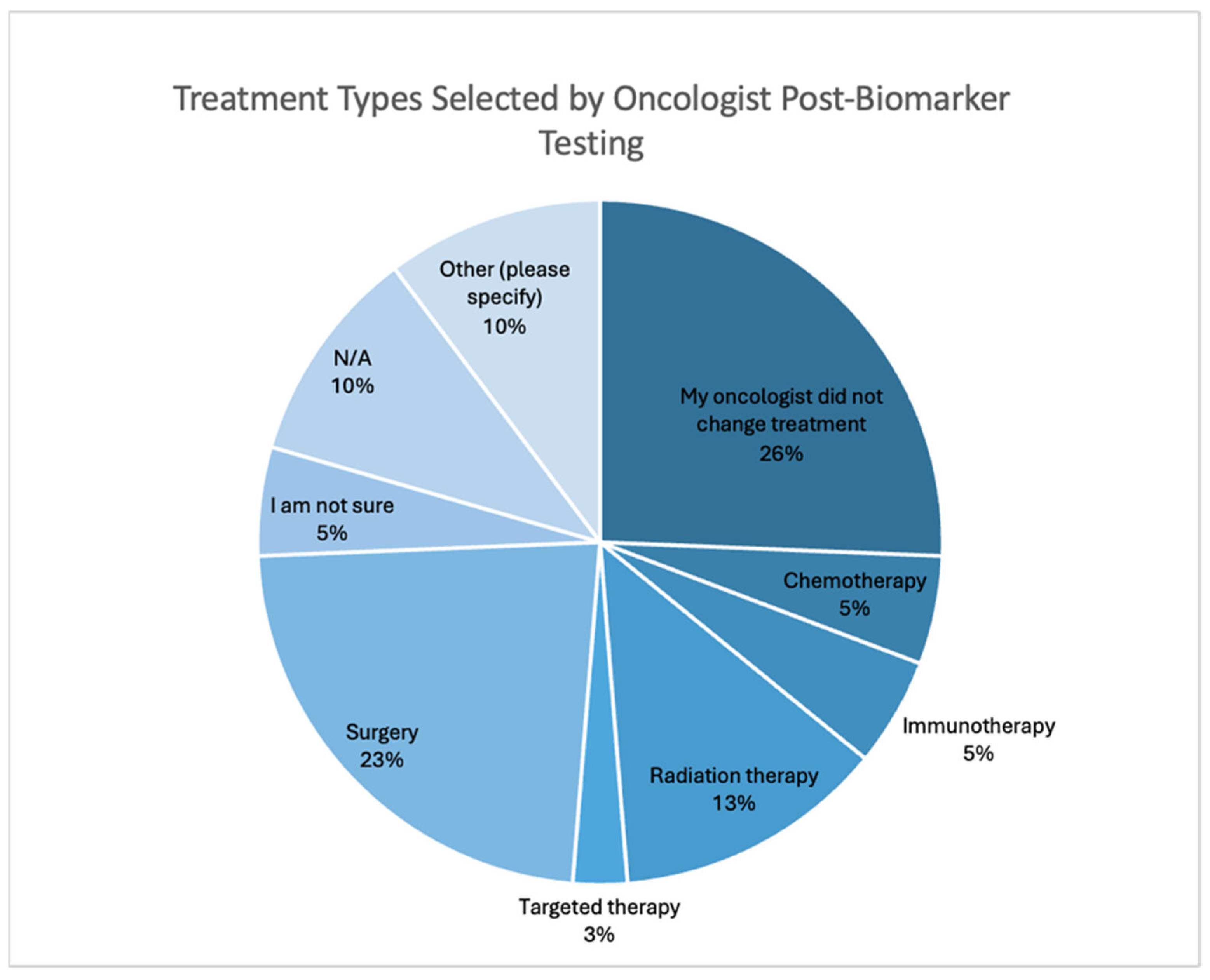
3.1.4. Biomarker Testing Informing Treatment
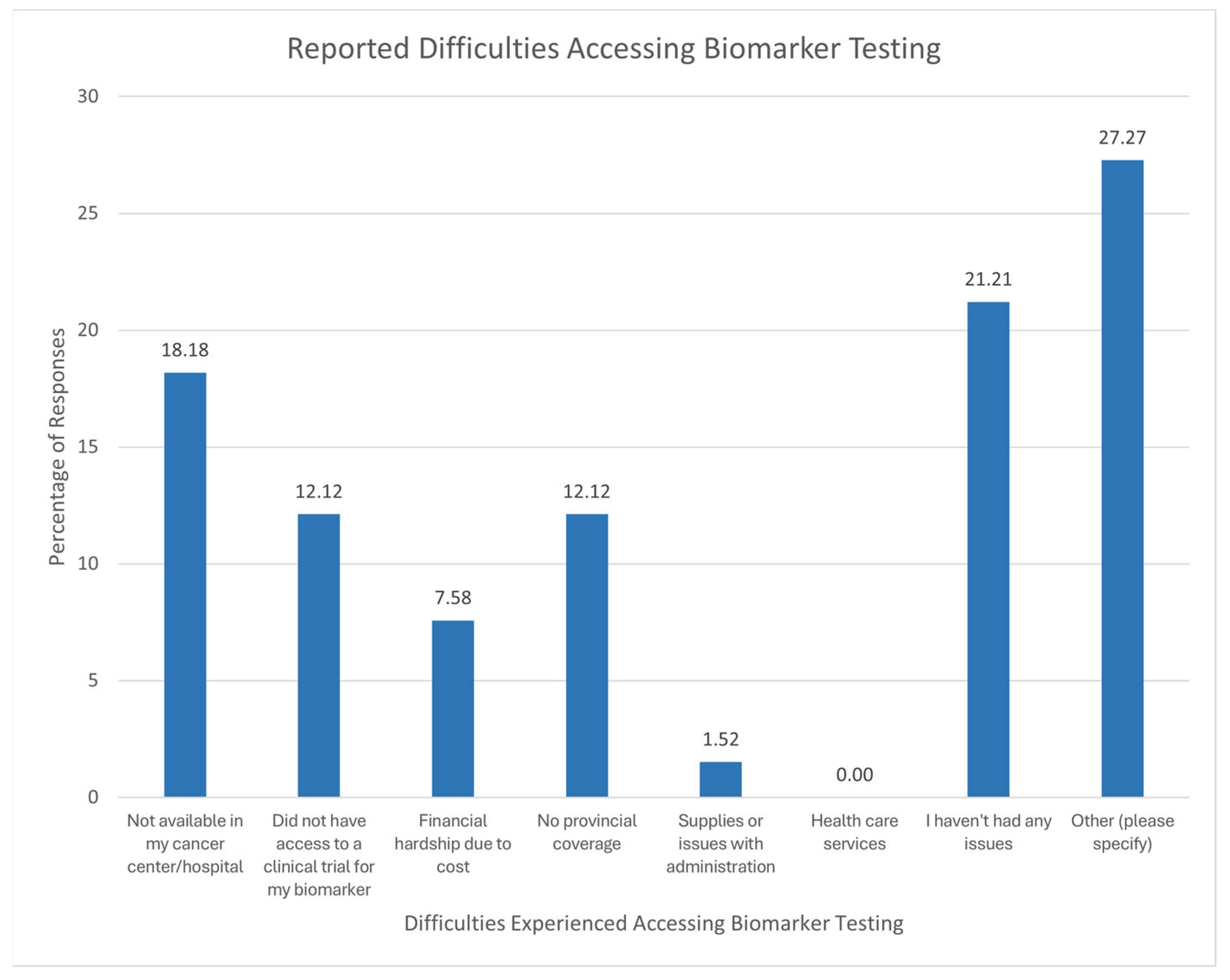
3.1.5. Difficulties Experienced During Biomarker Testing
3.2. Thematic Analysis
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Demographic | Respondents (n) | Percentage (%) |
|---|---|---|
| Gender | ||
| Female | 60 | 81.08 |
| Male | 14 | 18.92 |
| Age (years) | ||
| <20 | 0 | NA |
| 20–29 | 3 | 4.05 |
| 30–39 | 7 | 9.46 |
| 40–49 | 16 | 21.62 |
| 50–59 | 18 | 24.32 |
| 60–69 | 22 | 29.73 |
| 70–79 | 7 | 9.46 |
| 80+ | 1 | 1.35 |
| Residence | ||
| Alberta (AB) | 4 | 5.41 |
| British Columbia (BC) | 10 | 13.51 |
| Manitoba (MB) | 1 | 1.35 |
| New Brunswick (NB) | 2 | 2.70 |
| Newfoundland and Labrador (NL) | 2 | 2.70 |
| Northwest Territories (NT) | 0 | NA |
| Nova Scotia (NS) | 3 | 4.05 |
| Nunavut (NU) | 3 | 4.05 |
| Ontario (ON) | 30 | 40.54 |
| Prince Edward Island (PEI) | 0 | NA |
| Quebec (QB) | 20 | 27.03 |
| Saskatchewan (SK) | 2 | 2.70 |
| Yukon (YT) | 0 | NA |
| Employment Status | ||
| Full-time employment | 23 | 31.08 |
| Part-time employment | 7 | 9.46 |
| Not employed | 44 | 59.46 |
| Educational Attainment | ||
| High school diploma or equivalent | 7 | 9.46 |
| Some college credit, no degree | 8 | 10.81 |
| College certificate or diploma | 9 | 12.16 |
| Bachelor’s degree | 30 | 40.54 |
| Higher degree (master’s or doctorate) | 18 | 24.32 |
| Preferred not to say | 2 | 2.70 |
| Stage at Diagnosis | I | II | III | IV | I Don’t Know | Total |
|---|---|---|---|---|---|---|
| Age at Diagnosis | ||||||
| Under 20 years | NA | NA | NA | 1.39 | NA | 1.39 |
| 20–29 years | NA | 1.39 | 1.39 | 1.39 | NA | 4.17 |
| 30–39 years | 1.39 | 1.39 | 11.11 | 1.39 | NA | 15.28 |
| 40–49 years | 1.39 | 6.94 | 5.56 | 6.94 | 1.39 | 22.22 |
| 50–59 years | 5.56 | 6.94 | 8.33 | 2.78 | 1.39 | 25.00 |
| 60–69 years | 4.17 | 4.17 | 8.33 | 6.94 | NA | 23.61 |
| 70–79 years | NA | NA | 4.17 | 4.17 | NA | 8.33 |
| 80+ years | NA | NA | NA | NA | NA | NA |
| Total | 12.50 | 20.83 | 38.89 | 25.00 | 2.78 | 100.00 |
| Diagnostic Method | ||||||
| Incidental finding/physical exam by family doctor | 1.39 | 2.78 | 6.94 | 5.56 | 1.39 | |
| Biopsy | 5.56 | 13.89 | 25.00 | 11.11 | 1.39 | |
| Reporting of symptoms and/or discomfort | 2.78 | 12.50 | 16.67 | 18.06 | NA | |
| Blood work | NA | 4.17 | 15.28 | 5.56 | NA | |
| Colonoscopy | 1.39 | 4.17 | 9.72 | 1.39 | 1.39 | |
| Other (please specify) | 4.17 | 5.56 | 11.11 | 2.78 | 1.39 |
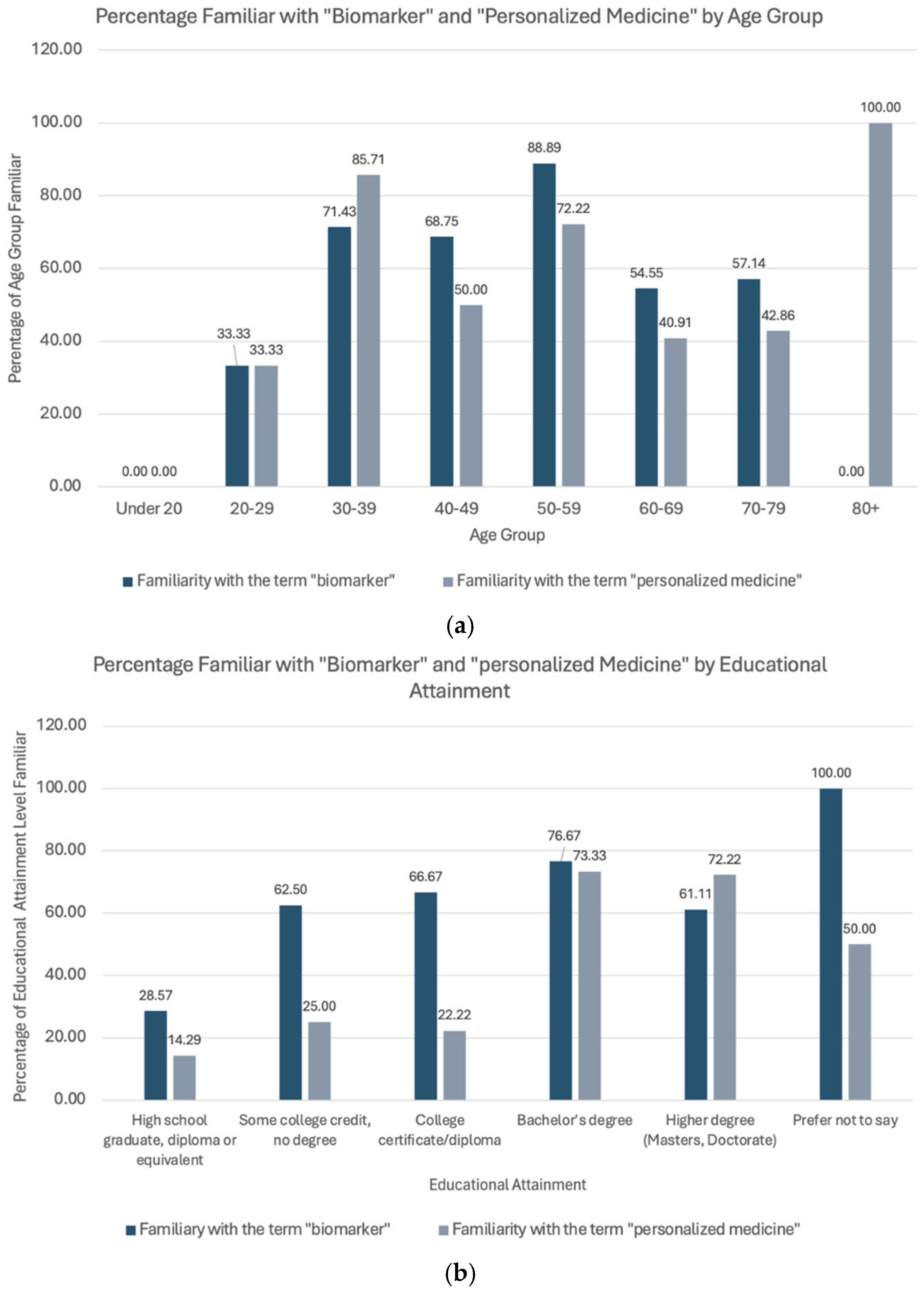
| Demographic | With “Biomarker” | With “Precision Medicine” | |||
|---|---|---|---|---|---|
| Respondents | “Yes” | Percentage | “Yes” | Percentage | |
| Age (years) | |||||
| Under 20 | 0 | 0 | NA | 0 | NA |
| 20–29 | 3 | 1 | 33.33 | 1 | 33.33 |
| 30–39 | 7 | 5 | 71.42 | 6 | 85.71 |
| 40–49 | 16 | 11 | 68.75 | 8 | 50.00 |
| 50–59 | 18 | 16 | 88.88 | 13 | 72.22 |
| 60–69 | 22 | 12 | 54.54 | 9 | 40.91 |
| 70–79 | 7 | 4 | 5.147 | 3 | 42.86 |
| 80+ | 1 | 0 | NA | 1 | 100.00 |
| Educational Attainment | |||||
| High school graduate, diploma or equivalent | 7 | 2 | 28.57 | 1 | 14.29 |
| Some college credit, no degree | 8 | 5 | 62.5 | 2 | 25.00 |
| College certificate/diploma | 9 | 6 | 66.67 | 2 | 22.22 |
| Bachelor’s degree | 30 | 23 | 76.67 | 22 | 73.33 |
| Higher degree (masters, doctorate) | 18 | 11 | 61.11 | 13 | 72.22 |
| Prefer not to say | 2 | 2 | 100.00 | 1 | 50.00 |
| Total | 74 | 49 | 41 | ||
| Oncologist | Media/ Internet/ Family and Friends | Patient Groups/ Other Cancer Patients | Other Healthcare Physician | Other (Please Specify) | Total | |
|---|---|---|---|---|---|---|
| At diagnosis | 3 | 1 | 0 | 2 | 4 | 10 |
| At treatment selection | 4 | 8 | 1 | 0 | 1 | 14 |
| When learning about clinical trials | 0 | 0 | 1 | 0 | 1 | 2 |
| When monitoring tumour recurrence | 2 | 0 | 1 | 0 | 1 | 4 |
| My cancer team has never discussed biomarkers | 1 | 9 | 3 | 1 | 1 | 15 |
| Other (please specify) | 4 | 3 | 2 | 1 | 3 | 13 |
| Total | 14 | 21 | 8 | 4 | 11 | 58 |
| Unaware | Somewhat Aware | Fully Aware | |||||
|---|---|---|---|---|---|---|---|
| Age (Years) | No. of Respondents | Percentage | No. of Respondents | Percentage | No. of Respondents | Percentage | Total |
| <20 | 0 | NA | 0 | NA | 0 | NA | 0 |
| 20–29 | 0 | NA | 2 | 100.00 | 0 | NA | 2 |
| 30–39 | 3 | 42.86 | 2 | 28.57 | 2 | 28.57 | 7 |
| 40–49 | 11 | 73.33 | 3 | 20.00 | 1 | 6.67 | 15 |
| 50–59 | 9 | 52.94 | 4 | 23.53 | 4 | 23.53 | 17 |
| 60–69 | 14 | 66.67 | 7 | 33.33 | 7 | NA | 21 |
| 70–79 | 6 | 85.71 | 0 | NA | 0 | 14.29 | 7 |
| 80+ | 1 | 100.00 | 0 | NA | 0 | NA | 1 |
| Gender | |||||||
| Female | 33 | 58.93 | 16 | 28.57 | 7 | 12.50 | 56 |
| Male | 11 | 78.57 | 2 | 14.29 | 1 | 7.14 | 14 |
| Number of Respondents | Percentage | |
|---|---|---|
| Stage when offered | ||
| 0 | 0 | NA |
| I | 5 | 15.15% |
| II | 5 | 15.15% |
| III | 6 | 18.18% |
| IV | 9 | 27.27% |
| I don’t know | 3 | 9.09% |
| N/A | 5 | 15.15% |
| Type of treatment undergoing | ||
| Chemotherapy | 9 | 34.61% |
| Immunotherapy | 1 | 3.85% |
| Radiation Therapy | 1 | 3.85% |
| Targeted Therapy | 2 | 7.69% |
| Surgery | 13 | 50.00% |
References
- Canadian Cancer Statistics. Available online: https://cancer.ca/en/research/cancer-statistics/canadian-cancer-statistics (accessed on 22 July 2024).
- Passaro, A.; Al Bakir, M.; Hamilton, E.G.; Diehn, M.; André, F.; Roy-Chowdhuri, S.; Mountzios, G.; Wistuba, I.I.; Swanton, C.; Peters, S. Cancer biomarkers: Emerging trends and clinical implications for personalized treatment. Cell 2024, 187, 1617–1635. [Google Scholar] [CrossRef] [PubMed]
- Definition of Biomarker. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomarker (accessed on 20 September 2024).
- Biomarker Testing for Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-cancer-treatment (accessed on 22 September 2024).
- Goetz, L.H.; Schork, N.J. Personalized Medicine: Motivation, Challenges, and Progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Ballman, K.V. Biomarker: Predictive or Prognostic? J. Clin. Oncol. 2015, 33, 3968–3971. [Google Scholar] [CrossRef]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2024, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hodroj, K.; Barthelemy, D.; Lega, J.-C.; Grenet, G.; Gagnieu, M.-C.; Walter, T.; Guitton, J.; Payen-Gay, L. Issues and Limitations of Available Biomarkers for Fluoropyrimidine-Based Chemotherapy Toxicity, a Narrative Review of the Literature. ESMO Open 2021, 6, 100125. [Google Scholar] [CrossRef]
- Biomarkers—DPYD. Available online: https://www.knowyourbiomarker.org/biomarkers/dpyd (accessed on 25 October 2024).
- Colorectal Cancer Statistics. Available online: https://cancer.ca/en/cancer-information/cancer-types/colorectal/statistics (accessed on 22 July 2024).
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463. [Google Scholar] [CrossRef]
- Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M.; the Alzheimer’s Disease Neuroimaging Initiative. Maternal Family History Is Associated with Alzheimer’s Disease Biomarkers. J. Alzheimer’s Dis. 2012, 31, 659–668. [Google Scholar] [CrossRef]
- Pigott, E.; Binder, L. Getting Better, Faster: The Case for Optimizing Access to Precision Medicines in the Wake of the Revolution in Cancer Care. Collective Oncology Network for Exchange, Cancer Care Innovation, Treatment Access & Education; CONECTed: Ottawa, ON, Canada, 5 July 2023. [Google Scholar]
- Comprehensive Cancer Biomarker Testing Program. Colorectal Cancer Screening During the COVID-19 Pandemic: Impact of Paused Screening and Evaluation of Strategies to Reduce Delays. Available online: https://www150.statcan.gc.ca/n1/en/pub/45-28-0001/2021001/article/00018-eng.pdf?st=KxGEjIx3 (accessed on 22 July 2024).
- A State of Readiness Progress Report. Available online: https://accesstogenomictesting.ca/wp-content/uploads/2023/11/Progress-Report-FINAL_w_ISBN-1.pdf (accessed on 20 March 2025).
- Delays in Diagnosis and Testing Results Are Complicating Canadian Cancer Care: Expert. Available online: https://www.ctvnews.ca/health/delays-in-diagnosis-and-testing-results-are-complicating-canadian-cancer-care-expert-1.6491715 (accessed on 22 July 2024).
- Canadian Cancer Statistics: A 2024 Special Report on the Economic Impact of Cancer in Canada. Available online: https://cdn.cancer.ca/-/media/files/cancer-information/resources/publications/canadian-cancer-statistics-a-2024-special-report-on-the-economic-impact-of-cancer-in-canada/0835-2976-2024-special-report-en.pdf (accessed on 20 March 2025).
- Zer, A.; Cutz, J.C.; Sekhon, H.; Hwang, D.M.; Sit, C.; Maganti, M.; Sung, M.; Binnie, M.; Brade, A.; Chung, T.B.; et al. Translation of Knowledge to Practice—Improving Awareness in NSCLC Molecular Testing. J. Thorac. Oncol. 2018, 13, 1004–1011. [Google Scholar] [CrossRef]
- Shirdarreh, M.; Aziza, O.; Pezo, R.C.; Jerzak, K.J.; Warner, E. Patients’ and Oncologists’ Knowledge and Expectations Regarding Tumor Multigene Next-Generation Sequencing: A Narrative Review. Oncologist 2021, 26, e1359–e1371. [Google Scholar] [CrossRef]
- Snow, S.; Brezden-Masley, C.; Carter, M.D.; Dhani, N.; Macaulay, C.; Ramjeesingh, R.; Raphael, M.J.; Slovinec D’Angelo, M.; Servidio-Italiano, F. Barriers and Unequal Access to Timely Molecular Testing Results: Addressing the Inequities in Cancer Care Delays across Canada. Curr. Oncol. 2024, 31, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Blais, N.; Cheema, P.; Couture, C.; Juergens, R.; Kamel-Reid, S.; Tsao, M.-S.; Wheatley-Price, P.; Xu, Z.; Ionescu, D.N. Standardizing Biomarker Testing for Canadian Patients with Advanced Lung Cancer. Curr. Oncol. 2018, 25, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fortune, E.E.; Zaleta, A.K.; Saxton, M.C. Biomarker Testing Communication, Familiarity, and Informational Needs among People Living with Breast, Colorectal, and Lung Cancer. Patient Educ. Couns. 2023, 112, 107720. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V.; Boulton, E.; Davey, L.; McEvoy, C. The online survey as a qualitative research tool. Int. J. Soc. Res. Methodol. 2020, 24, 641–654. [Google Scholar] [CrossRef]
- Huber, M.; Knottnerus, J.A.; Green, L.; Van Der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; Van Der Meer, J.W.; et al. How should we define health? BMJ 2011, 343, d4163. [Google Scholar] [CrossRef]
- Van de Velde, D.; De Zutter, F.; Satink, T.; Costa, U.; Janquart, S.; Senn, D.; De Vriendt, P. Delineating the concept of self-management in chronic conditions: A concept analysis. BMJ Open 2019, 9, e027775. [Google Scholar] [CrossRef]
- El Bizri, M.E.; Sukkarieh, L.; Stein, B.D. Patients’ and Caregivers’ Perspective on Biomarker Testing Across Canada. Colorectal Cancer Canada. Available online: https://www.colorectalcancercanada.com/wp-content/uploads/2023/07/Patient-and-Caregiver-Perspective-Poster.pdf (accessed on 10 November 2024).
- Screening for Colorectal Cancer. Available online: https://www.canada.ca/en/health-canada/services/healthy-living/your-health/diseases/screening-colorectal-cancer.html (accessed on 19 March 2025).
- Pichler, T.; Mumm, F.; Dehar, N.; Dickman, E.; Díez de Los Ríos de la Serna, C.; Dinkel, A.; Heinrich, K.; Hennink, M.; Parviainen, A.D.; Raske, V.; et al. Understanding Communication between Patients and Healthcare Professionals Regarding Comprehensive Biomarker Testing in Precision Oncology: A Scoping Review. Cancer Med. 2024, 13, e6913. [Google Scholar] [CrossRef]
- Normanno, N.; Apostolidis, K.; Wolf, A.; Al Dieri, R.; Deans, Z.; Fairley, J.; Maas, J.; Martinez, A.; Moch, H.; Nielsen, S.; et al. Access and Quality of Biomarker Testing for Precision Oncology in Europe. Eur. J. Cancer 2022, 176, 70–77. [Google Scholar] [CrossRef]
- Stacey, D.; Paquet, L.; Samant, R. Exploring Cancer Treatment Decision-Making by Patients: A Descriptive Study. Curr. Oncol. 2010, 17, 85–93. [Google Scholar] [CrossRef]
- Kaur, J.S. How Should We “Empower” Cancer Patients? Cancer 2014, 120, 3108–3110. [Google Scholar] [CrossRef]
- Sheffield, B.S.; Banerji, S.; Chankowsky, A.; Dudani, S.; Gill, S.; Gorski, Z.; Kassam, S.; Macaulay, C.; Manna, M.; Perdrizet, K.; et al. Toward Timely and Equitable Advanced Biomarker Testing for Patients with Metastatic Cancer in Canada. Curr. Oncol. 2025, 32, 141. [Google Scholar] [CrossRef] [PubMed]
- Anggondowati, T.; Ganti, A.K.; Watanabe-Galloway, S.; Haynatzki, G.; Islam, K.M. Effect of Time to Treatment on Survival in Non-Small Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 8542. [Google Scholar] [CrossRef]
- Bertakis, K.D.; Azari, R. Determinants and Outcomes of Patient-Centered Care. Patient Educ. Couns. 2011, 85, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Constantinidou, A.; Chatzittofis, A. Patients’ Perspectives Related to Ethical Issues and Risks in Precision Medicine: A Systematic Review. Front. Med. 2023, 10, 1215663. [Google Scholar] [CrossRef]
- The Economic Burden of Cancer in Canada. Available online: https://www.partnershipagainstcancer.ca/topics/economic-burden-cancer/ (accessed on 26 June 2024).
- Foundation Medicine Patient FAQs|Foundation Medicine. Available online: https://www.foundationmedicine.com/faq/patient-faqs (accessed on 26 June 2024).
- Clement, F.; Memedovich, K.A. Drug Coverage in Canada: Gaps and Opportunities. J. Psychiatry Neurosci. 2018, 43, 148–150. [Google Scholar] [CrossRef]
- Schönbrodt, F.D.; Perugini, M. At what sample size do correlations stabilize? J. Res. Personal. 2013, 47, 609–612. [Google Scholar] [CrossRef]
- Bauhoff, S. Self-Report Bias in Estimating Cross-Sectional and Treatment Effects. In Encyclopedia of Quality of Life and Well-Being Research; Michalos, A.C., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 5798–5800. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mksyartinian, P.; Xu, S.; Barroma, C.; Peláez, S.; Stein, B.D. Exploring Cancer Patients’ and Caregivers’ Perspectives and Knowledge Regarding Biomarker Testing in Canada. Curr. Oncol. 2025, 32, 292. https://doi.org/10.3390/curroncol32060292
Mksyartinian P, Xu S, Barroma C, Peláez S, Stein BD. Exploring Cancer Patients’ and Caregivers’ Perspectives and Knowledge Regarding Biomarker Testing in Canada. Current Oncology. 2025; 32(6):292. https://doi.org/10.3390/curroncol32060292
Chicago/Turabian StyleMksyartinian, Patil, Selina Xu, Chrissa Barroma, Sandra Peláez, and Barry D. Stein. 2025. "Exploring Cancer Patients’ and Caregivers’ Perspectives and Knowledge Regarding Biomarker Testing in Canada" Current Oncology 32, no. 6: 292. https://doi.org/10.3390/curroncol32060292
APA StyleMksyartinian, P., Xu, S., Barroma, C., Peláez, S., & Stein, B. D. (2025). Exploring Cancer Patients’ and Caregivers’ Perspectives and Knowledge Regarding Biomarker Testing in Canada. Current Oncology, 32(6), 292. https://doi.org/10.3390/curroncol32060292







