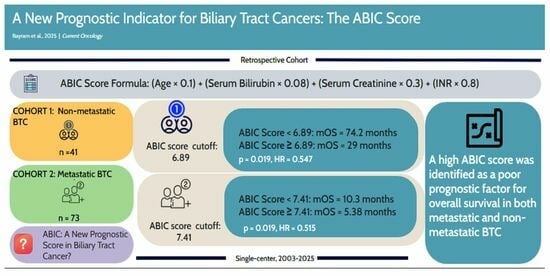
A New Prognostic Indicator for Biliary Tract Cancers: The ABIC Score
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Study Design
2.2. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Treatment Modalities
3.3. Survival Analysis and Treatment Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Moustafa, M.; Linecker, M.; Lurje, G.; Capobianco, I.; Baumgart, J.; Ratti, F.; Rauchfuss, F.; Balci, D.; Fernandes, E.; et al. ALPPS for Locally Advanced Intrahepatic Cholangiocarcinoma: Did Aggressive Surgery Lead to the Oncological Benefit? An International Multi-center Study. Ann. Surg. Oncol. 2020, 27, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, M.H.; Kim, K.P.; Park, D.H.; Moon, S.H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver 2009, 3, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Wozniak, A.; Cook, P.; Adaniel, C.; Acevedo, J.; Azócar, L.; Hsing, A.W.; Roa, J.C.; Pasetti, M.F.; Miquel, J.F.; et al. Salmonella enterica serovar Typhi and gallbladder cancer: A case-control study and meta-analysis. Cancer Med. 2016, 5, 3235–3310. [Google Scholar] [CrossRef] [PubMed]
- Forrest, E.H.; Atkinson, S.R.; Richardson, P.; Masson, S.; Ryder, S.; Thursz, M.R.; Allison, M. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J. Hepatol. 2018, 68, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Rincón, D.; Abraldes, J.G.; Miquel, R.; Colmenero, J.; Bellot, P.; García-Pagán, J.C.; Fernández, R.; Moreno, M.; Bañares, R.; et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am. J. Gastroenterol. 2008, 103, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018, 67, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Pan, Y.; Zheng, Y.Y.; Yang, Y.; Hou, X.G.; Deng, C.J.; Ma, Y.T.; Xie, X. Age-Bilirubin-International Normalized Ratio (INR)-Creatinine (ABIC) Score, a Potential Prognostic Model for Long-Term Mortality of CAD Patients After PCI. J. Inflamm. Res. 2023, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch. Pathol. Lab. Med. 2021, 145, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, R.; Zhang, X.; Zhou, T.; Sun, D.; Yang, N.; Luo, Z. Increased age, bilirubin, international normalized ratio, and creatinine score to triglyceride ratio are associated with alcohol-associated primary liver carcinoma: A single-centered retrospective study. Lipids Health Dis. 2023, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, J.; Cai, J.; Jie, Y.; Zhang, Y.; Li, H.; Lu, T.; He, L.; Xiao, C.; Zeng, K.; et al. Predictive Value of Age-Bilirubin-International Normalized Ratio-Creatinine Score in Short-Term Survival of Acute-on-Chronic Hepatitis B Liver Failure. Cell. Physiol. Biochem. 2018, 51, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Heijnen, M.L.; Houterman, S.; Lemmens, V.E.; Louwman, M.W.; Maas, H.A.; Coebergh, J.W. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit. Rev. Oncol./Hematol. 2005, 55, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.M.; Endo, Y.; Lima, H.A.; Alaimo, L.; Moazzam, Z.; Shaikh, C.; Poultsides, G.A.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; et al. Albumin-Bilirubin Grade and Tumor Burden Score Predict Outcomes Among Patients with Intrahepatic Cholangiocarcinoma After Hepatic Resection: A Multi-Institutional Analysis. J. Gastrointest. Surg. 2023, 27, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pang, Q.; Jin, H.; Zhou, L.; Hu, X.; Qian, Z.; Man, Z.; Yang, S.; Liu, H. Albumin-Bilirubin Grade as a Novel Predictor of Survival in Advanced Extrahepatic Cholangiocarcinoma. Gastroenterol. Res. Pract. 2018, 2018, 8902146. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, I.M.; Subbiah, V.; Tsimberidou, A.M.; Naing, A.; Kaseb, A.O.; Javle, M.; Fu, S.; Hong, D.S.; Piha-Paul, S.; Wheler, J.J.; et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: Eliciting early response signals from phase 1 trials. Oncotarget 2013, 4, 156–165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kriengkrai, W.; Somjaivong, B.; Titapun, A.; Wonggom, P. Predictive Factors for Post-Hepatectomy Liver Failure in Patients with Cholangiocarcinoma. Asian Pac. J. Cancer Prev. APJCP 2023, 24, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, C.; Zhou, H.; Hu, S.; Wang, H.; Xie, Q.; Lei, L.; Wang, P.; Liu, G.; Hu, H. Comparison of Modified Child-pugh (MCP), Albumin-bilirubin (ALBI), and Child-pugh (CP) score for predicting of survival in Hepatocellular Carcinoma Patients Treated with Transcatheter Arterial Chemoembolization. Bull. Cancer 2021, 108, 931–939. [Google Scholar] [CrossRef] [PubMed]


| Non-Metastatic Group | Metastatic Group | ||||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Number of Patients | 41 | 73 | |||
| Median Age (min–max) | 62 (30–78) | 63 (36–82) | |||
| Gender | Female | 30 | (73.2) | 32 | (43.8) |
| Male | 11 | (26.8) | 41 | (56.2) | |
| Location | GBC | 31 | (75.6) | 38 | (52.1) |
| IHCC and EHCC | 10 | (24.4) | 35 | (47.9) | |
| Stage at diagnosis | I | 4 | (9.8) | ||
| II | 23 | (56.1) | |||
| III | 14 | (34.1) | |||
| IV | 0 | (0) | 73 | (100) | |
| Comorbidity | Yes | 23 | (56.1) | 39 | (53.4) |
| No | 18 | (53.9) | 34 | (46.6) | |
| Smoking History | Yes (former smoker or current smoker) | 16 | (39) | 34 | (46.6) |
| No | 25 | (61) | 39 | (53.4) | |
| CEA | Normal | 35 | (85.4) | 43 | (58.9) |
| Elevated | 6 | (14.6) | 30 | (41.1) | |
| CA 19-9 | Normal | 30 | (73.2) | 14 | (19.2) |
| Elevated | 11 | (2.8) | 59 | (80.8) | |
| GGT | Normal | 19 | (46.3) | 6 | (8.2) |
| Elevated | 22 | (53.7) | 67 | (91.8) | |
| T. Bilirubin | Normal | 35 | (85.4) | 39 | (53.4) |
| Elevated | 6 | (14.6) | 34 | (46.6) | |
| ALP | Normal | 32 | (78) | 27 | (37) |
| Elevated | 9 | (22) | 46 | (63) | |
| ALT | Normal | 36 | (87.8) | 34 | (46.6) |
| Elevated | 5 | (12.2) | 39 | (53.4) | |
| AST | Normal | 32 | (78) | 33 | (45.2) |
| Elevated | 9 | (22) | 40 | (54.8) | |
| ECOG | 0−1 | 30 | (73.2) | 49 | (67.1) |
| 2−4 | 11 | (26.8) | 24 | (32.9) | |
| ABIC Score (ROC Analysis) | 6.89 | 7.41 | |||
| ABIC Score | Low | 13 | (31.7) | 23 | (31.5) |
| High | 28 | (68.3) | 50 | (68.5) | |
| Non-Metastatic Group | Metastatic Group | ||||
|---|---|---|---|---|---|
| n: 41 | (%) | n: 73 | (%) | ||
| Curative Surgery | Yes | 41 | (100) | 0 | (0) |
| No | 0 | (0) | 73 | (100) | |
| Neoadjuvant CT | Gemcitabine-Cisplatin | 3 | (7.31) | 0 | (0) |
| Adjuvant CT | 28 | (68.2) | 0 | (0) | |
| Gemcitabine | 8 | (28.6) | |||
| Gemcitabine-Cisplatin | 6 | (21.4) | |||
| Gemcitabine-Capecitabine | 3 | (10.7) | |||
| FOLFOX | 6 | (21.4) | |||
| Cisplatin-5 Fluorouracil | 4 | (14.3) | |||
| Capecitabine | 1 | (3.6) | |||
| Adjuvant CRT | 13 | (31.7) | 0 | (0) | |
| Relapse | 21 | (51.2) | |||
| First-Line CT | Yes | 14 | (34.1) | 57 | (78.1) |
| First-Line CT Regimen | Gemcitabine-Cisplatin | 7 | (50) | 26 | (45.6) |
| Gemcitabine | 3 | (21.4) | 13 | (22.8) | |
| FOLFOX | 2 | (14.2) | 7 | (12.3) | |
| Cisplatin-5 Fluorouracil | 1 | (7.1) | 6 | (10.5) | |
| Gemcitabine-Capecitabine | 0 | 2 | (3.5) | ||
| Capecitabine | 1 | (7.1) | 2 | (3.5) | |
| Gemcitabine-Oxaliplatin | 0 | 1 | (1.8) | ||
| Progressive disease after first-line CT | 12 | (29.2) | 51 | (69.9) | |
| Second-Line CT | Yes | 3 | (7.3) | 11 | (15) |
| Second-Line CT Regimen | Gemcitabine-Cisplatin | 1 | (33.3) | 4 | (36.4) |
| Gemcitabine-Carboplatin | 2 | (66.7) | 2 | (18.2) | |
| FOLFOX | 0 | 3 | (27.2) | ||
| Capecitabine | 0 | 2 | (18.2) | ||
| Median OS (Months) | Univariate (p Value) | Multivariate p Value | HR | ||
|---|---|---|---|---|---|
| Gender | Female | 31.08 | 0.786 | ||
| Male | 21.48 | ||||
| Age Group | ≤62 | 53.9 | 0.116 | ||
| >62 | 23.5 | ||||
| Comorbidity | Yes | 30.3 | 0.193 | ||
| No | 40.3 | ||||
| Smoking | Yes (former smoker or current smoker) | 29.0 | 0.650 | ||
| No | 40.3 | ||||
| Location | GBC | 53.9 | 0.013 | 0.012 | 0.320 |
| IHCC and EHCC | 18.0 | ||||
| Stage | I–II | 58.4 | 0.012 | 0.009 | 0.322 |
| III | 21.7 | ||||
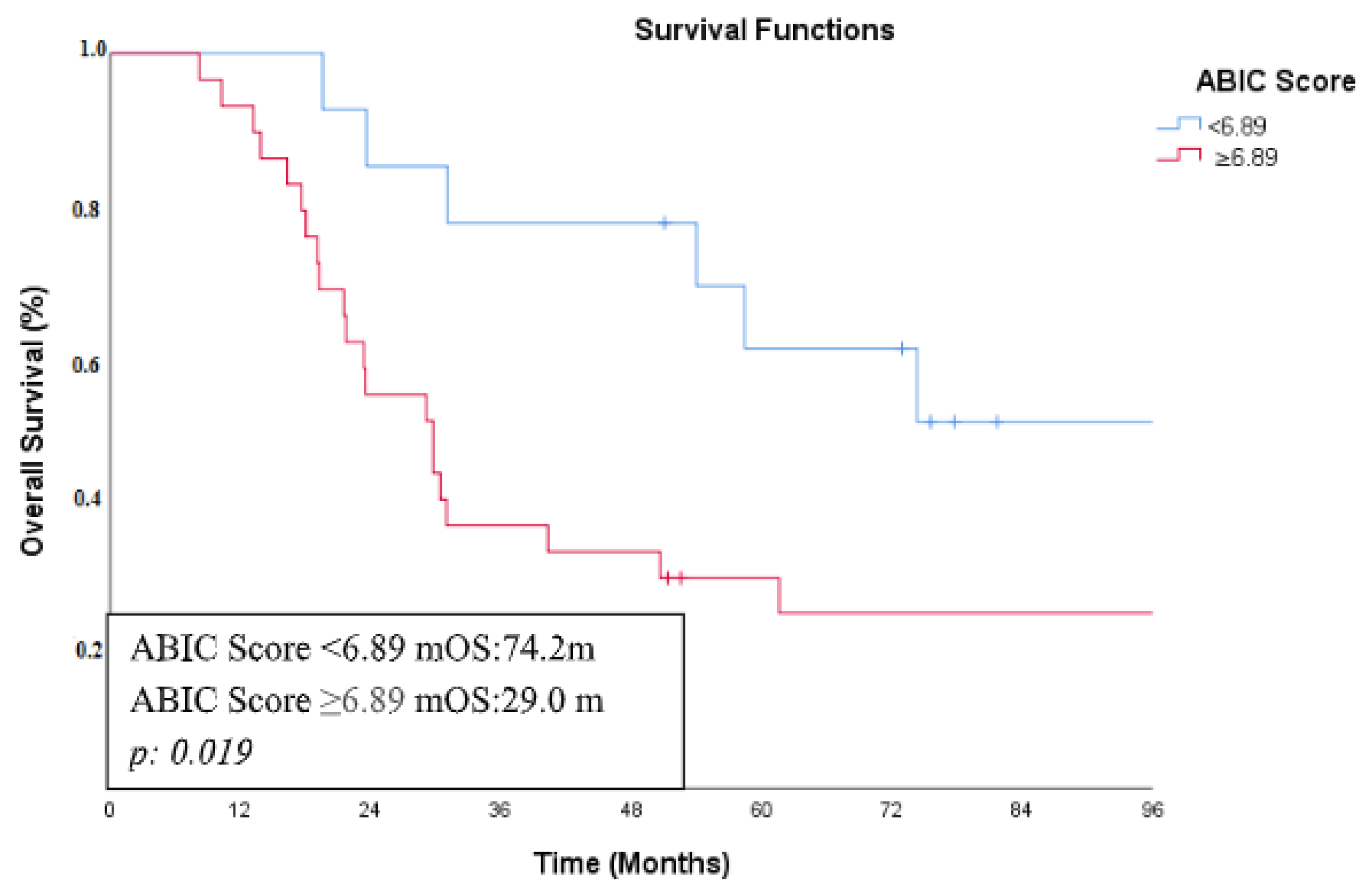
| ABIC Score | <6.89 | 74.2 | 0.019 | 0.031 | 0.361 |
| ≥6.89 | 29.0 | ||||
| CEA | Normal | 50.6 | 0.146 | ||
| Elevated | 17.6 | ||||
| CA 19-9 | Normal | 31.0 | 0.542 | ||
| Elevated | 19.2 | ||||
| AST | Normal | 31.0 | 0.144 | ||
| Elevated | 29.7 | ||||
| GGT | Normal | 58.4 | 0.432 | ||
| Elevated | 29.73 | ||||
| ALT | Normal | 50.6 | 0.750 | ||
| Elevated | 30.3 | ||||
| ALP | Normal | 61.5 | 0.622 | ||
| Elevated | 30.3 | ||||
| ECOG | 0−1 | 31.6 | 0.054 | ||
| 2−4 | 30.3 | ||||
| Adjuvant CT | Yes | 58.4 | 0.138 | ||
| No | 29.7 | ||||
| Adjuvant CRT | Yes | 30.9 | 0.478 | ||
| No | 40.3 |
| Median OS (Months) | Univariate (p Value) | Multivariate p Value | HR | ||
|---|---|---|---|---|---|
| Gender | Female | ||||
| Male | |||||
| Age Group | ≤64 | 7.91 | 0.985 | ||
| >64 | 7.35 | ||||
| Comorbidity | Yes | 7.85 | 0.60 | ||
| No | 6.93 | ||||
| Smoking | Yes (former smoker or current smoker) | 6.43 | 0.294 | ||
| No | 7.85 | ||||
| Location | GBC | 7.91 | 0.485 | ||
| IHCC and EHCC | 5.78 | ||||
| ABIC Score | <7.41 | 10.3 | 0.019 | 0.021 | 0.515 |
| ≥7.41 | 5.38 | ||||
| CEA | Normal | 7.91 | 0.038 | 0.022 | 0.547 |
| Elevated | 3.87 | ||||
| CA19.9 | Normal | 8.18 | 0.207 | ||
| Elevated | 6.93 | ||||
| AST | Normal | 8.18 | 0.245 | ||
| Elevated | 4.76 | ||||
| ALT | Normal | 7.91 | 0.533 | ||
| Elevated | 6.93 | ||||
| GGT | Normal | 10.3 | 0.142 | ||
| Elevated | 6.93 | ||||
| ALP | Normal | 7.91 | 0.407 | ||
| Elevated | 7.35 | ||||
| ECOG | 0−1 | 8.18 | 0.002 | 0.519 | |
| 2−4 | 3.71 | ||||
| First-Line CT | Yes | 8.08 | 0.001 | 0.001 | 0.283 |
| No | 3.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayram, D.; Bal, Ö.; Karaman, K.; Bardakçı, M.; Demirtaş Esmer, D.; Seven, İ.; Sekmek, S.; Perkin, P.; Köş, F.T.; Algın, E.; et al. A New Prognostic Indicator for Biliary Tract Cancers: The ABIC Score. Curr. Oncol. 2025, 32, 200. https://doi.org/10.3390/curroncol32040200
Bayram D, Bal Ö, Karaman K, Bardakçı M, Demirtaş Esmer D, Seven İ, Sekmek S, Perkin P, Köş FT, Algın E, et al. A New Prognostic Indicator for Biliary Tract Cancers: The ABIC Score. Current Oncology. 2025; 32(4):200. https://doi.org/10.3390/curroncol32040200
Chicago/Turabian StyleBayram, Doğan, Öznur Bal, Kemal Karaman, Murat Bardakçı, Derya Demirtaş Esmer, İsmet Seven, Serhat Sekmek, Perihan Perkin, Fahriye Tuğba Köş, Efnan Algın, and et al. 2025. "A New Prognostic Indicator for Biliary Tract Cancers: The ABIC Score" Current Oncology 32, no. 4: 200. https://doi.org/10.3390/curroncol32040200
APA StyleBayram, D., Bal, Ö., Karaman, K., Bardakçı, M., Demirtaş Esmer, D., Seven, İ., Sekmek, S., Perkin, P., Köş, F. T., Algın, E., & Uncu, D. (2025). A New Prognostic Indicator for Biliary Tract Cancers: The ABIC Score. Current Oncology, 32(4), 200. https://doi.org/10.3390/curroncol32040200