Non-Small-Cell Lung Cancer Patients Harboring ROS1 Rearrangement: Real World Testing Practices, Characteristics and Treatment Patterns (ROS1REAL Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Eligibility
2.3. Molecular Profiling
2.4. Treatment
2.5. Data Analysis
3. Results
3.1. Patient Characteristics
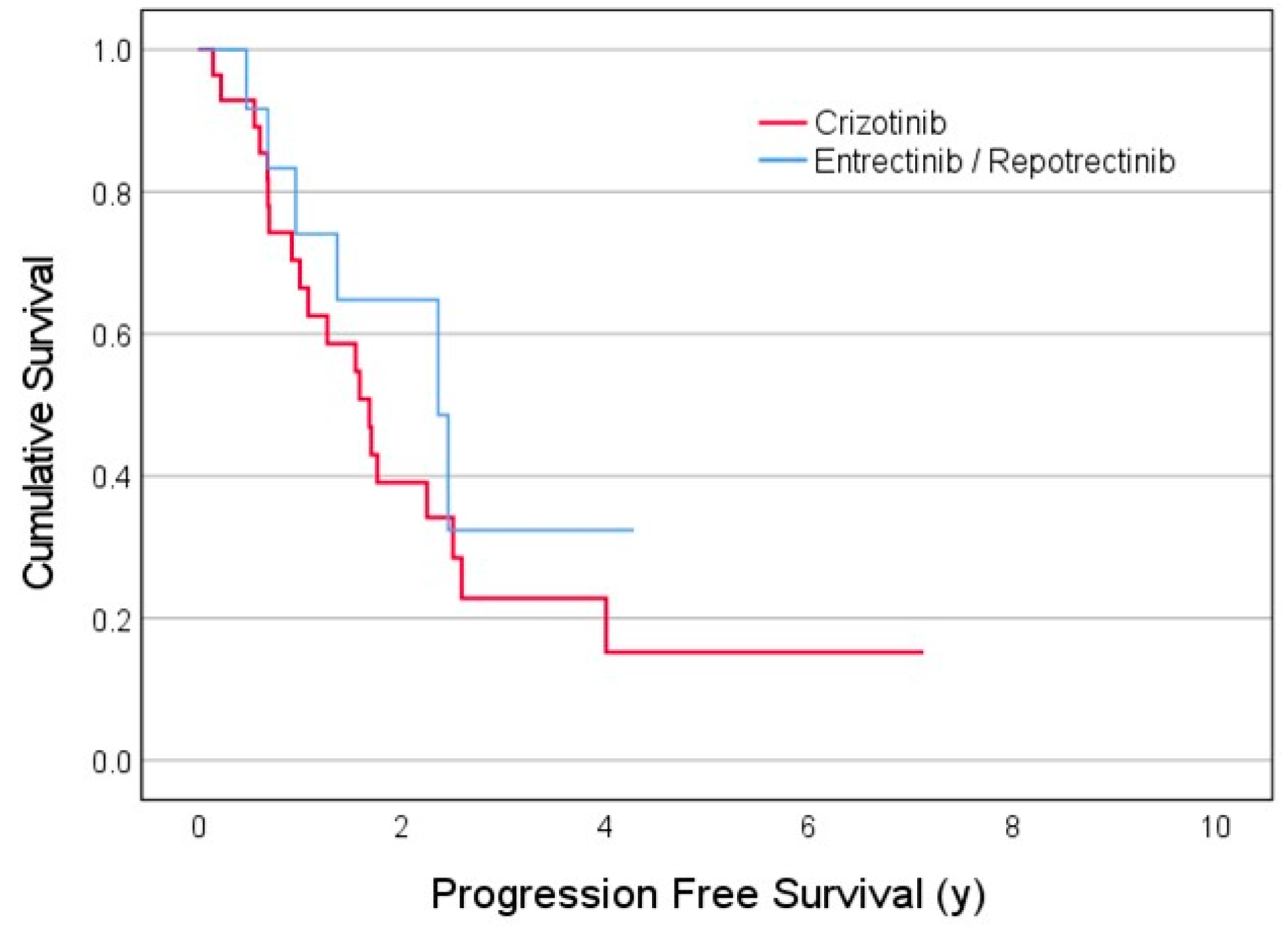
3.2. Treatment Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 27 January 2023).
- Kim, H.H.; Lee, J.C.; Oh, I.-J.; Kim, E.Y.; Yoon, S.H.; Lee, S.Y.; Lee, M.K.; Lee, J.E.; Park, C.K.; Lee, K.Y.; et al. Real-World Outcomes of Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer. Cancers 2024, 16, 528. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tang, Z.; Li, J.; Jiang, J.; Liu, Y. Progress of non-small cell lung cancer with ROS1 rearrangement. Front. Mol. Biosci. 2023, 10, 1238093. [Google Scholar] [CrossRef] [PubMed]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. 2022, 29, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.; Khoury, R.; Brenner, R.; Nasrallah, H.; Shofaniyeh, I.; Yousef, S.; Agbarya, A. Treatment of Rare Mutations in Patients with Lung Cancer. Biomedicines 2021, 9, 534. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef] [PubMed]
- Ten Berge, D.M.H.J.; Damhuis, R.A.M.; Aerts, J.G.J.V.; Dingemans, A.C. Real-world treatment patterns and survival of patients with ROS1 rearranged stage IV non-squamous NSCLC in the Netherlands. Lung Cancer 2023, 181, 107253. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, B.; Del Conte, A.; Bertoli, E.; De Carlo, E.; Revelant, A.; Spina, M.; Bearz, A. Therapeutical Options in ROS1-Rearranged Advanced Non Small Cell Lung Cancer. Int. J. Mol. Sci. 2023, 24, 11495. [Google Scholar] [CrossRef] [PubMed]
- Almquist, D.; Ernani, V. The Road Less Traveled: A Guide to Metastatic ROS1-Rearranged Non-Small-Cell Lung Cancer. JCO Oncol. Pract. 2021, 17, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, K.; Patell, R.; Rangachari, D.; Costa, D.B. Targeting ROS1 rearrangements in non-small cell lung cancer with crizotinib and other kinase inhibitors. Transl. Cancer Res. 2018, 7 (Suppl. 7), S779–S786. [Google Scholar] [CrossRef]
- Mian, A.A.; Haberbosch, I.; Khamaisie, H.; Agbarya, A.; Pietsch, L.; Eshel, E.; Najib, D.; Chiriches, C.; Ottmann, O.G.; Hantschel, O.; et al. Crizotinib acts as ABL1 inhibitor combining ATP-binding with allosteric inhibition and is active against native BCR-ABL1 and its resistance and compound mutants BCR-ABL1T315I and BCR-ABL1T315I-E255K. Ann. Hematol. 2021, 100, 2023–2029. [Google Scholar] [CrossRef]
- Shaw, A.; Riely, G.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.; Solomon, B.; Varella-Garcia, M.; Iafrate, A.; Shapiro, G.; Usari, T.; et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): Updated results, including overall survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive Cancer Network (NCCN) Version 1. Non Small Cell Lung Cancer. ROS1 Rearrangement. 2024. Available online: https://mail.google.com/mail/u/1/#inbox/FMfcgzGwJcfdHlLfGMxMRHlMsPLVtBkC?projector=1&messagePartId=0.1 (accessed on 3 February 2024).
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Chiu, C.-H.; Fan, Y.; Cho, B.C.; Lu, S.; Ahn, M.-J.; Krebs, M.G.; Liu, S.V.; John, T.; Otterson, G.A.; et al. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion-Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100332. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Camidge, D.R.; Lin, J.J.; Kim, S.-W.; Solomon, B.J.; Dziadziuszko, R.; Besse, B.; Goto, K.; de Langen, A.J.; Wolf, J.; et al. Repotrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 118–131. [Google Scholar] [CrossRef] [PubMed]
- FDA Approved Repotrectinib for ROS1-Positive Non-Small Cell Lung Cancer. U.S. Food & Drug Administration. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-repotrectinib-ros1-positive-non-small-cell-lung-cancer (accessed on 3 February 2024).
- Sidaway, P. Repotrectinib effective in ROS1-fusion-positive NSCLC. Nat. Rev. Clin. Oncol. 2024, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Janzic, U.; Shalata, W.; Szymczak, K.; Dziadziuszko, R.; Jakopovic, M.; Mountzios, G.; Płużański, A.; Araujo, A.; Charpidou, A.; Agbarya, A. Real-World Experience in Treatment of Patients with Non-Small-Cell Lung Cancer with BRAF or cMET Exon 14 Skipping Mutations. Int. J. Mol. Sci. 2023, 24, 12840. [Google Scholar] [CrossRef]
- Lee, J.; Park, C.K.; Yoon, H.; Sa, Y.J.; Woo, I.S.; Kim, H.R.; Kim, S.Y.; Kim, T. PD-L1 expression in ROS1-rearranged non-small cell lung cancer: A study using simultaneous genotypic screening of EGFR, ALK, and ROS1. Thorac. Cancer 2019, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Crizotinib Dosage. Available online: https://www.ema.europa.eu/en/search?search_api_fulltest=crizotinib (accessed on 22 February 2024).
- Entrectinib Dosage. Available online: https://www.ema.europa.eu/en/search?search_api_fulltest=entrectinib (accessed on 22 February 2024).
- Repotrectinib Dosage. Available online: https://www.ema.europa.eu/en/search?search_api_fulltest=repotrectinib (accessed on 22 February 2024).
- Baxevanos, P.; Mountzios, G. Novel chemotherapy regimens for advanced lung cancer: Have we reached a plateau? Ann. Transl. Med. 2018, 6, 139. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Rare Mutations and Fusions in NSCLC. Navigating Rare Mutations & Fusions. Available online: https://rare-mutations.lungevity.org/rare-mutations/about-rare-mutations-and-fusions-nsclc#:~:text=Approximately%203%25%2D4%25%20of,a%20minority%20are%20never%2Dsmokers (accessed on 18 July 2024).
- EGFR and Lung Cancer. American Lung Association. Available online: http://www.lung.org/lung-health-diseases-lookup/lung-cancer/symptoms-diagnosis/biomarker-testing/egfr#:~:text=Who%20is%20likely%20to%20have,minimal%20to%20no%20smoking%20history (accessed on 18 July 2024).
- Blackhall, F.H.; Peters, S.; Bubendorf, L.; Dafni, U.; Kerr, K.M.; Hager, H.; Soltermann, A.; O'Byrne, K.J.; Dooms, C.; Sejda, A.; et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I to III Adenocarcinoma: Results From the European Thoracic Oncology Platform Lungscape Project. J. Clin. Oncol. 2014, 32, 2780–2787. [Google Scholar] [CrossRef]
- Li, W.; Fei, K.; Guo, L.; Wang, Y.; Shu, C.; Wang, J.; Ying, J. CD74/SLC34A2-ROS1 Fusion Variants Involving the Transmembrane Region Predict Poor Response to Crizotinib in NSCLC Independent of TP53 Mutation. J. Thorac. Oncol. 2024, 19, 613–625. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Jung, H.A.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Park, K.; Ahn, M.-J. Evaluating entrectinib as a treatment option for non-small cell lung cancer. Expert Opin. Pharmacother. 2020, 21, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Entrectinib: A Review in NTRK+ Solid Tumours and ROS1 + NSCLC. Drugs 2021, 81, 697–708. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; A Soo, R.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in Advanced ROS1-Positive Non-Small-Cell Lung Cancer: A Multicentre, Open-Label, Single-Arm, Phase 1–2 Trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, M.; Li, H.; Chen, L. Entrectinib, a new multi-target inhibitor for cancer therapy. Biomed Pharmacother. 2022, 150, 112974. [Google Scholar] [CrossRef]
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers-biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Menichincheri, M.; Ardini, E.; Magnaghi, P.; Avanzi, N.; Banfi, P.; Bossi, R.; Buffa, L.; Canevari, G.; Ceriani, L.; Colombo, M.; et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J. Med. Chem. 2016, 59, 3392–3408. [Google Scholar] [CrossRef] [PubMed]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef]
- Lin, J.J.; Shaw, A.T. Recent Advances in Targeting ROS1 in Lung Cancer. J. Thorac. Oncol. 2017, 12, 1611–1625. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, R.; Zhang, X.; Huang, L.; Sun, L.; Zhao, J.; Ma, J.; Han, C. Recent Progress in Rare Oncogenic Drivers and Targeted Therapy for Non-Small Cell Lung Cancer. Onco. Targets Ther. 2019, 12, 10343–10360. [Google Scholar] [CrossRef] [PubMed]
- Jóri, B.; Falk, M.; Hövel, I.; Weist, P.; Tiemann, M.; Heukamp, L.; Griesinger, F. Acquired G2032R Resistance Mutation in ROS1 to Lorlatinib Therapy Detected with Liquid Biopsy. Curr. Oncol. 2022, 29, 6628–6634. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhai, D.; Deng, W.; Rogers, E.; Huang, Z.; Whitten, J.; Li, Y. TPX-0005, a novel ALK/ROS1/TRK inhibitor, effectively inhibited a broad spectrum of mutations including solvent front ALK G1202R, ROS1 G2032R and TRKA G595R mutants. Eur. J. Cancer 2016, 69, S32. [Google Scholar] [CrossRef]
- Dhillon, S. Repotrectinib: First Approval. Drugs 2024, 84, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.; Iadeluca, L.; Sura, S.; Wilner, K.; Emir, B.; Krulewicz, S.; Espirito, J.; Bartolome, L. Real-World Outcomes Among Crizotinib-Treated Patients with ROS1-Positive Advanced Non-Small-Cell Lung Cancer: A Community Oncology-Based Observational Study. Target Oncol. 2022, 17, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, E.; the Israel Lung Cancer Group; Agbarya, A.; Grinberg, R.; Cyjon, A.; Bar, J.; Moskovitz, M.; Peled, N. Clinical activity of brigatinib in ROS1-rearranged non-small cell lung cancer. Clin. Transl. Oncol. 2020, 22, 2303–2311. [Google Scholar] [CrossRef]
- Roys, A.; Chang, X.; Liu, Y.; Xu, X.; Wu, Y.; Zuo, D. Resistance Mechanisms and Potent-Targeted Therapies of ros1-Positive Lung Cancer. Cancer Chemother. Pharmacol. 2019, 1284, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Katayama, R.; McTigue, M.; Liu, W.; Deng, Y.-L.; Brooun, A.; Friboulet, L.; Huang, D.; Falk, M.D.; Timofeevski, S.; et al. Acquired Resistance to Crizotinib from a Mutation in CD74–ROS1. N. Engl. J. Med. 2013, 368, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Morris, T.A.; Khoo, C.; Solomon, B.J. Targeting ROS1 Rearrangements in Non-small Cell Lung Cancer: Crizotinib and Newer Generation Tyrosine Kinase Inhibitors. Drugs 2019, 79, 1277–1286. [Google Scholar] [CrossRef]
- Zou, H.Y.; Li, Q.; Engstrom, L.D.; West, M.; Appleman, V.; Wong, K.A.; McTigue, M.; Deng, Y.-L.; Liu, W.; Brooun, A.; et al. PF-06463922 is a Potent and Selective Next-Generation ROS1/ALK Inhibitor Capable of Blocking Crizotinib-Resistant ROS1 Mutations. Proc. Natl. Acad. Sci. USA 2015, 112, 3493–3498. [Google Scholar] [CrossRef]
- Pfizer’s Next-Generation ALK/ROS1 Inhibitor, Lorlatinib, Granted Breakthrough Therapy Designation from FDA for ALK-Positive Metastatic Non-Small Cell Lung Cancer, Pfizer. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_s_next_generation_alk_ros1_inhibitor_lorlatinib_granted_breakthrough_therapy_designation_from_fda_for_alk_positive_metastatic_non_small_cell_lung_cancer. (accessed on 22 February 2024).
- Weng, Y.; Cai, M. ROS1 resistance mutations and co-occurring genetic alterations to the ROS1 protein-tyrosine kinase inhibitors (crizotinib) in lung cancer. Ann. Oncol. 2021, 32 (Suppl. 5), S989. [Google Scholar] [CrossRef]
- Garcia-Pardo, M.; Calles, A. ROS-1 NSCLC therapy resistance mechanism. PCM 2021, 4, 16. [Google Scholar] [CrossRef]
- Belaroussi, Y.; Bouteiller, F.; Bellera, C.; Pasquier, D.; Perol, M.; Debieuvre, D.; Filleron, T.; Girard, N.; Schott, R.; Mathoulin-Pélissier, S.; et al. Survival outcomes of patients with metastatic non-small cell lung cancer receiving chemotherapy or immunotherapy as first-line in a real-life setting. Sci. Rep. 2023, 13, 9584. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Q.; Liang, L.; Li, J.; Liu, Z.; Li, W.; Yang, L.; Yang, G.; Xu, F.; Ying, J.; et al. Crizotinib vs. platinum-based chemotherapy as first-line treatment for advanced non-small cell lung cancer with different ROS1 fusion variants. Cancer Med. 2019, 9, 3328–3336. [Google Scholar] [CrossRef]
- A Study of Repotrectinib Versus Crizotinib with Locally Advanced or Metastatic TKI-Naïve ROS1 Positive Non-Small Cell Lung Cancer TRIDENT-3. Available online: https://clinicaltrials.gov/study/NCT06140836 (accessed on 22 February 2024).
- Lorlatinib after Failure of First-Line TKI in Patients with Advanced ROS1-Positive NSCLC (ALBATROS). Available online: https://clinicaltrials.gov/study/NCT04621188 (accessed on 22 February 2024).
- A Study to Compare the Efficacy and Safety of Entrectinib and Crizotinib in Participants with Advanced or Metastatic ROS1 NSCLC with and without CNS Metastases [NCT 04603807]. Available online: https://clinicaltrials.gov/study/NCT04603807 (accessed on 22 February 2024).

| Characteristics | Crizotinib a n = 28 | Entrectinib /Repotrectinib a n = 14 | Platinum-Doublet n = 7 | Total n = 49 | p a |
|---|---|---|---|---|---|
| Age at diagnosis (years) b | 62.6 ± 10.1 | 59.7 ± 14.9 | 59.3 ± 10.9 | 61.6 ± 11.8 | 0.46 |
| Gender | 0.72 | ||||
| Female | 15 (54%) | 9 (64%) | 4 (57%) | 28 (57%) | |
| Male | 13 (46%) | 5 (36%) | 3 (43%) | 21 (43%) | |
| Smoking habits | 0.88 | ||||
| Current | 3 (11%) | 1 (7%) | 0 | 4 (8%) | |
| Former | 7 (25%) | 3 (21%) | 3 (43%) | 13 (27%) | |
| Never | 18 (64%) | 10 (72%) | 4 (57%) | 32 (65%) | |
| Comorbidities | |||||
| Hypertension | 9 (32%) | 3 (21%) | 4 (57%) | 16 (33%) | 0.72 |
| DM c | 3 (11%) | 0 | 0 | 3 (6%) | 0.54 |
| PVD c | 1 (3.6%) | 0 | 1 (14%) | 2 (4%) | NA |
| Other chronic conditions: | 7 (25%) | 2 (14%) | 2 (29%) | 11 (22%) | 0.69 |
| Hypothyroidism | 0 | 1 (7.14%) | 1 (14.3%) | 2 (4%) | NA |
| COPD c | 2 (7.14%) | 0 | 0 | 2 (4%) | NA |
| Stage at initial diagnosis | 0.31 | ||||
| II d | 1 | 0 | 1 | 2 | |
| III d | 0 | 2 | 0 | 2 | |
| IV | 27 (96%) | 12 (86%) | 6 (86%) | 45 (92%) | |
| Mutation type: | n = 13 | 0.60 | |||
| Fusion | 17 (60.7%) | 6 (46.2%) | 4 (57.1%) | 27 (56%) | |
| Rearrangement | 6 (21%) | 3 (23.1%) | 1 (14.3%) | 10 (21%) | |
| Translocation | 5 (17.9%) | 4 (30.8%) | 2 (28.6%) | 11 (23%) | |
| PD-L1 c expression | n = 22 | n = 9 | n = 6 | 0.15 | |
| <1% | 4 (18.2%) | 4 (44.4%) | 3 (50%) | 11 (30%) | |
| 1–49% | 5 (22.7%) | 3 (33.3%) | 3 (50%) | 11 (30%) | |
| 50 % | 13 (59.1%) | 2 (22.2%) | 0 | 15 (41%) | |
| ECOG c Performance Status | 0.76 | ||||
| 0 | 9 (32.1%) | 3 (21.4%) | 1 (14.3%) | 13 (27%) | |
| 1 | 15 (53.6%) | 9 (64.3%) | 5(71.4%) | 29 (59%) | |
| 2 | 3 (10.7%) | 2 (14.3%) | 1 (14.3%) | 6 (12%) | |
| 3 | 1 (3.6%) | 0 | 0 | 1 (2%) | |
| Metastatic sites | |||||
| Brain | 3 (10.7%) | 3 (21.4%) | 3 (42.9%) | 9 (18%) | 0.38 |
| Contralateral Lung | 15 (53.6%) | 10 (71.4%) | 5 (71.4%) | 30 (61%) | 0.33 |
| Lymph nodes—extrathoracic | 12 (42.9%) | 7 (50.0%) | 4 (57.1%) | 23 (47%) | 0.75 |
| Pleura | 16 (57.1%) | 2 (14.3%) | 3 (42.9%) | 21 (43%) | 0.01 |
| Pericardial | 3 (10.7%) | 0 | 0 | 3 (6%) | NA |
| Bone | 10 (35.7%) | 5 (35.7%) | 2 (28.6%) | 17 (35%) | 1.00 |
| Adrenal | 4 (14.3%) | 3 (21.4%) | 1 (14.3%) | 8 (16%) | 0.67 |
| Liver | 6 (21.4%) | 3 921.4%) | 1 (14.3%) | 10 (20%) | 1.00 |
| Spleen | 1 | 0 | 0 | 1 | NA |
| Peritoneal | 1 | 1 | 0 | 2 | NA |
| Clinical Characteristics | Crizotinib a n = 28 | Entrectinib + Repotrectinib a n = 14 | Platinum-Doublet + Chemotherapy n = 7 | Total n = 49 | p a |
|---|---|---|---|---|---|
| Median no. of cycles b | 11 [7–12] | 15.5 [6.5–3.2] | 6 [5–12] | 11.5 [6–25] | 0.55 |
| Best Response | n = 5 | 0.48 | |||
| Complete response | 4 (14%) | 1 (7%) | 1 (20%) | 6 (13%) | |
| Partial response | 15 (54%) | 11 (79%) | 4 (80%) | 30 (64%) | |
| Stable disease | 4 (14%) | 1 (7%) | 0 | 5 (11%) | |
| Progressive disease | 5 (18%) | 1 (7%) | 0 | 6 (13%) | |
| Toxicity c | n = 16 | n = 11 | n = 4 | 0.27 | |
| Grades 1/2 | 15 (94%) | 8 (73%) | 1 (25%) | 24 (77%) | |
| Grades 3/4 | 1 (6%) | 3 (27%) | 3 (75%) | 7 (23%) | |
| Reason for discontinuation | n = 17 | n = 5 | n = 4 | 0.54 | |
| Death | 2 (12%) | 1 (20%) | 0 | 3 (11%) | |
| Progressive disease | 15 (88%) | 4 (80%) | 2 (50%) | 22 (82%) | |
| Progression in the CNS d | 3/15 (20%) | 1/4 (25%) | 0 | ||
| Second-line treatment | 14/18 (78%) | 3/8 (37.5%) | 7/7 (100%) | 27 (73%) | 0.078 |
| Clinical Trials.gov ID | Official Title | Drug | Study Start | Estimated Completion | Phase. |
|---|---|---|---|---|---|
| NCT06140836 | A Study of Repotrectinib Versus Crizotinib in Participants With Locally Advanced or Metastatic Tyrosine Kinase Inhibitor (TKI)-naïve ROS1-positive Non-Small Cell Lung Cancer (NSCLC) (TRIDENT-3) | Repotrectinib Crizotinib | 2023-12-21 | 2031-01-27 | 3 |
| NCT03093116 | A Phase 1/2, Open-Label, Multi-Center, First-in-Human Study of the Safety, Tolerability, Pharmacokinetics, and Anti-Tumor Activity of TPX-0005 in Patients With Advanced Solid Tumors Harboring ALK, ROS1, or NTRK1-3 Rearrangements (TRIDENT-1) | Repotrectinib | 2017-03-07 | 2028-02-29 | 1 2 |
| NCT04603807 | A Study to Compare the Efficacy and Safety of Entrectinib and Crizotinib in Participants With Advanced or Metastatic ROS1 Non-small Cell Lung Cancer (NSCLC) With and Without Central Nervous System (CNS) Metastases | Entrectinib Crizotinib | 2021-09-30 | 2027-12-01 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janzic, U.; Maimon Rabinovich, N.; Shalata, W.; Kian, W.; Szymczak, K.; Dziadziuszko, R.; Jakopovic, M.; Mountzios, G.; Pluzanski, A.; Araujo, A.; et al. Non-Small-Cell Lung Cancer Patients Harboring ROS1 Rearrangement: Real World Testing Practices, Characteristics and Treatment Patterns (ROS1REAL Study). Curr. Oncol. 2024, 31, 4369-4381. https://doi.org/10.3390/curroncol31080326
Janzic U, Maimon Rabinovich N, Shalata W, Kian W, Szymczak K, Dziadziuszko R, Jakopovic M, Mountzios G, Pluzanski A, Araujo A, et al. Non-Small-Cell Lung Cancer Patients Harboring ROS1 Rearrangement: Real World Testing Practices, Characteristics and Treatment Patterns (ROS1REAL Study). Current Oncology. 2024; 31(8):4369-4381. https://doi.org/10.3390/curroncol31080326
Chicago/Turabian StyleJanzic, Urska, Natalie Maimon Rabinovich, Walid Shalata, Waleed Kian, Katarzyna Szymczak, Rafal Dziadziuszko, Marko Jakopovic, Giannis Mountzios, Adam Pluzanski, Antonio Araujo, and et al. 2024. "Non-Small-Cell Lung Cancer Patients Harboring ROS1 Rearrangement: Real World Testing Practices, Characteristics and Treatment Patterns (ROS1REAL Study)" Current Oncology 31, no. 8: 4369-4381. https://doi.org/10.3390/curroncol31080326
APA StyleJanzic, U., Maimon Rabinovich, N., Shalata, W., Kian, W., Szymczak, K., Dziadziuszko, R., Jakopovic, M., Mountzios, G., Pluzanski, A., Araujo, A., Charpidou, A., Daher, S., & Agbarya, A. (2024). Non-Small-Cell Lung Cancer Patients Harboring ROS1 Rearrangement: Real World Testing Practices, Characteristics and Treatment Patterns (ROS1REAL Study). Current Oncology, 31(8), 4369-4381. https://doi.org/10.3390/curroncol31080326








