New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices
Abstract
1. Introduction
2. The Link between High Drug Prices and High Drug Development Costs
3. Controlling Drug Prices
4. Endpoints: Problems with ICERs
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Patented Medicine Prices Review Board. Annual Report 2021. Available online: https://www.canada.ca/en/patented-medicine-prices-review/services/annual-reports/annual-report-2021.html (accessed on 18 March 2024).
- Siddiqui, M.; Rajkumar, S.V. The high cost of cancer drugs and what we can do about it. Mayo Clin. Proc. 2012, 87, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Carrera, P.M.; Kantarjian, H.M.; Blinder, V.S. The financial burden and distress of patients with cancer: Understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J. Clin. 2018, 68, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J. A Short Primer on Why Cancer Still Sucks (Chapters 3 and 11–15); Tellwell: Victoria, BC, Canada, 2022. [Google Scholar]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Rajkumar, S.V. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clin. Proc. 2015, 90, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Wouters, O.J. Lobbying Expenditures and Campaign Contributions by the Pharmaceutical and Health Product Industry in the United States, 1999–2018. JAMA Intern. Med. 2020, 180, 688–697. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Human Drug Imports 2024. Available online: https://www.fda.gov/drugs/guidance-compliance-regulatory-information/human-drug-imports (accessed on 24 April 2024).
- Labrie, Y. Is There Any Evidence That Regulating Pharmaceutical Prices Negatively Affects R&D or Access to New Medicines? A Systematic Literature Review. Canadian Health Policy 2020. Available online: https://www.researchgate.net/publication/342783080 (accessed on 21 March 2024).
- LaMattina, J. Early Impact of The Inflation Reduction Act on Drug Discovery. Forbes 2024. Available online: https://www.forbes.com/sites/johnlamattina/2024/03/06/early-impact-of-the-inflation-reduction-act-on-drug-discovery/?sh=6a12733951d0 (accessed on 23 March 2024).
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A.; Hansen, R.W.; Grabowski, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef] [PubMed]
- Schlander, M.; Hernandez-Villafuerte, K.; Cheng, C.Y.; Mestre-Ferrandiz, J.; Baumann, M. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. Pharmacoeconomics 2021, 39, 1243–1269. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, N.A.; Ellis, P. Drug development: From concept to marketing! Nephron Clin. Pract. 2009, 113, c125–c131. [Google Scholar] [CrossRef]
- Stewart, D.J.; Whitney, S.N.; Kurzrock, R. Equipoise lost: Ethics, costs, and the regulation of cancer clinical research. J. Clin. Oncol. 2010, 28, 2925–2935. [Google Scholar] [CrossRef]
- International Council for harmonisation of Technical Requirements of Pharmaceuticals for Human Use. ICH Harmonised Guideline. Good Clinical Practice. E6(R3). 2023. Available online: https://database.ich.org/sites/default/files/ICH_E6%28R3%29_DraftGuideline_2023_0519.pdf (accessed on 24 January 2024).
- DiMasi, J.A.; Grabowski, H.G. Economics of new oncology drug development. J. Clin. Oncol. 2007, 25, 209–216. [Google Scholar] [CrossRef]
- Newell, D.R.; Burtles, S.S.; Fox, B.W.; Jodrell, D.I.; Connors, T.A. Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br. J. Cancer 1999, 81, 760–768. [Google Scholar] [CrossRef]
- Newell, D.R.; Silvester, J.; McDowell, C.; Burtles, S.S.; Cancer Research, U.K. The Cancer Research UK experience of pre-clinical toxicology studies to support early clinical trials with novel cancer therapies. Eur. J. Cancer 2004, 40, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Kurzrock, R.; Kantarjian, H.; Stewart, D.J. A cancer trial scandal and its regulatory backlash. Nat. Biotechnol. 2014, 32, 27–31. [Google Scholar] [CrossRef]
- Bright, K.; Mills, A.; Bradford, J.P.; Stewart, D.J. RAPID framework for improved access to precision oncology for lethal disease: Results from a modified multi-round delphi study. Front. Health Serv. 2023, 3, 1015621. [Google Scholar] [CrossRef]
- Dilts, D.M.; Sandler, A.B.; Baker, M.; Cheng, S.K.; George, S.L.; Karas, K.S.; McGuire, S.; Menon, G.S.; Reusch, J.; Sawyer, D.; et al. Processes to activate phase III clinical trials in a Cooperative Oncology Group: The Case of Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 24, 4553–4557. [Google Scholar] [CrossRef]
- Dilts, D.M.; Cheng, S.K.; Crites, J.S.; Sandler, A.B.; Doroshow, J.H. Phase III clinical trial development: A process of chutes and ladders. Clin. Cancer Res. 2010, 16, 5381–5389. [Google Scholar] [CrossRef]
- Humphreys, K.; Trafton, J.; Wagner, T.H. The cost of institutional review board procedures in multicenter observational research. Ann. Intern. Med. 2003, 139, 77. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.E.; Hanusa, B.H.; Stone, R.A.; Ling, B.S.; Arnold, R.M. Time required for institutional review board review at one Veterans Affairs medical center. JAMA Surg. 2015, 150, 103–109. [Google Scholar] [CrossRef]
- Whitney, S.N. From Oversight to Overkill: Inside the Broken System That Blocks Medical Breakthroughs- and How We Can Fit It; Rivertowns Books: Irvington, NY, USA, 2023. [Google Scholar]
- Getz, K.A.; Stergiopoulos, S.; Short, M.; Surgeon, L.; Krauss, R.; Pretorius, S.; Desmond, J.; Dunn, D. The Impact of Protocol Amendments on Clinical Trial Performance and Cost. Ther. Innov. Regul. Sci. 2016, 50, 436–441. [Google Scholar] [CrossRef]
- Bonomi, P.; Blumenthal, G.; Ferris, A.S.; Stewart, D.J.; Selig, W.K.D.; Krug, L.M.; Allen, J.; Ison, G.; Langer, C.J.; Melemed, A.; et al. Making Lung Cancer Clinical Trials More Inclusive: Recommendations for Expanding Eligibility Criteria. J. Thorac. Oncol. 2018, 13, 748–751. [Google Scholar] [CrossRef]
- Kurzrock, R.; Stewart, D.J. Compliance in early-phase cancer clinical trials research. Oncologist 2013, 18, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Sateren, W.B.; Trimble, E.L.; Abrams, J.; Brawley, O.; Breen, N.; Ford, L.; McCabe, M.; Kaplan, R.; Smith, M.; Ungerleider, R.; et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J. Clin. Oncol. 2002, 20, 2109–2117. [Google Scholar] [CrossRef]
- Comis, R.L.; Miller, J.D.; Aldige, C.R.; Krebs, L.; Stoval, E. Public attitudes toward participation in cancer clinical trials. J. Clin. Oncol. 2003, 21, 830–835. [Google Scholar] [CrossRef]
- Global Oncology Trends 2019. The IQVIA Institute. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019 (accessed on 17 March 2024).
- Increase in Clinical Trials’ Complexicity 2001–2005 and 2011–2015. Statista. Available online: https://www.statista.com/statistics/732558/complexity-of-clinical-trials-increase/ (accessed on 23 January 2024).
- Roberts, D.A.; Kantarjian, H.M.; Steensma, D.P. Contract research organizations in oncology clinical research: Challenges and opportunities. Cancer 2016, 122, 1476–1482. [Google Scholar] [CrossRef]
- O’Leary, E.; Seow, H.; Julian, J.; Levine, M.; Pond, G.R. Data collection in cancer clinical trials: Too much of a good thing? Clin. Trials 2013, 10, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Linnell, S.K.; Stewart, D.J.; Kurzrock, R.U.S. Food and Drug Administration inspections of clinical investigators: Overview of results from 1977 to 2009. Clin. Cancer Res. 2014, 20, 3364–3370. [Google Scholar] [CrossRef]
- Roche, K.; Paul, N.; Smuck, B.; Whitehead, M.; Zee, B.; Pater, J.; Hiatt, M.A.; Walker, H. Factors affecting workload of cancer clinical trials: Results of a multicenter study of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2002, 20, 545–556. [Google Scholar] [CrossRef]
- Craft, B.S.; Kurzrock, R.; Lei, X.; Herbst, R.; Lippman, S.; Fu, S.; Karp, D.D. The changing face of phase 1 cancer clinical trials: New challenges in study requirements. Cancer 2009, 115, 1592–1597. [Google Scholar] [CrossRef]
- Wipke-Tevis, D.D.; Pickett, M.A. Impact of the Health Insurance Portability and Accountability Act on participant recruitment and retention. West. J. Nurs. Res. 2008, 30, 39–53. [Google Scholar] [CrossRef]
- Goss, E.; Link, M.P.; Bruinooge, S.S.; Lawrence, T.S.; Tepper, J.E.; Runowicz, C.D.; Schilsky, R.L. The impact of the privacy rule on cancer research: Variations in attitudes and application of regulatory standards. J. Clin. Oncol. 2009, 27, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Heyward, J.; Anderson, G.; Alexander, G.C. Variation in the estimated costs of pivotal clinical benefit trials supporting the US approval of new therapeutic agents, 2015–2017: A cross-sectional study. BMJ Open 2020, 10, e038863. [Google Scholar] [CrossRef] [PubMed]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Value of $100 from 2006 to 2016. Available online: https://www.in2013dollars.com/us/inflation/2006?endYear=2016&amount=100 (accessed on 17 March 2024).
- Stewart, D.J.; Batist, G.; Kantarjian, H.M.; Bradford, J.P.; Schiller, J.H.; Kurzrock, R. The Urgent Need for Clinical Research Reform to Permit Faster, Less Expensive Access to New Therapies for Lethal Diseases. Clin. Cancer Res. 2015, 21, 4561–4568. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J.; Avorn, J.; Kesselheim, A.S. New FDA breakthrough-drug category—Implications for patients. N. Engl. J. Med. 2014, 370, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Horning, S.J.; Haber, D.A.; Selig, W.K.; Ivy, S.P.; Roberts, S.A.; Allen, J.D.; Sigal, E.V.; Sawyers, C.L. Developing standards for breakthrough therapy designation in oncology. Clin. Cancer Res. 2013, 19, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Hudson, K.L.; Collins, F.S. The 21st Century Cures Act—A View from the NIH. N. Engl. J. Med. 2017, 376, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S. Winners and Losers of the 21st Century Cures Act. 2016. Available online: https://www.statnews.com/2016/12/05/21st-century-cures-act-winners-losers/ (accessed on 17 March 2024).
- Burris, J.F.; Puglisi, J.T. Impact of Federal Regulatory Changes on Clinical Pharmacology and Drug Development: The Common Rule and the 21st Century Cures Act. J. Clin. Pharmacol. 2018, 58, 281–285. [Google Scholar] [CrossRef]
- Biden, J. Cancer Moonshot. 2016. Available online: https://obamawhitehouse.archives.gov/sites/default/files/docs/finalvp_exec_report_10-17-16final_3.pdf (accessed on 17 March 2024).
- Stewart, D.J.; Kurzrock, R. Cancer: The road to Amiens. J. Clin. Oncol. 2009, 27, 328–333. [Google Scholar] [CrossRef]
- Stewart, D.J.; Batist, G. Redefining cancer: A new paradigm for better and faster treatment innovation. J. Popul. Ther. Clin. Pharmacol. 2014, 21, e56–e65. [Google Scholar]
- Pinnow, C.L. Lessons from the Pandemic: We need Increased Collaboration between the Biopharmaceutical Sector and Government. National Newswatch. 20 November 2020. Available online: https://www.nationalnewswatch.com/2020/11/25/lessons-from-the-pandemic-a-call-to-action-for-increased-collaboration-between-the-canadian-biopharmaceutical-sector-and-government-for-all-innovative-products/ (accessed on 7 February 2024).
- Hilton, J.; Mazzarello, S.; Fergusson, D.; Joy, A.A.; Robinson, A.; Arnaout, A.; Hutton, B.; Vandermeer, L.; Clemons, M. Novel Methodology for Comparing Standard-of-Care Interventions in Patients with Cancer. J. Oncol. Pract. 2016, 12, e1016–e1024. [Google Scholar] [CrossRef]
- Stewart, D.J.; Bosse, D.; Goss, G.; Hilton, J.F.; Jonker, D.; Fung-Kee-Fung, M. A novel, more reliable approach to use of progression-free survival as a predictor of gain in overall survival: The Ottawa PFS Predictive Model. Crit. Rev. Oncol. Hematol. 2020, 148, 102896. [Google Scholar] [CrossRef]
- European Medicines Agency. Report on Budgetary and Financial Management. Financial Year 2018. 2019. Available online: https://www.europarl.europa.eu/cmsdata/185228/Report%20on%20Budg%20and%20financial%20management.pdf#:~:text=Authorised%20appropriations%20in%20the%20European%20Medicines%20Agency%E2%80%99s%20initial,so%20the%20final%20budget%20remained%20at%20EUR%20337%2C761%2C000 (accessed on 23 January 2024).
- FY 2022 FDA Budget Request. 2022. Available online: https://www.fda.gov/media/149613/download?attachment (accessed on 23 January 2024).
- 20 Pros and Cons of Zero Tolerance Policy. Ablison. Available online: https://www.ablison.com/pros-and-cons-of-zero-tolerance-policy/ (accessed on 13 January 2024).
- Bell, C. The Hidden Side of Zero Tolerance Policies: The African American Perspective. Sociol. Compass 2015, 9, 14–22. [Google Scholar] [CrossRef]
- Juvenile Law Center. Zero-Tolerance Policies: The Good, the Bad, and the Ugly. 2014. Available online: https://jlc.org/news/zero-tolerance-policies-good-bad-and-ugly (accessed on 23 January 2024).
- Stewart, D.J.; Stewart, A.A.; Wheatley-Price, P.; Batist, G.; Kantarjian, H.M.; Schiller, J.; Clemons, M.; Bradford, J.P.; Gillespie, L.; Kurzrock, R. The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018, 7, 1824–1836. [Google Scholar] [CrossRef]
- Broder, S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010, 85, 1–18. [Google Scholar] [CrossRef]
- Bok, K.; Sitar, S.; Graham, B.S.; Mascola, J.R. Accelerated COVID-19 vaccine development: Milestones, lessons, and prospects. Immunity 2021, 54, 1636–1651. [Google Scholar] [CrossRef]
- Crimp, D. Before Occupy: How AIDS Activists Seized Control of the FDA in 1988. The Atlantic 2011. Available online: https://www.theatlantic.com/health/archive/2011/12/before-occupy-how-aids-activists-seized-control-of-the-fda-in-1988/249302/ (accessed on 5 February 2024).
- Pronker, E.S.; Weenen, T.C.; Commandeur, H.; Claassen, E.H.; Osterhaus, A.D. Risk in vaccine research and development quantified. PLoS ONE 2013, 8, e57755. [Google Scholar] [CrossRef]
- Roos, D. How a New Vaccine Was Developed in Record Time in the 1960s. 2023. Available online: https://www.history.com/news/mumps-vaccine-world-war-ii (accessed on 6 February 2024).
- CanCertainty. Equal and Fair Cancer Treatment for All. Available online: https://www.cancertaintyforall.ca/ (accessed on 6 February 2024).
- Slevin, M.L.; Stubbs, L.; Plant, H.J.; Wilson, P.; Gregory, W.M.; Armes, P.J.; Downer, S.M. Attitudes to chemotherapy: Comparing views of patients with cancer with those of doctors, nurses, and general public. BMJ 1990, 300, 1458–1460. [Google Scholar] [CrossRef]
- Carr, J. Wage and Price Controls: Panacea for Inflation or Prescription for Disaster? The Fraser Institute. 1976. Available online: https://www.fraserinstitute.org/sites/default/files/wage-and-price-controls.pdf (accessed on 24 January 2024).
- Lakdawalla, D.N.; Doshi, J.A.; Garrison, L.P., Jr.; Phelps, C.E.; Basu, A.; Danzon, P.M. Defining Elements of Value in Health Care-A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value Health 2018, 21, 131–139. [Google Scholar] [CrossRef]
- Glaus, C.E.G.; Kloeti, A.; Vokinger, K.N. Defining ‘therapeutic value’ of medicines: A scoping review. BMJ Open 2023, 13, e078134. [Google Scholar] [CrossRef]
- Terkola, R.; Antonanzas, F.; Postma, M. Stakeholder opinions on value in healthcare. Eur. J. Hosp. Pharm. 2019, 26, 79–84. [Google Scholar] [CrossRef]
- Antonanzas, F.; Terkola, R.; Postma, M. The Value of Medicines: A Crucial but Vague Concept. Pharmacoeconomics 2016, 34, 1227–1239. [Google Scholar] [CrossRef]
- Gordon, J.; Stainthorpe, A.; Jones, B.; Jacob, I.; Hertel, N.; Diaz, J.; Yuan, Y.; Borrill, J. Non-Price-Related Determinants of Value and Access for Novel Non-small Cell Lung Cancer Treatments: A Cross-Country Review of HTA Decision Making. Pharmacoecon Open 2021, 5, 701–713. [Google Scholar] [CrossRef]
- Allen, N.; Walker, S.R.; Liberti, L.; Salek, S. Health Technology Assessment (HTA) Case Studies: Factors Influencing Divergent HTA Reimbursement Recommendations in Australia, Canada, England, and Scotland. Value Health 2017, 20, 320–328. [Google Scholar] [CrossRef]
- Nabhan, C.; Feinberg, B.A. Value-Based Calculators in Cancer: Current State and Challenges. J. Oncol. Pract. 2017, 13, 499–506. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Davidson, N.E.; Wollins, D.S.; Blayney, D.W.; Dicker, A.P.; Ganz, P.A.; Hoverman, J.R.; Langdon, R.; Lyman, G.H.; Meropol, N.J.; et al. Updating the American Society of Clinical Oncology Value Framework: Revisions and Reflections in Response to Comments Received. J. Clin. Oncol. 2016, 34, 2925–2934. [Google Scholar] [CrossRef]
- Beresniak, A.; Dupont, D. Is there an alternative to quality-adjusted life years for supporting healthcare decision making? Expert Rev. Pharmacoecon. Outcomes Res. 2016, 16, 351–357. [Google Scholar] [CrossRef]
- Rand, L.Z.; Melendez-Torres, G.J.; Kesselheim, A.S. Alternatives to the quality-adjusted life year: How well do they address common criticisms? Health Serv. Res. 2023, 58, 433–444. [Google Scholar] [CrossRef]
- Campbell, J.D.; Whittington, M.D.; Pearson, S.D. An Alternative Measure of Health for Value Assessment: The Equal Value Life-Year. Pharmacoeconomics 2023, 41, 1175–1182. [Google Scholar] [CrossRef]
- Turner, H.C.; Archer, R.A.; Downey, L.E.; Isaranuwatchai, W.; Chalkidou, K.; Jit, M.; Teerawattananon, Y. An Introduction to the Main Types of Economic Evaluations Used for Informing Priority Setting and Resource Allocation in Healthcare: Key Features, Uses, and Limitations. Front. Public Health 2021, 9, 722927. [Google Scholar] [CrossRef]
- Simoens, S. How to assess the value of medicines? Front. Pharmacol. 2010, 1, 115. [Google Scholar] [CrossRef]
- Binder, L.; Ghadban, M.; Sit, C.; Barnard, K. Health Technology Assessment Process for Oncology Drugs: Impact of CADTH Changes on Public Payer Reimbursement Recommendations. Curr. Oncol. 2022, 29, 1514–1526. [Google Scholar] [CrossRef]
- Beresniak, A.; Medina-Lara, A.; Auray, J.P.; De Wever, A.; Praet, J.C.; Tarricone, R.; Torbica, A.; Dupont, D.; Lamure, M.; Duru, G. Validation of the underlying assumptions of the quality-adjusted life-years outcome: Results from the ECHOUTCOME European project. Pharmacoeconomics 2015, 33, 61–69. [Google Scholar] [CrossRef]
- Bobinac, A.; Van Exel, N.J.; Rutten, F.F.; Brouwer, W.B. Willingness to pay for a quality-adjusted life-year: The individual perspective. Value Health 2010, 13, 1046–1055. [Google Scholar] [CrossRef]
- Nimdet, K.; Chaiyakunapruk, N.; Vichansavakul, K.; Ngorsuraches, S. A systematic review of studies eliciting willingness-to-pay per quality-adjusted life year: Does it justify CE threshold? PLoS ONE 2015, 10, e0122760. [Google Scholar] [CrossRef]
- Gyrd-Hansen, D. Willingness to pay for a QALY: Theoretical and methodological issues. Pharmacoeconomics 2005, 23, 423–432. [Google Scholar] [CrossRef]
- Antonanzas, F.; Terkola, R.; Overton, P.M.; Shalet, N.; Postma, M. Defining and Measuring the Affordability of New Medicines: A Systematic Review. Pharmacoeconomics 2017, 35, 777–791. [Google Scholar] [CrossRef]
- Skinner, B.J. New Cancer Drugs in Canada 2012 to 2021: An Economic Analysis of Cost, Benefit, Availability, and Public Insurance Coverage. Canadian Health Policy 2023. Available online: https://www.canadianhealthpolicy.com/product/new-cancer-drugs-in-canada-2012-to-2021-an-economic-analysis-of-cost-benefit-availability-and-public-insurance-coverage/ (accessed on 11 February 2024).
- OECD Data: Hospital Beds. 2022. Available online: https://data.oecd.org/healtheqt/hospital-beds.htm#indicator-chart (accessed on 29 January 2024).
- Statistics Canada. Demographic Estimates by Age and Sex, Provinces and Territories. 2023. Available online: https://www150.statcan.gc.ca/n1/pub/71-607-x/71-607-x2020018-eng.htm (accessed on 29 January 2024).
- Vreman, R.A.; Strigkos, G.; Leufkens, H.G.M.; Schunemann, H.J.; Mantel-Teeuwisse, A.K.; Goettsch, W.G. Addressing uncertainty in relative effectiveness assessments by HTA organizations. Int. J. Technol. Assess. Health Care 2022, 38, e17. [Google Scholar] [CrossRef]
- Bloem, L.T.; Vreman, R.A.; Peeters, N.W.L.; Hoekman, J.; van der Elst, M.E.; Leufkens, H.G.M.; Klungel, O.H.; Goettsch, W.G.; Mantel-Teeuwisse, A.K. Associations between uncertainties identified by the European Medicines Agency and national decision making on reimbursement by HTA agencies. Clin. Transl. Sci. 2021, 14, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Barr, H.K.; Guggenbickler, A.M.; Hoch, J.S.; Dewa, C.S. Real-World Cost-Effectiveness Analysis: How Much Uncertainty Is in the Results? Curr. Oncol. 2023, 30, 4078–4093. [Google Scholar] [CrossRef] [PubMed]
- Wakutsu, N.; Hirose, E.; Yonemoto, N.; Demiya, S. Assessing Definitions and Incentives Adopted for Innovation for Pharmaceutical Products in Five High-Income Countries: A Systematic Literature Review. Pharmaceut Med. 2023, 37, 53–70. [Google Scholar] [CrossRef]
- Rejon-Parrilla, J.C.; Espin, J.; Epstein, D. How innovation can be defined, evaluated and rewarded in health technology assessment. Health Econ. Rev. 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- de Sola-Morales, O.; Cunningham, D.; Flume, M.; Overton, P.M.; Shalet, N.; Capri, S. Defining Innovation with Respect to New Medicines: A Systematic Review from a Payer Perspective. Int. J. Technol. Assess. Health Care 2018, 34, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Synnott, P.G.; Voehler, D.; Enright, D.E.; Kowal, S.; Ollendorf, D.A. The Value of New: Consideration of Product Novelty in Health Technology Assessments of Pharmaceuticals. Appl. Health Econ. Health Policy 2023, 21, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Fojo, T.; Mailankody, S.; Lo, A. Unintended consequences of expensive cancer therapeutics-the pursuit of marginal indications and a me-too mentality that stifles innovation and creativity: The John Conley Lecture. JAMA Otolaryngol. Head. Neck Surg. 2014, 140, 1225–1236. [Google Scholar] [CrossRef]
- Hughes-Wilson, W.; Palma, A.; Schuurman, A.; Simoens, S. Paying for the Orphan Drug System: Break or bend? Is it time for a new evaluation system for payers in Europe to take account of new rare disease treatments? Orphanet J. Rare Dis. 2012, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Postma, M.J.; Noone, D.; Rozenbaum, M.H.; Carter, J.A.; Botteman, M.F.; Fenwick, E.; Garrison, L.P. Assessing the value of orphan drugs using conventional cost-effectiveness analysis: Is it fit for purpose? Orphanet J. Rare Dis. 2022, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Berdud, M.; Drummond, M.; Towse, A. Establishing a reasonable price for an orphan drug. Cost. Eff. Resour. Alloc. 2020, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Adkins, E.M.; Nicholson, L.; Floyd, D.; Ratcliffe, M.; Chevrou-Severac, H. Oncology drugs for orphan indications: How are HTA processes evolving for this specific drug category? Clinicoecon Outcomes Res. 2017, 9, 327–342. [Google Scholar] [CrossRef]
- Gronde, T.V.; Uyl-de Groot, C.A.; Pieters, T. Addressing the challenge of high-priced prescription drugs in the era of precision medicine: A systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks. PLoS ONE 2017, 12, e0182613. [Google Scholar] [CrossRef]
- Cohen, B.L. Society’s valuation of life saving in radiation protection and other contexts. Health Phys. 1980, 38, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Bussgen, M.; Stargardt, T. Does health technology assessment compromise access to pharmaceuticals? Eur. J. Health Econ. 2023, 24, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Cowling, T.; Nayakarathna, R.; Wills, A.L.; Tankala, D.; Paul Roc, N.; Barakat, S. Early access for innovative oncology medicines: A different story in each nation. J. Med. Econ. 2023, 26, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.Y.; Cameron, C.; Mitha, A.; Wills, A. Building infrastructure for outcomes-based agreements in Canada: Can administrative health data be used to support an outcomes-based agreement in oncology? Support. Care Cancer 2022, 31, 5. [Google Scholar] [CrossRef] [PubMed]
- Sehdev, S.R.; Rawson, N.S.B.; Aseyev, O.I.; Buick, C.J.; Butler, M.O.; Edwards, S.; Gill, S.; Gotfrit, J.M.; Hsia, C.C.; Juergens, R.A.; et al. Access to Oncology Medicines in Canada: Consensus Forum for Recommendations for Improvement. Curr. Oncol. 2024, 31, 1803–1816. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Chabot, I.; Glennie, J. Evolution of health technology assessment: Best practices of the pan-Canadian Oncology Drug Review. Clinicoecon. Outcomes Res. 2015, 7, 287–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gotfrit, J.; Dempster, W.; Chambers, J.; Wheatley-Price, P. The Pathway for New Cancer Drug Access in Canada. Curr. Oncol. 2022, 29, 455–464. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.; Charles, C.; Elit, L.; Gafni, A. Is there an economic rationale for cancer drugs to have a separate reimbursement review process for resource allocation purposes? Pharmacoeconomics 2015, 33, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.R.; Mai, H.; Trudeau, M.E.; Mittmann, N.; Chiasson, K.; Chan, K.K.W.; Cheung, M.C. Reimbursement recommendations for cancer drugs supported by phase II evidence in Canada. Curr. Oncol. 2020, 27, e495–e500. [Google Scholar] [CrossRef]
- CADTH Reimbursement Recommendation: Tepotinib (Tepmetko). 2022. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0255%20Tepmetko%20-%20CADTH%20Final%20Recommendation%20Final.pdf#:~:text=The%20CADTH%20pan-Canadian%20Oncology%20Drug%20Review%20Expert%20Review,unresectable%20or%20metastatic%20NSCLC%20harbouring%20METex14%20skipping%20alterations (accessed on 12 February 2024).
- CADTH Reimbursement Recommendation. Pemigatinib (Pemazyre). 2022. Available online: https://www.cadth.ca/sites/default/files/DRR/2022/PC0252%20Pemazyre%20-%20CADTH%20Final%20Rec.pdf (accessed on 12 February 2024).
- Skedgel, C.; Wranik, D.; Hu, M. The Relative Importance of Clinical, Economic, Patient Values and Feasibility Criteria in Cancer Drug Reimbursement in Canada: A Revealed Preferences Analysis of Recommendations of the Pan-Canadian Oncology Drug Review 2011–2017. Pharmacoeconomics 2018, 36, 467–475. [Google Scholar] [CrossRef]
- CPI Inflation Calculator. Value of $1 from 1984 to 2024. 2024. Available online: https://www.in2013dollars.com/us/inflation/1984?amount=1#:~:text=%241%20in%201984%20is%20equivalent%20in%20purchasing%20power,today%2C%20an%20increase%20of%20%241.95%20over%2040%20years (accessed on 25 January 2024).
- Macrotrends. Canada GDP Per Capita 1960–2024. 2022. Available online: https://www.macrotrends.net/countries/CAN/canada/gdp-per-capita#:~:text=Canada%20gdp%20per%20capita%20for%202022%20was%20%2454%2C966%2C,2019%20was%20%2446%2C374%2C%20a%200.37%25%20decline%20from%202018 (accessed on 28 January 2024).
- The Canadian Dollar Has Lost 30% Its Value Since 2007. 2024. Available online: https://www.in2013dollars.com/canada/inflation/2007#:~:text=The%20Canadian%20dollar%20has%20lost%2030%25%20its%20value,today%2C%20an%20increase%20of%20%2442.49%20over%2017%20years (accessed on 12 February 2024).
- Simoens, S.; Huys, I. How much do the public sector and the private sector contribute to biopharmaceutical R&D? Drug Discov. Today 2022, 27, 939–945. [Google Scholar] [CrossRef]
- Ledley, F.D.; McCoy, S.S.; Vaughan, G.; Cleary, E.G. Profitability of Large Pharmaceutical Companies Compared with Other Large Public Companies. JAMA 2020, 323, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Batta, A.; Kalra, B.S.; Khirasaria, R. Trends in FDA drug approvals over last 2 decades: An observational study. J. Fam. Med. Prim. Care 2020, 9, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Fees Payable to the European Medicines Agency. 2023. Available online: https://www.ema.europa.eu/en/about-us/fees-payable-european-medicines-agency (accessed on 29 January 2024).
- Dickson, M.; Gagnon, J.P. The cost of new drug discovery and development. Discov. Med. 2004, 4, 172–179. [Google Scholar]
- Samuel, N.; Verma, S. Cross-comparison of cancer drug approvals at three international regulatory agencies. Curr. Oncol. 2016, 23, e454–e460. [Google Scholar] [CrossRef]
- Roth, L.K. Prescription Drug User Fee Rates for Fiscal Year 2023. Federl Register 2022. Available online: https://www.federalregister.gov/documents/2022/10/07/2022-21968/prescription-drug-user-fee-rates-for-fiscal-year-2023#:~:text=This%20document%20provides%20fee%20rates%20for%20FY%202023,will%20remain%20in%20effect%20through%20September%2030%2C%202023 (accessed on 22 April 2024).
- Kagan, J. European Medicines Agency (EMA): Meaning and Examples. Investopedia. 2023. Available online: https://www.investopedia.com/terms/e/european-medicines-agency-ema.asp (accessed on 29 January 2024).
- Spicer, O.; Grootendorst, P. An Empirical Examination of the Patented Medicine Prices Review Board Price Control Amendments on Drug Launches in Canada. Canadian Centre for Health Economics 2020. Working Paper No. 200003. Available online: https://www.canadiancentreforhealtheconomics.ca/wp-content/uploads/2020/08/Spicer-Grootendorst-2020.pdf (accessed on 29 January 2024).
- Government of Canada: Pharmaceutical Industry Profile. 2024. Available online: https://ised-isde.canada.ca/site/canadian-life-science-industries/en/biopharmaceuticals-and-pharmaceuticals/pharmaceutical-industry-profile (accessed on 23 April 2024).
- Government of Canada. Fees for Health Canada. 2023. Available online: https://www.canada.ca/en/health-canada/services/funding/fees-health-canada.html#a1.1 (accessed on 29 January 2024).
- Government of Canada. Fact Sheet: Cancer in Canada. 2019. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/fact-sheet-cancer-canada.html (accessed on 23 April 2024).
- OECD Data: Pharmaceutical Spending. 2024. Available online: https://data.oecd.org/healthres/pharmaceutical-spending.htm#indicator-chart (accessed on 18 March 2024).
- Patented Medicine Prices Review Board. New PMPRB Guidelines. 2020. Available online: https://www.canada.ca/content/dam/pmprb-cepmb/documents/legislation/guidelines/PMPRB%20final%20Guidelines%20-%20Public%20Webinar%20Deck%20November-20-2020-EN.pdf (accessed on 29 January 2024).
- Revised PMPRB Guidelines. Overview of Key Changes. Public Webinar. Patented Medicine Prices Review Board 2020/07/08. Slide 43. 2020. Available online: https://www.canada.ca/content/dam/pmprb-cepmb/documents/consultations/draft-guidelines/2020/PMPRB-Public-Webinar-July8-2020.pdf (accessed on 29 January 2024).
- Government of Canada. Project Orbis. 2022. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/international-activities/project-orbis.html (accessed on 6 February 2024).
- Government of Canada. Regulatory Innovation for Health Products: Agile Licensing for Drugs. 2023. Available online: https://www.canada.ca/en/health-canada/corporate/about-health-canada/activities-responsibilities/strategies-initiatives/health-products-food-regulatory-modernization/agile-licensing-drugs.html (accessed on 11 February 2024).
- Bellehumeur, C. Increasing Access to Innovative Medicines. Innovative Medicines Canada. 2022. Available online: https://innovativemedicines.ca/wp-content/uploads/2022/10/20221007_FINAL_PreBudget_Consultation-1.pdf (accessed on 29 January 2024).
- The Conference Boad of Canada. Access and Time to Patient Prescription Drugs in Canada. 2024. Available online: https://www.bing.com/search?q=conference+board+of+Canada+access+and+time&form=ANNH02&refig=cbeacb6f6df141b6893867f9d4acb0c9&pc=LCTS (accessed on 11 February 2024).
- Gotfrit, J.; Shin, J.J.W.; Mallick, R.; Stewart, D.J.; Wheatley-Price, P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncologist 2020, 25, e130–e137. [Google Scholar] [CrossRef]
- MacPhail, C.; Snow, S. Not All Canadian Cancer Patients Are Equal-Disparities in Public Cancer Drug Funding across Canada. Curr. Oncol. 2022, 29, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Surge in Medically Assisted Deaths under Canada’s MAID Program Outpaces Every Other Country. Toronto Star 2024. Available online: https://www.thestar.com/news/investigations/surge-in-medically-assisted-deaths-under-canada-s-maid-program-outpaces-every-other-country/article_29028f96-bc6b-11ee-8f67-03bf29ac7d34.html (accessed on 29 January 2024).
- Trachtenberg, A.J.; Manns, B. Cost analysis of medical assistance in dying in Canada. CMAJ 2017, 189, E101–E105. [Google Scholar] [CrossRef]
- Booth, C.M.; Eisenhauer, E.A.; Gyawali, B.; Tannock, I.F. Progression-Free Survival Should Not Be Used as a Primary End Point for Registration of Anticancer Drugs. J. Clin. Oncol. 2023, 41, 4968–4972. [Google Scholar] [CrossRef]
- Garassino, M.C.; Gadgeel, S.; Speranza, G.; Felip, E.; Esteban, E.; Domine, M.; Hochmair, M.J.; Powell, S.F.; Bischoff, H.G.; Peled, N.; et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes from the Phase 3 KEYNOTE-189 Study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, R.; Ahuja, A.; Ademisoye, E.; Juarez-Garcia, A.; Shaw, J.W. Relative value assessment: Characterizing the benefit of oncology therapies through diverse survival metrics from a US perspective. Clinicoecon. Outcomes Res. 2019, 11, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Karweit, J.; Kotapati, S.; Wagner, S.; Shaw, J.W.; Wolfe, S.W.; Abernethy, A.P. An expanded portfolio of survival metrics for assessing anticancer agents. Am. J. Manag. Care 2017, 23, 54–61. [Google Scholar] [PubMed]
- Shafrin, J.; Schwartz, T.T.; Okoro, T.; Romley, J.A. Patient Versus Physician Valuation of Durable Survival Gains: Implications for Value Framework Assessments. Value Health 2017, 20, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Cole, K.; Bosse, D.; Brule, S.; Fergusson, D.; Ramsay, T. Population Survival Kinetics Derived from Clinical Trials of Potentially Curable Lung Cancers. Curr. Oncol. 2024, 31, 1600–1617. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.M.; Sengar, M.; Goodman, A.; Wilson, B.; Aggarwal, A.; Berry, S.; Collingridge, D.; Denburg, A.; Eisenhauer, E.A.; Ginsburg, O.; et al. Common Sense Oncology: Outcomes that matter. Lancet Oncol. 2023, 24, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Kesselheim, A.S.; Ross, J.S. The Accelerated Approval Program for Cancer Drugs–Finding the Right Balance. N. Engl. J. Med. 2023, 389, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R. The arrangement of field experiments. J. Minist. Agric. Great Br. 1926, 33, 503–513. [Google Scholar]
- Ziliak, S.T.; McCloskey, D.N. The Cult of Statistical Significance: How the Standard Error Costs Us Jobs, Justice, and Lives; The University of Michigan Press: Ann Arbor, MI, USA, 2011. [Google Scholar]
- Wasserstein, R.L.; Lazar, N.A. The ASA statement on p-values: Context, process, and purpose. Am. Statist. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Senn, S. Contribution to the discussion of “A critical evaluation of the current p-value controversy”. Biom. J. 2017, 59, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Morgenstern, H. Misconceptions, Misuses, and Misinterpretations of P Values and Significance Testing. J. Bone Jt. Surg. Am. 2017, 99, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Poole, C.; Kuss, O. The ongoing tyranny of statistical significance testing in biomedical research. Eur. J. Epidemiol. 2010, 25, 225–230. [Google Scholar] [CrossRef] [PubMed]
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N. Engl. J. Med. 1993, 329, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, A.; D’Agostino, R.B., Sr. Multiplicity Considerations in Clinical Trials. N. Engl. J. Med. 2018, 378, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csoszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Wears, R.L. An introduction to the Bayesian analysis of clinical trials. Ann. Emerg. Med. 1993, 22, 1328–1336. [Google Scholar] [CrossRef]
- Del Paggio, J.C.; Berry, J.S.; Hopman, W.M.; Eisenhauer, E.A.; Prasad, V.; Gyawali, B.; Booth, C.M. Evolution of the Randomized Clinical Trial in the Era of Precision Oncology. JAMA Oncol. 2021, 7, 728–734. [Google Scholar] [CrossRef]
- Broglio, K.R.; Berry, D.A. Detecting an overall survival benefit that is derived from progression-free survival. J. Natl. Cancer Inst. 2009, 101, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Ramsay, T.; Navani, V.; Liu, G.; Jiang, D.M.; Batist, G. Progression-Free Survival Gain: A Reliable Primary End Point for Drug Registration That Can Accelerate Patient Access to Urgently Needed Therapies. J. Clin. Oncol. 2024, 42, 973–974. [Google Scholar] [CrossRef]
- Stewart, D.J.; Kurzrock, R. Fool’s gold, lost treasures, and the randomized clinical trial. BMC Cancer 2013, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, T.; Wilkerson, J.; Simon, R.; Bates, S.E.; Fojo, T. Stable disease is not preferentially observed with targeted therapies and as currently defined has limited value in drug development. Cancer J. 2009, 15, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, P.; Del Mastro, L.; Sormani, M.P.; Bastholt, L.; Danova, M.; Focan, C.; Fountzilas, G.; Paul, J.; Rosso, R.; Venturini, M. Objective response to chemotherapy as a potential surrogate end point of survival in metastatic breast cancer patients. J. Clin. Oncol. 2005, 23, 5117–5125. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghi, R.H.; Eisenhauer, E.A. Review of phase II trial designs used in studies of molecular targeted agents: Outcomes and predictors of success in phase III. J. Clin. Oncol. 2008, 26, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Braiteh, F.; Stewart, D.J.; Kurzrock, R. Ultimate fate of oncology drugs approved by the us food and drug administration without a randomized Trial. J. Clin. Oncol. 2009, 27, 6243–6250. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Zhao, B.; Sima, C.S.; Ginsberg, M.S.; James, L.P.; Lefkowitz, R.A.; Guo, P.; Kris, M.G.; Schwartz, L.H.; Riely, G.J. Variability of lung tumor measurements on repeat computed tomography scans taken within 15 minutes. J. Clin. Oncol. 2011, 29, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Blome, C.; Augustin, M. Measuring change in quality of life: Bias in prospective and retrospective evaluation. Value Health 2015, 18, 110–115. [Google Scholar] [CrossRef]
- Mooney, A. Quality of life: Questionnaires and questions. J. Health Commun. 2006, 11, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Haraldstad, K.; Wahl, A.; Andenaes, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Pequeno, N.P.F.; Cabral, N.L.A.; Marchioni, D.M.; Lima, S.; Lyra, C.O. Quality of life assessment instruments for adults: A systematic review of population-based studies. Health Qual. Life Outcomes 2020, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Wheatley-Price, P. Randomized Clinical Trials in the Era of Precision Oncology-The Role of End Points, Industry Funding, and Medical Writing Integrity. JAMA Oncol. 2021, 7, 1577–1578. [Google Scholar] [CrossRef]
- Farah, E.; Kenney, M.; Kica, A.; Haddad, P.; Stewart, D.J.; Bradford, J.P. Beyond Participation: Evaluating the Role of Patients in Designing Oncology Clinical Trials. Curr. Oncol. 2023, 30, 8310–8327. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.J.; Bosse, D.; Robinson, A.; Ong, M.; Fung-Kee-Fung, M.; Brule, S.; Hilton, J.F.; Ocana, A. Potential insights from population kinetic assessment of progression-free survival curves. Crit. Rev. Oncol. Hematol. 2020, 153, 103039. [Google Scholar] [CrossRef] [PubMed]
- Wedam, S.; Fashoyin-Aje, L.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; Theoret, M.R.; Amiri-Kordestani, L.; Pazdur, R.; Beaver, J.A. FDA Approval Summary: Palbociclib for Male Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2020, 26, 1208–1212. [Google Scholar] [CrossRef]
- Kim, T.E.; Park, S.I.; Shin, K.H. Incorporation of real-world data to a clinical trial: Use of external controls. Transl. Clin. Pharmacol. 2022, 30, 121–128. [Google Scholar] [CrossRef]
- Advancing Cancer Therapy. Nat. Cancer 2021, 2, 245–246. [CrossRef]
- Healthcare Resources: Medical Technology. OECD Stat. 2024. Available online: https://stats.oecd.org/Index.aspx?DataSetCode=HEALTH_REAC (accessed on 23 April 2024).
- Radiotherapy Equipment. OECD Data 2024. Available online: https://data.oecd.org/healtheqt/radiotherapy-equipment.htm#indicator-chart (accessed on 23 April 2024).
- Computed Tomography (CT) Scanners. OECD Data 2024. Available online: https://data.oecd.org/healtheqt/computed-tomography-ct-scanners.htm#indicator-chart (accessed on 23 April 2024).
- Magentic Resonance Imaging (MRI) Units. OECD Data 2024. Available online: https://data.oecd.org/healtheqt/magnetic-resonance-imaging-mri-units.htm#indicator-chart (accessed on 23 April 2024).
| Issue | Recommendations |
|---|---|
| Issues with Clinical Research Oversight: | |
| There are too many impediments to rapid progress against lethal diseases. | We need revised oversight of clinical research for lethal diseases that will:
|
| Preclinical toxicology requirements are excessive. | We only need LD10 in rodents (i.e., the drug dose that kills 10% of rodents). |
| Study review and activation processes are too slow and inefficient. | We need:
|
| Trial eligibility criteria are irrationally rigid. | We need to:
|
| Requiring an accredited test for molecular screening/selection for a clinical trial can greatly delay trial activation and escalate costs (if test is not already active and accredited). | Permit screening using a research lab test, with later refinement/accreditation of the test if it is determined to predict therapy benefit. |
| Regulatory burden of running a trial is excessive. |
|
| Trial data collection requirements are expensive, burdensome, wasteful, and excessive. | Rationalize data collection. |
| We cannot change clinical research regulation locally since it is “harmonized” internationally. | We need rapid international collaboration on making research regulation much more efficient, much less costly, and much more progress-centered for lethal diseases. |
| General issues with delayed access to new therapies in Canada: | |
| Companies delay applying to Health Canada for drug marketing approval. | Generally, Canada should approve a drug for a lethal disease as soon as the US FDA or the EMA approves it (e.g., through Project Orbis) without further extensive review. |
| Public funding of new drugs takes much too long in Canada. | Canada needs:
|
| Issues with ICER and QALY calculation and value in Canada | |
| General factors that are considered in calculating an ICER and/or the value of a QALY for a new therapy need to be updated. | For ICER and QALY calculations for therapies for lethal diseases, we need:
|
| CADTH assigns the same value for a QALY to all disease types. | For lethal diseases (and particularly for uncommon malignancy subtypes and rare or orphan diseases), the upper value assigned per QALY should be at the higher end of either 3 times Canada’s GDP per capita or the upper limit of the Canadian government’s “Value per Statistical Life-Year”. |
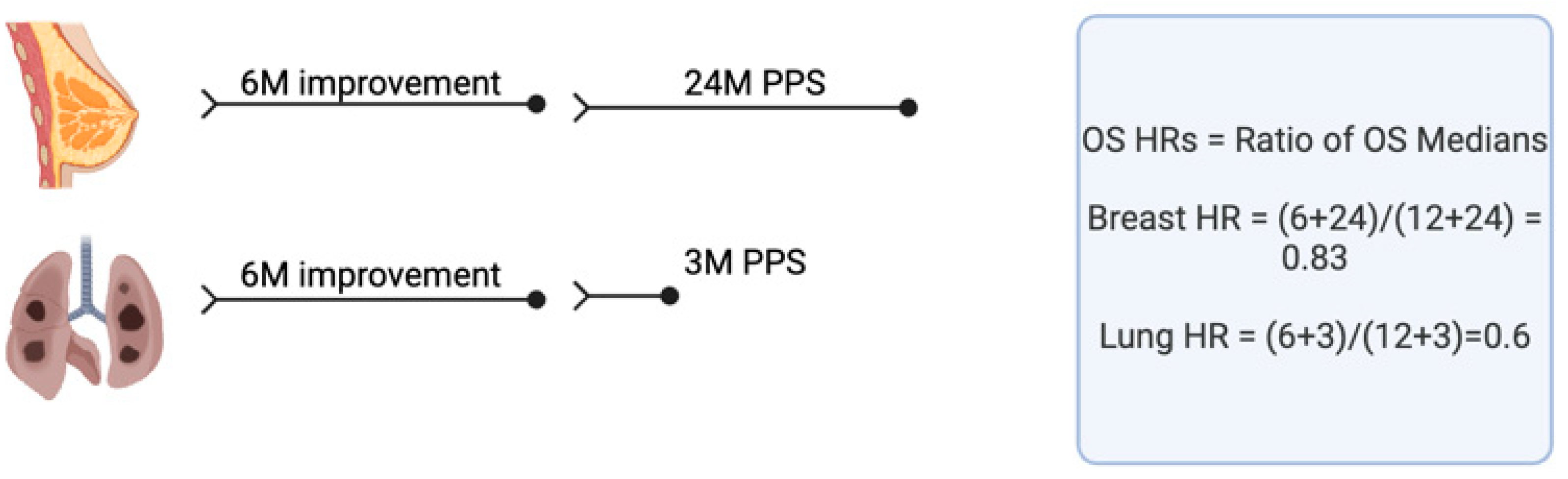
| Different trial endpoints are not adequately considered in calculating QALYs, ICERs and therapy efficacy and value. | In calculating QALYs, ICERs and therapy efficacy and value, we should consider:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, D.J.; Bradford, J.-P.; Sehdev, S.; Ramsay, T.; Navani, V.; Rawson, N.S.B.; Jiang, D.M.; Gotfrit, J.; Wheatley-Price, P.; Liu, G.; et al. New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices. Curr. Oncol. 2024, 31, 2453-2480. https://doi.org/10.3390/curroncol31050184
Stewart DJ, Bradford J-P, Sehdev S, Ramsay T, Navani V, Rawson NSB, Jiang DM, Gotfrit J, Wheatley-Price P, Liu G, et al. New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices. Current Oncology. 2024; 31(5):2453-2480. https://doi.org/10.3390/curroncol31050184
Chicago/Turabian StyleStewart, David J., John-Peter Bradford, Sandeep Sehdev, Tim Ramsay, Vishal Navani, Nigel S. B. Rawson, Di Maria Jiang, Joanna Gotfrit, Paul Wheatley-Price, Geoffrey Liu, and et al. 2024. "New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices" Current Oncology 31, no. 5: 2453-2480. https://doi.org/10.3390/curroncol31050184
APA StyleStewart, D. J., Bradford, J.-P., Sehdev, S., Ramsay, T., Navani, V., Rawson, N. S. B., Jiang, D. M., Gotfrit, J., Wheatley-Price, P., Liu, G., Kaplan, A., Spadafora, S., Goodman, S. G., Auer, R. A. C., & Batist, G. (2024). New Anticancer Drugs: Reliably Assessing “Value” While Addressing High Prices. Current Oncology, 31(5), 2453-2480. https://doi.org/10.3390/curroncol31050184







