The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 2018, 78, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.; Schmults, C.D. Management of high-risk cutaneous squamous cell carcinoma. J. Clin. Aesthetic Dermatol. 2010, 3, 39–48. [Google Scholar]
- Knatko, E.V.; Praslicka, B.; Higgins, M.; Evans, A.; Purdie, K.J.; Harwood, C.A.; Proby, C.M.; Ooi, A.; Dinkova-Kostova, A.T. Whole-Exome Sequencing Validates a Preclinical Mouse Model for the Prevention and Treatment of Cutaneous Squamous Cell Carcinoma. Cancer Prev. Res. 2017, 10, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khachemoune, A. Reappraising basosquamous carcinoma: A summary of histologic features, diagnosis, and treatment. Arch. Dermatol. Res. 2020, 312, 605–609. [Google Scholar] [CrossRef]
- Organisation Mondiale de la Santé, Centre International de Recherche sur le Cancer (Ed.) WHO Classification of Skin Tumors, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Wermker, K.; Roknic, N.; Goessling, K.; Klein, M.; Schulze, H.J.; Hallermann, C. Basosquamous Carcinoma of the Head and Neck: Clinical and Histologic Characteristics and Their Impact on Disease Progression. Neoplasia 2015, 17, 301–305. [Google Scholar] [CrossRef][Green Version]
- Garcia, C.; Poletti, E.; Crowson, A.N. Basosquamous carcinoma. J. Am. Acad. Dermatol. 2009, 60, 137–143. [Google Scholar] [CrossRef]
- Gualdi, G.; Soglia, S.; Fusano, M.; Monari, P.; Giuliani, F.; Porreca, A.; Nicola, M.; Calzavara-Pinton, P.; Amerio, P. Characterization of Basosquamous Cell Carcinoma: A Distinct Type of Keratinizing Tumor. Acta Derm. Venereol. 2021, 101, adv00353. [Google Scholar] [CrossRef]
- Martin, R.C.G.; Edwards, M.J.; Cawte, T.G.; Sewell, C.L.; McMasters, K.M. Basosquamous carcinoma: Analysis of prognostic factors influencing recurrence. Cancer 2000, 88, 1365–1369. [Google Scholar] [CrossRef]
- Bowman, P.H.; Ratz, J.L.; Knoepp, T.G.; Barnes, C.J.; Finley, E.M. Basosquamous Carcinoma. Dermatol. Surg. 2003, 29, 830–833. [Google Scholar]
- Alam, M.; Ratner, D. Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. Organo Hosp. Enfermedades Nutr. 2016, 68, 84–91. [Google Scholar]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef]
- Setlow, R.B. The Wavelengths in Sunlight Effective in Producing Skin Cancer: A Theoretical Analysis. Proc. Natl. Acad. Sci. USA 1974, 71, 3363–3366. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Marconi, A.; Sandhu, S.K.; Kiss, A.; Efimova, T.; Palazzo, E. Investigating Cutaneous Squamous Cell Carcinoma in vitro and in vivo: Novel 3D Tools and Animal Models. Front. Med. 2022, 9, 875517. [Google Scholar] [CrossRef]
- Murgia, G.; Denaro, N.; Boggio, F.; Nazzaro, G.; Benzecry, V.; Bortoluzzi, P.; Passoni, E.; Garrone, O.; Marzano, A. Basosquamous Carcinoma: Comprehensive Clinical and Histopathological Aspects, Novel Imaging Tools, and Therapeutic Approaches. Cells 2023, 12, 2737. [Google Scholar] [CrossRef] [PubMed]
- Pleasance, E.D.; Cheetham, R.K.; Stephens, P.J.; McBride, D.J.; Humphray, S.J.; Greenman, C.D.; Varela, I.; Lin, M.L.; Ordóñez, G.R.; Bignell, G.R.; et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010, 463, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Karin, M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010, 20, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef]
- Mantovani, A. Cancer: Inflaming metastasis. Nature 2009, 457, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P.; Lee, T.; Kimlin, M.G.; Kitahara, C.M.; Zhang, R.; Alexander, B.H.; Linet, M.S.; Cahoon, E.K. Lifetime Ambient UV Radiation Exposure and Risk of Basal Cell Carcinoma by Anatomic Site in a Nationwide, U.S. Cohort, 1983–2005. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1932–1946. [Google Scholar] [CrossRef] [PubMed]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Mai, Z.M.; Byrne, S.N.; Little, M.P.; Sargen, M.R.; Cahoon, E.K. Solar UVR and Variations in Systemic Immune and Inflammation Markers. JID Innov. 2021, 1, 100055. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Ghamar Talepoor, A.; Doroudchi, M. Immunosenescence in atherosclerosis: A role for chronic viral infections. Front. Immunol. 2022, 13, 945016. [Google Scholar] [CrossRef]
- Lecot, P.; Sarabi, M.; Pereira Abrantes, M.; Mussard, J.; Koenderman, L.; Caux, C.; Bendriss-Vermare, N.; Michallet, M.C. Neutrophil Heterogeneity in Cancer: From Biology to Therapies. Front. Immunol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Ravindranathan, D.; Master, V.A.; Bilen, M.A. Inflammatory Markers in Cancer Immunotherapy. Biology 2021, 10, 325. [Google Scholar] [CrossRef]

| Variable | High Sun Exposure (n = 106, 80%) ± SD | Low Sun Exposure (n = 26, 20%) ± SD | t | df | p |
|---|---|---|---|---|---|
| LY% | 24.22 ± 7.64 | 20.71 ± 8.10 | 2.067 | 130 | 0.041 |
| NLR | 3.08 ± 1.47 | 3.94 ± 2.43 | −2.309 | 130 | 0.023 |
| Variable | SCC Group (n = 88) ± SD | BSC Group (n = 44) ± SD | t | df | p |
|---|---|---|---|---|---|
| Age (years) | 76.14 ± 12.50 | 74.86 ± 9.53 | 0.594 | 130 | 0.554 |
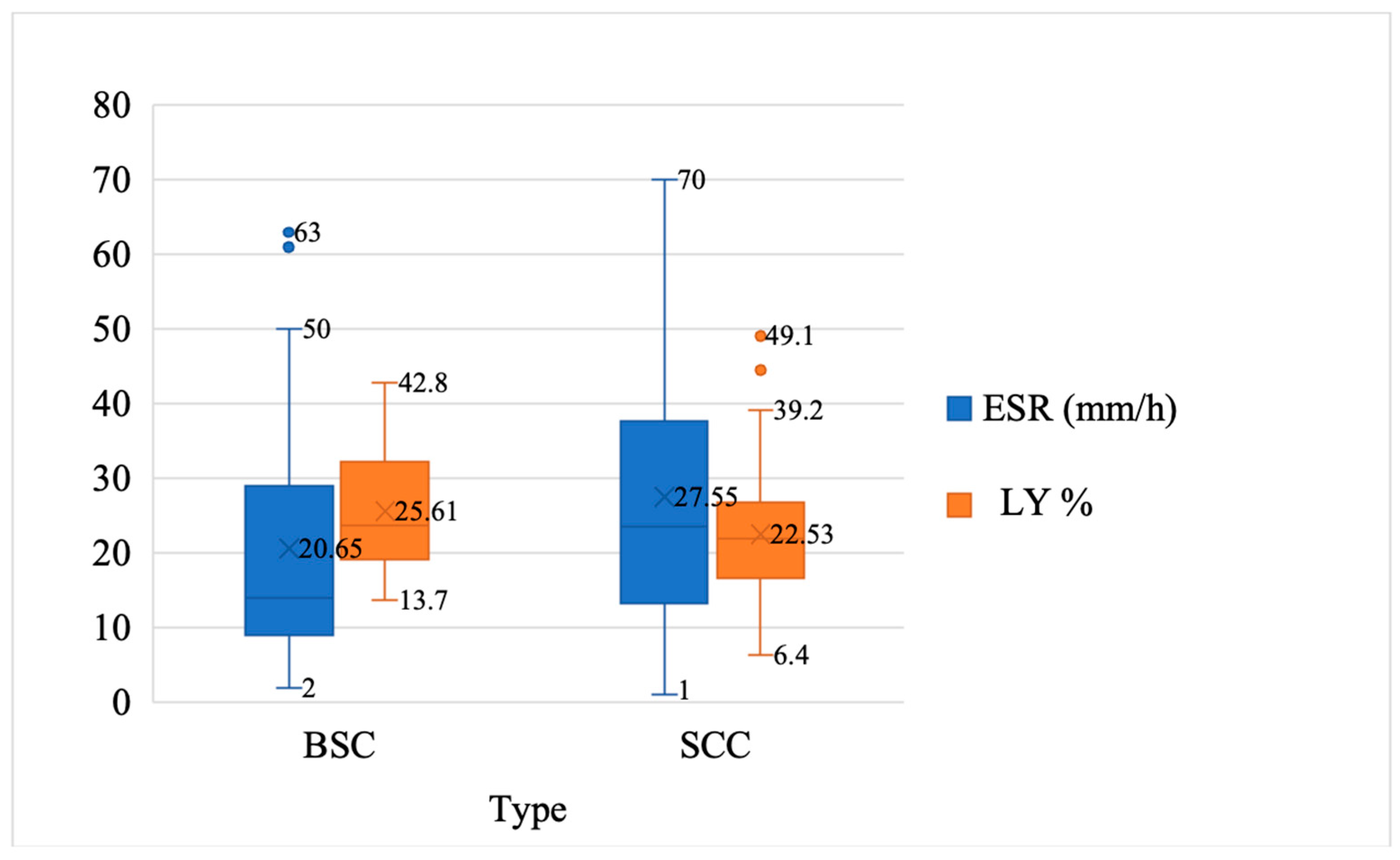
| ESR (mm/h) | 27.54 ± 18.48 | 20.65 ± 16.92 | 2.059 | 130 | 0.041 |
| LY% | 25.54 ± 7.90 | 22.53 ± 7.38 | −2.108 | 130 | 0.037 |
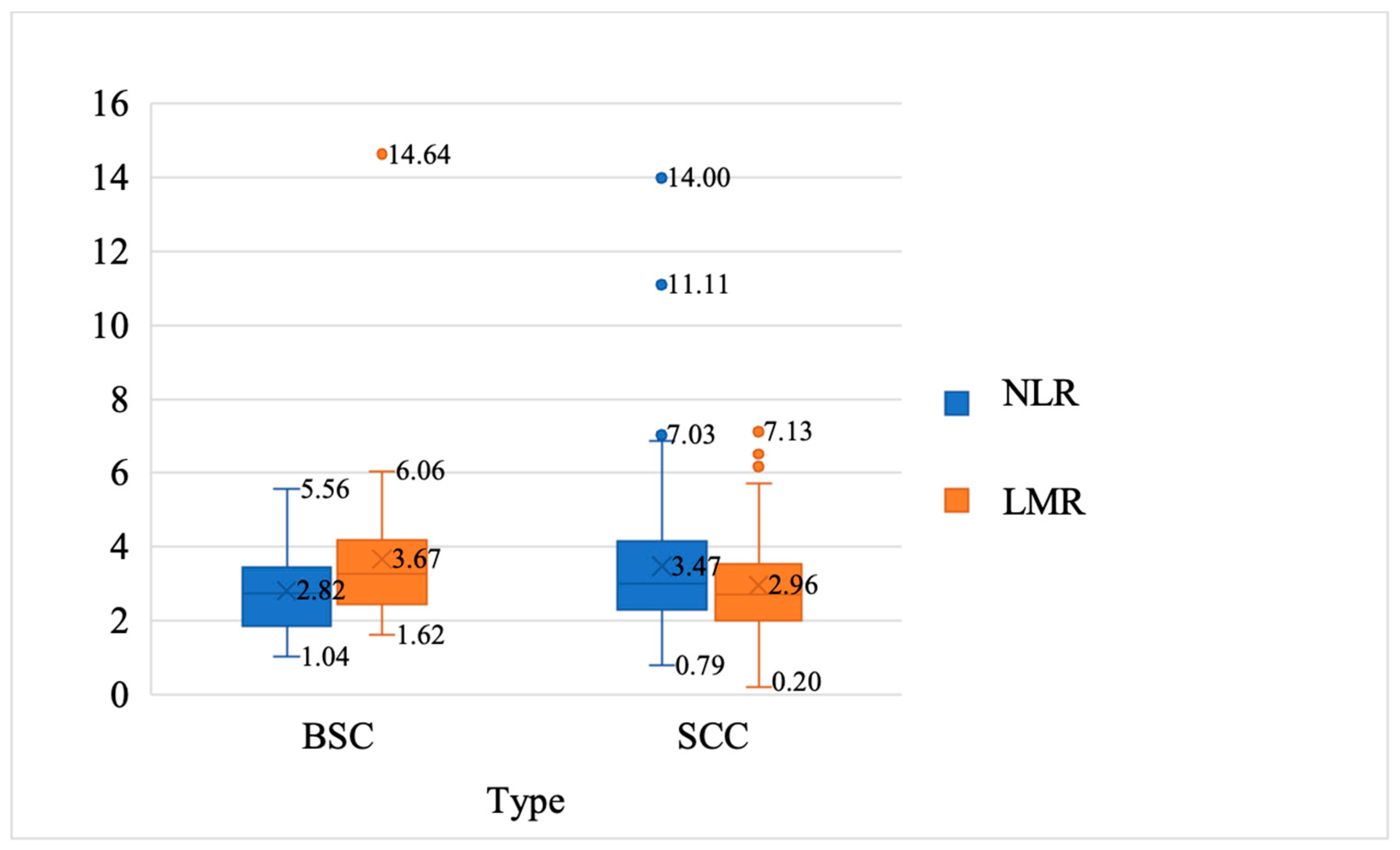
| NLR | 3.47 ± 1.93 | 2.81 ± 1.12 | 2.062 | 130 | 0.041 |
| LMR | 2.96 ± 1.45 | 3.67 ± 2.10 | −2.263 | 130 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore, A.; Oproiu, A.-M.; Iancu, I.; Florescu, I.-P. The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure. Curr. Oncol. 2024, 31, 2481-2487. https://doi.org/10.3390/curroncol31050185
Grigore A, Oproiu A-M, Iancu I, Florescu I-P. The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure. Current Oncology. 2024; 31(5):2481-2487. https://doi.org/10.3390/curroncol31050185
Chicago/Turabian StyleGrigore, Anamaria, Ana-Maria Oproiu, Ioana Iancu, and Ioan-Petre Florescu. 2024. "The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure" Current Oncology 31, no. 5: 2481-2487. https://doi.org/10.3390/curroncol31050185
APA StyleGrigore, A., Oproiu, A.-M., Iancu, I., & Florescu, I.-P. (2024). The Immune Response of Cutaneous Basosquamous- and Squamous-Cell Carcinoma Associated with Sun Exposure. Current Oncology, 31(5), 2481-2487. https://doi.org/10.3390/curroncol31050185





