Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
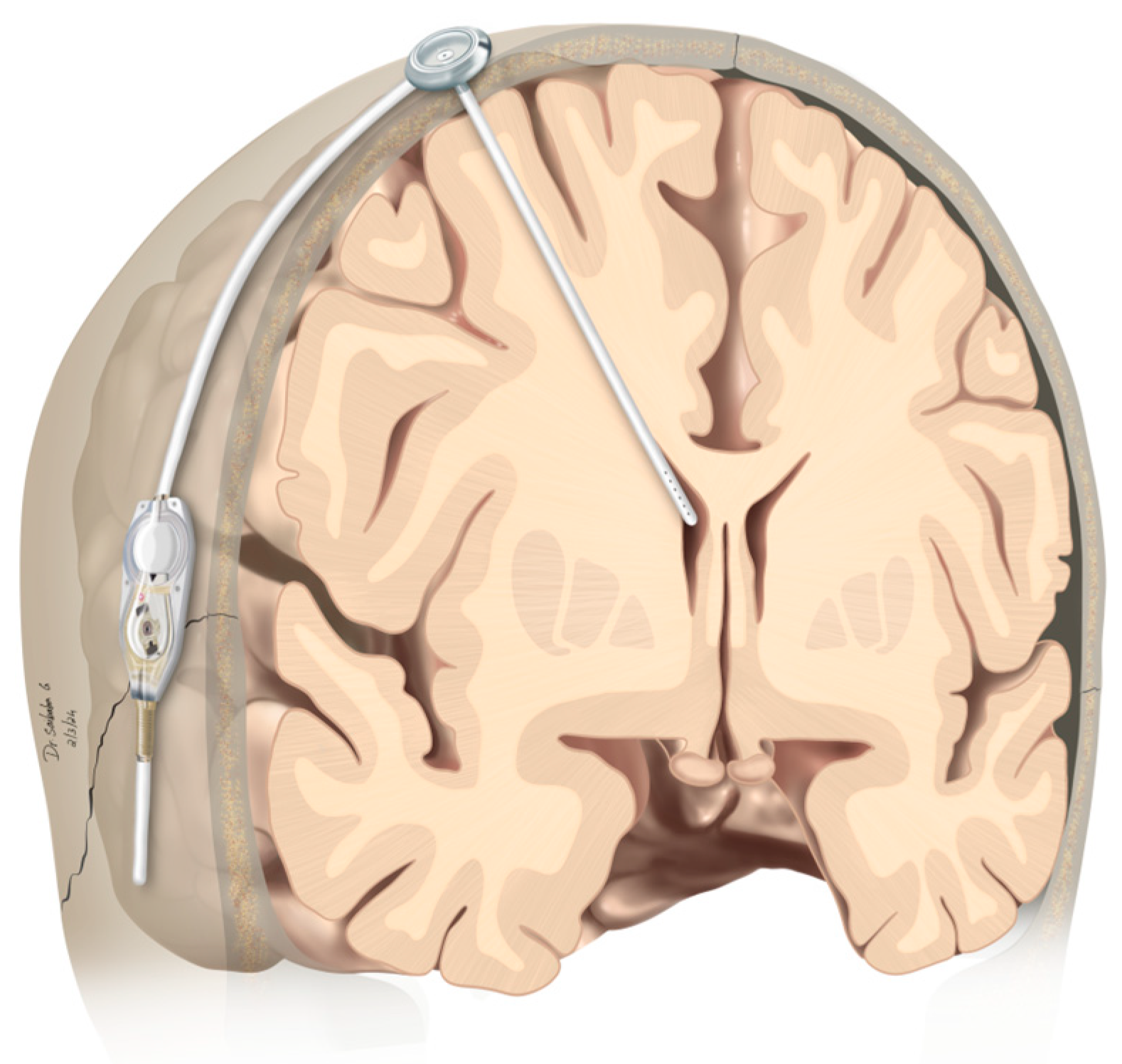
2.2. Operative Procedure
2.3. Intrathecal Chemotherapy Protocol
2.4. Data Collection and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Hydrocephalus
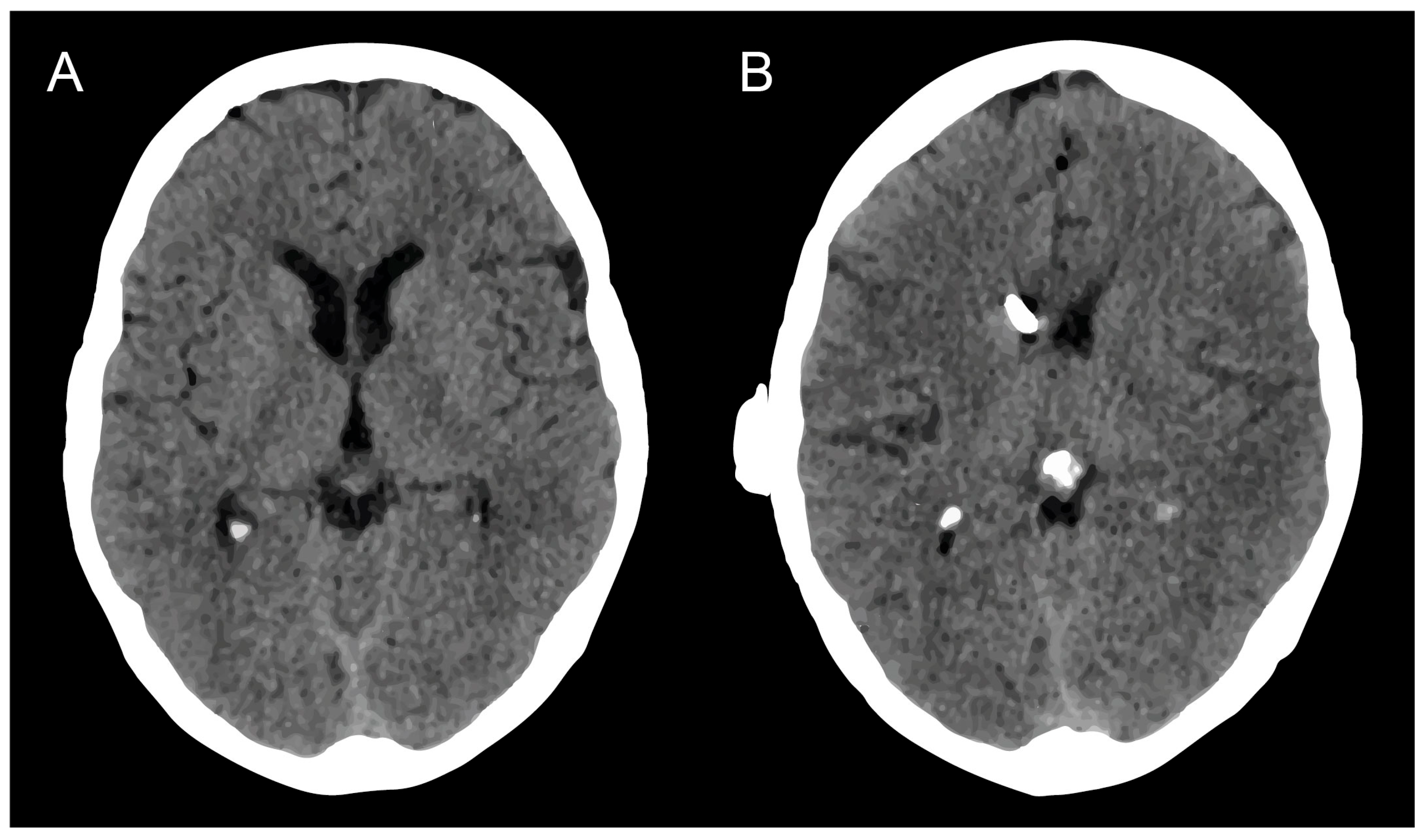
3.3. Surgical Management of mS/R
3.4. Feasibility/Procedure of ITC with mS/R
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cifarelli, C.P.; Brehmer, S.; Vargo, J.A.; Hack, J.D.; Kahl, K.H.; Sarria-Vargas, G.; Giordano, F.A. Intraoperative radiotherapy (IORT) for surgically resected brain metastases: Outcome analysis of an international cooperative study. J. Neuro-Oncol. 2019, 145, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.; Fröhlich, A.; Schäfer, N.; Keil, V.C.; Landsberg, J.; Herrlinger, U.; Sirokay, J. Treatment of metastasized melanoma with combined checkpoint inhibition in a patient with highly active multiple sclerosis. J. Dermatol. 2020, 47, e184–e185. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Weller, M.; Brandsma, D.; Van den Bent, M.; de Azambuja, E.; Henriksson, R.; Boulanger, T.; Peters, S.; Watts, C.; Wick, W.; et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann. Oncol. 2017, 28, iv84–iv99. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Radioisotope CSF flow studies in leptomeningeal metastases. J. Neuro-Oncol. 1998, 38, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Niwinska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: Propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med. Oncol. 2013, 30, 408. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, J.B.; Gwak, H.S.; Kwon, J.W.; Shin, S.H.; Yoo, H. Clinical outcome of cerebrospinal fluid shunts in patients with leptomeningeal carcinomatosis. World J. Surg. Oncol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Fick, T.; Nandoe Tewarie, R.; Broekman, M.L. Management of hydrocephalus in patients with leptomeningeal metastases: An ethical approach to decision-making. J. Neuro-Oncol. 2018, 140, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Le Rhun, E.; Devos, P.; Weller, J.; Seystahl, K.; Mo, F.; Compter, A.; Berghoff, A.S.; Jongen, J.L.M.; Wolpert, F.; Ruda, R.; et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro-Oncology 2021, 23, 1100–1112. [Google Scholar] [CrossRef]
- Sandberg, D.I.; Bilsky, M.H.; Souweidane, M.M.; Bzdil, J.; Gutin, P.H. Ommaya reservoirs for the treatment of leptomeningeal metastases. Neurosurgery 2000, 47, 49–54; discussion 54–55. [Google Scholar] [CrossRef]
- Lin, N.; Dunn, I.F.; Glantz, M.; Allison, D.L.; Jensen, R.; Johnson, M.D.; Friedlander, R.M.; Kesari, S. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: A retrospective, case-controlled study. J. Neurosurg. 2011, 115, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Schuss, P.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; Heimann, M.; Ko, Y.D.; Landsberg, J.; et al. The Impact of Prolonged Mechanical Ventilation on Overall Survival in Patients with Surgically Treated Brain Metastases. Front. Oncol. 2021, 11, 658949. [Google Scholar] [CrossRef] [PubMed]
- Bolton, L. Surgical Site Infection in Cancer Patients. Wounds 2021, 33, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, A.L.; Heimann, M.; Lehmann, F.; Ilic, I.; Paech, D.; Borger, V.; Radbruch, A.; Schafer, N.; Schuss, P.; Vatter, H.; et al. Survival after resection of brain metastasis: Impact of synchronous versus metachronous metastatic disease. J. Neuro-Oncol. 2023, 161, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Schafer, N.; Bumes, E.; Eberle, F.; Fox, V.; Gessler, F.; Giordano, F.A.; Konczalla, J.; Onken, J.; Ottenhausen, M.; Scherer, M.; et al. Implementation, relevance, and virtual adaptation of neuro-oncological tumor boards during the COVID-19 pandemic: A nationwide provider survey. J. Neuro-Oncol. 2021, 153, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Schuss, P.; Borger, V.; Güresir, A.; Vatter, H.; Güresir, E. Cranioplasty and Ventriculoperitoneal Shunt Placement after Decompressive Craniectomy: Staged Surgery Is Associated with Fewer Postoperative Complications. World Neurosurg. 2015, 84, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Hadjiathanasiou, A.; Kilinc, F.; Behmanesh, B.; Bernstock, J.; Güresir, E.; Heimann, M.; Konczalla, J.; Scharnböck, E.; Schneider, M.; Weinhold, L.; et al. Impact of Comorbidities and Frailty on Early Shunt Failure in Geriatric Patients with Normal Pressure Hydrocephalus. Front. Med. 2020, 7, 596270. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Mack, F.; Baumert, B.G.; Schäfer, N.; Hattingen, E.; Scheffler, B.; Herrlinger, U.; Glas, M. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat. Rev. 2016, 43, 83–91. [Google Scholar] [CrossRef]
- Shapiro, W.R.; Johanson, C.E.; Boogerd, W. Treatment modalities for leptomeningeal metastases. Semin. Oncol. 2009, 36, S46–S54. [Google Scholar] [CrossRef]
- Burger, M.C.; Wagner, M.; Franz, K.; Harter, P.N.; Bähr, O.; Steinbach, J.P.; Senft, C. Ventriculoperitoneal Shunts Equipped with On-Off Valves for Intraventricular Therapies in Patients with Communicating Hydrocephalus due to Leptomeningeal Metastases. J. Clin. Med. 2018, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, N.; Solheim, O.; Fredriksli, O.A.; Gulati, S. Revision and complication rates in adult shunt surgery: A single-institution study. Acta Neurochir. 2021, 163, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.Y.; Chung, W.K.; Oh, I.J. The prognostic significance of surgically treated hydrocephalus in leptomeningeal metastases. Clin. Neurol. Neurosurg. 2014, 119, 80–83. [Google Scholar] [CrossRef] [PubMed]
| Patient No | Age, Sex | Underlying Malignancy | Symptoms before VPS Surgery | Improvement of Symptoms after VPS | Used Intrathecal Chemotherapy Agent | SRC |
|---|---|---|---|---|---|---|
| 1 | 51, male | gastrointestinal | H/A, lethargy, N/V, gait disturbance, urinary incontinence | none | no ITC | none |
| 2 | 50, female | breast | H/A, lethargy, N/V | H/A | MTX | none |
| 3 | 50, female | breast | cognitive impairment, H/A, lethargy, N/V, visual disturbance | cognitive impairment, lethargy, N/V | no ITC | none |
| 4 | 63, male | lung | cognitive impairment, lethargy, visual disturbance, gait disturbance, hemiparesis, seizures | lethargy, hemiparesis | no ITC | none |
| 5 | 64, male | melanoma | cognitive impairment, H/A, lethargy, gait disturbance | none | MTX | yes |
| 6 | 66, female | breast | H/A, lethargy, visual disturbance, gait disturbance | H/A, lethargy, gait disturbance | MTX | yes |
| 7 | 42, male | gastrointestinal | radiological HC | radiological HC | MTX | none |
| 8 | 66, female | lung | H/A, lethargy, N/V | H/A, lethargy, N/V | MTX | none |
| 9 | 52, male | gastrointestinal | H/A, lethargy, visual disturbance, gait disturbance | lethargy | no ITC | none |
| 10 | 55, female | breast | H/A, lethargy, visual disturbance, gait disturbance | H/A, lethargy | no ITC | yes |
| 11 | 60, female | breast | H/A, visual disturbance, gait disturbance | H/A | no ITC | none |
| 12 | 72, female | breast | H/A, lethargy, gait disturbance | H/A, lethargy | MTX | none |
| 13 | 66, female | urogenital | cognitive impairment, H/A, gait disturbance, hemiparesis | cognitive impairment, H/A, gait disturbance, hemiparesis | no ITC | none |
| 14 | 65, female | breast | cognitive impairment, lethargy, gait disturbance | cognitive impairment, lethargy | no ITC | none |
| 15 | 51, male | lung | cognitive impairment, gait disturbance, hemiparesis | cognitive impairment | no ITC | none |
| 16 | 43, female | breast | radiological HC | radiological HC | no ITC | none |
| Patient No. | Postoperative Complications | Clavien-Dindo Classification |
|---|---|---|
| 5 | data cranial wound healing disturbance, shunt infection | grade IIIb |
| 6 | data cranial wound healing disturbance without infection, abdominal catheter dislocation | grade IIIb |
| 10 | abdominal catheter dislocation, shunt infection | grade IIIb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.; Wispel, C.; Potthoff, A.-L.; Heimann, M.; Borger, V.; Schaub, C.; Herrlinger, U.; Vatter, H.; Schuss, P.; Schäfer, N. Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy. Curr. Oncol. 2024, 31, 2410-2419. https://doi.org/10.3390/curroncol31050180
Schneider M, Wispel C, Potthoff A-L, Heimann M, Borger V, Schaub C, Herrlinger U, Vatter H, Schuss P, Schäfer N. Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy. Current Oncology. 2024; 31(5):2410-2419. https://doi.org/10.3390/curroncol31050180
Chicago/Turabian StyleSchneider, Matthias, Christian Wispel, Anna-Laura Potthoff, Muriel Heimann, Valeri Borger, Christina Schaub, Ulrich Herrlinger, Hartmut Vatter, Patrick Schuss, and Niklas Schäfer. 2024. "Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy" Current Oncology 31, no. 5: 2410-2419. https://doi.org/10.3390/curroncol31050180
APA StyleSchneider, M., Wispel, C., Potthoff, A.-L., Heimann, M., Borger, V., Schaub, C., Herrlinger, U., Vatter, H., Schuss, P., & Schäfer, N. (2024). Patients with Leptomeningeal Carcinomatosis and Hydrocephalus-Feasibility of Combined Ventriculoperitoneal Shunt and Reservoir Insertion for Intrathecal Chemotherapy. Current Oncology, 31(5), 2410-2419. https://doi.org/10.3390/curroncol31050180







