Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data
Abstract
1. Introduction
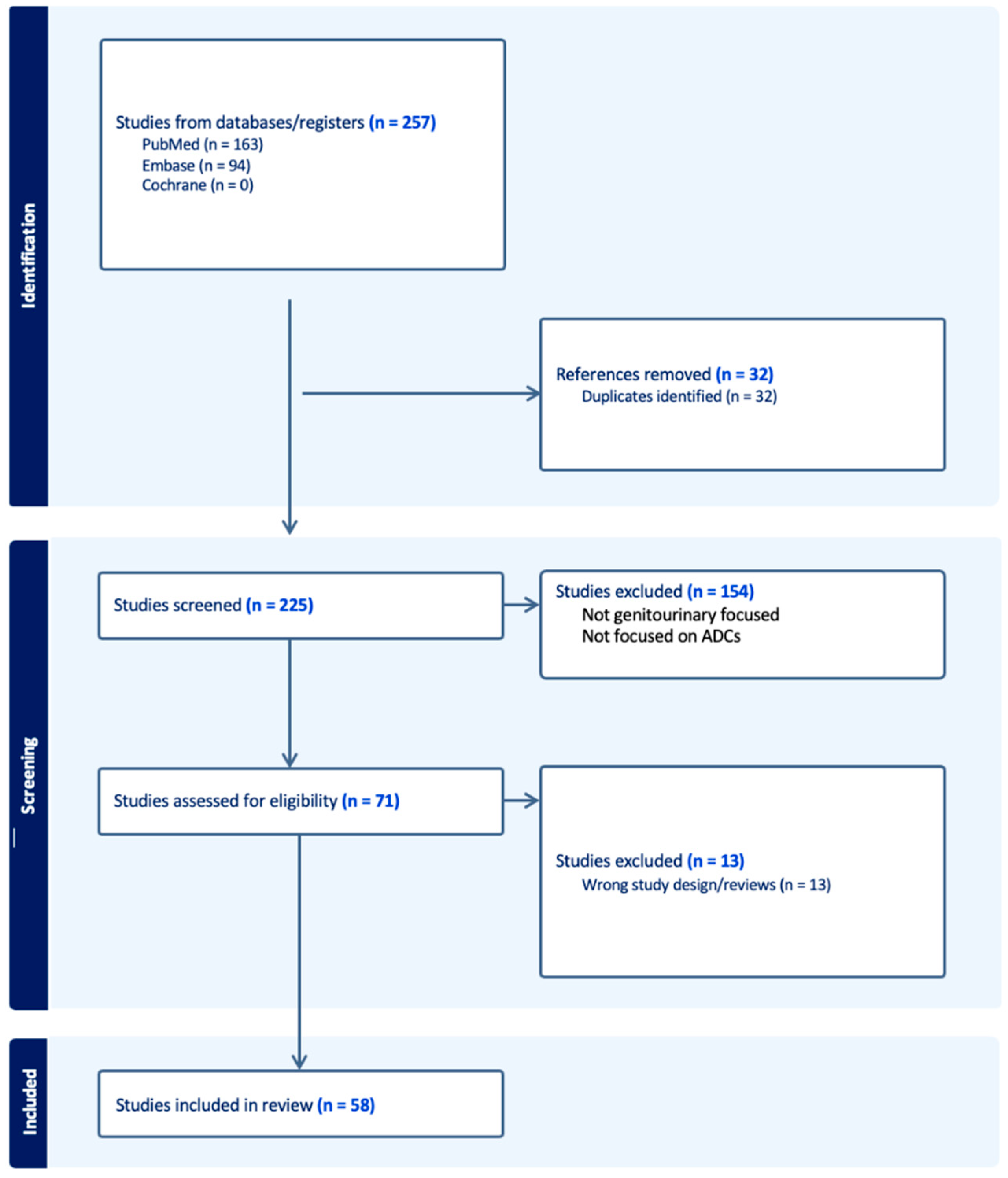
2. Methods
3. Results
3.1. ADCs in the Treatment of GU Cancers: Time for Loaded Guns
3.2. Enfortumab Vedotin
3.3. Trastuzumab Deruxtecan
3.4. Other HER2-Targeting ADCs
3.5. Sacituzumab Govitecan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gurney, H.; Clay, T.D.; Oliveira, N.; Wong, S.; Tran, B.; Harris, C. Systemic treatment of advanced and metastatic urothelial cancer: The landscape in Australia. Asia-Pac. J. Clin. Oncol. 2023, 19, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.S.; Powles, T.; Gupta, S.; Bedke, J.; Kikuchi, E.; De Wit, R.; Galsky, M.D.; Duran, I.; Necchi, A.; Retz, M.; et al. Enfortumab vedotin (EV) in combination with pembrolizumab (P) versus chemotherapy in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC): Subgroup analyses results from EV-302, a phase 3 global study. J. Clin. Oncol. 2024, 42 (Suppl. S4), LBA530. [Google Scholar] [CrossRef]
- Chalouni, C.; Doll, S. Fate of Antibody-Drug Conjugates in Cancer Cells. J. Exp. Clin. Cancer Res. 2018, 37, 20. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Brown, S. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef]
- Ranchon, F.; Chatelut, É.; Lambert, J.; Sesques, P.; Thibault, C.; Madelaine, I.; Rioufol, C.; Dieras, V.; Cazin, J.L. Anticorps monoclonaux conjugués et bispécifiques en cancérologie—Compte rendu de la Journée de Saint Louis 2022. Bull. Du Cancer 2023, 110, 1343–1351. [Google Scholar] [CrossRef]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel Anti-B-Cell Maturation Antigen Antibody-Drug Conjugate (GSK2857916) Selectively Induces Killing of Multiple Myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef]
- Corogeanu, D.; Zaki, K.; Beavil, A.J.; Arnold, J.N.; Diebold, S.S. Antibody conjugates for targeted delivery of Toll-like receptor 9 agonist to the tumor tissue. PLoS ONE 2023, 18, e0282831. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q.; Shen, Q.; Liu, Z.; Zhang, Z.; Zhou, T.; Yu, W.; He, Z.; He, Q.; Zhang, Q. Head-to-Head Comparison of the Expression Differences of NECTIN-4, TROP-2, and HER2 in Urothelial Carcinoma and Its Histologic Variants. Front. Oncol. 2022, 12, 858865. [Google Scholar] [CrossRef]
- Ghali, F.; Vakar-Lopez, F.; Roudier, M.P.; Garcia, J.; Arora, S.; Cheng, H.H.; Schweizer, M.T.; Haffner, M.C.; Lee, J.K.; Yu, E.Y.; et al. Metastatic Bladder Cancer Expression and Subcellular Localization of Nectin-4 and Trop-2 in Variant Histology: A Rapid Autopsy Study. Clin. Genitourin. Cancer 2023, 21, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhang, D. Linker design impacts Antibody-Drug conjugate pharmacokinetics and efficacy via modulating the stability and payload release efficiency. Front. Pharmacol. 2021, 12, 687926. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, D.; Xie, F.; Liu, L.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Antibody–drug conjugates: Recent advances in linker chemistry. Acta Pharm. Sin. B 2021, 11, 3889–3907. [Google Scholar] [CrossRef]
- McCombs, J.R.; Owen, S.C. Antibody Drug Conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Niegisch, G. Antibody-Drug-Conjugates (ADC): A Novel Treatment Option in Urothelial Carcinoma. Methods Mol. Biol. 2023, 2684, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Guevara-Patino, J.A.; Wang, X.; Li, R.; Sonpavde, G.; Jain, R.K. Antibody–Drug Conjugates in the Treatment of Urothelial Cancer. BioDrugs 2023, 37, 505–520. [Google Scholar] [CrossRef]
- Sonnet, M. Enfortumab Vedotin: Antibody drug conjugate for the treatment of locally advanced or metastatic urothelial cancer. Arzneimitteltherapie 2022, 20, 313–316. [Google Scholar]
- Choi, W.; Lombardo, K.; Patel, S.; Epstein, G.; Feng, M.; Gabrielson, A.; Hahn, N.M.; Hoffman-Censits, J.; McConkey, D.; Bivalacqua, T.J.; et al. A Molecular Inquiry into the Role of Antibody-Drug Conjugates in Bacillus Calmette-Guérin-exposed Non–muscle-invasive Bladder Cancer. Eur. Urol. 2022, 81, 138–142. [Google Scholar] [CrossRef]
- Sganga, S.; Riondino, S.; Iannantuono, G.M.; Rosenfeld, R.; Roselli, M.; Torino, F. Antibody–Drug Conjugates for the Treatment of Renal Cancer: A Scoping Review on Current Evidence and Clinical Perspectives. J. Pers. Med. 2023, 13, 1339. [Google Scholar] [CrossRef]
- Rosellini, M.; Santoni, M.; Mollica, V.; Rizzo, A.; Cimadamore, A.; Scarpelli, M.; Storti, N.; Battelli, N.; Montironi, R.; Massari, F. Treating prostate cancer by Antibody–Drug conjugates. Int. J. Mol. Sci. 2021, 22, 1551. [Google Scholar] [CrossRef]
- Maiorano, B.A.; Catalano, M.; Maiello, E.; Roviello, G. Enfortumab vedotin in metastatic urothelial carcinoma: The solution EVentually? Front. Oncol. 2023, 13, 1254906. [Google Scholar] [CrossRef]
- Razzaghdoust, A.; Muhammadnejad, S.; Parvin, M.; Bahram, B.; Zangeneh, M.; Basiri, A. Combination of T-DM1 and platinum-based chemotherapy in patient-derived xenograft models of muscle-invasive bladder cancer. DOAJ Dir. Open Access J. 2022, 25, 816–821. [Google Scholar] [CrossRef]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Cabaud, O.; Berger, L.; Crompot, E.; Adélaide, J.; Finetti, P.; Garnier, S.; Guille, A.; Carbuccia, N.; Farina, A.; Agavnian, E.; et al. Overcoming Resistance to Anti–Nectin-4 Antibody-Drug Conjugate. Mol. Cancer Ther. 2022, 21, 1227–1235. [Google Scholar] [CrossRef]
- Heath, E.I.; Rosenberg, J.E. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat. Rev. Urol. 2020, 18, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rodler, S.; Eismann, L.; Schlenker, B.; Casuscelli, J.; Brinkmann, I.; Sendelhofert, A.; Waidelich, R.; Buchner, A.; Stief, C.; Schulz, G.B.; et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value—Need for Biomarker Testing in High-Risk Patients? Cancers 2022, 14, 4411. [Google Scholar] [CrossRef]
- Isoda, B.; Shiga, M.; Kandori, S.; Nagumo, Y.; Yoshino, T.; Ikeda, A.; Kawahara, T.; Kimura, T.; Negoro, H.; Hoshi, A.; et al. Complete Response to Enfortumab Vedotin in a Hemodialysis Patient with Metastatic Urothelial Carcinoma: A Case Report. Case Rep. Oncol. 2023, 16, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Jindal, T.; Zhang, L.; Deshmukh, P.; Reyes, K.; Chan, E.; Kumar, V.; Zhu, X.; Maldonado, E.; Feng, S.; Johnson, M.; et al. Impact of Squamous Histology on Clinical Outcomes and Molecular Profiling in Metastatic Urothelial Carcinoma Patients Treated with Immune Checkpoint Inhibitors or Enfortumab Vedotin. Clin. Genitourin. Cancer 2023, 21, e394–e404. [Google Scholar] [CrossRef] [PubMed]
- Matte, P.; Campedel, L. Drug approval: Enfortumab vedotin-advanced urothelial carcinoma (which have previously received platinum-containing chemotherapy and immunotherapy). Bull. Cancer 2022, 109, 738–740. [Google Scholar] [CrossRef]
- Jindal, T.; Zhu, X.; Bose, R.; Kumar, V.; Maldonado, E.; Deshmukh, P.; Shipp, C.; Feng, S.; Johnson, M.S.; Angelidakis, A.; et al. Somatic alterations of TP53 and MDM2 associated with response to enfortumab vedotin in patients with advanced urothelial cancer. Front. Oncol. 2023, 13, 1161089. [Google Scholar] [CrossRef]
- Vulsteke, C.; De Cocker, L.; de Liaño, A.G.; Montesdeoca, C.; De Meulenaere, A.; Croes, L.; Delombaerde, D.; Szabados, B.; Powles, T. First Evidence of Activity of Enfortumab Vedotin on Brain Metastases in Urothelial Cancer Patients. Pharmaceuticals 2023, 16, 375. [Google Scholar] [CrossRef] [PubMed]
- Collette, K.R.; Myint, Z.W.; Parasramka, S.V.; Ellis, C.S. Case Report: Safety and Efficacy of Enfortumab Vedotin in a Patient with Metastatic Urothelial Carcinoma Undergoing Peritoneal Dialysis. Front. Oncol. 2022, 12, 892793. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients with Nectin-4–Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Uemura, M.; Kimura, T.; Kawasaki, Y.; Takamoto, A.; Yamaguchi, A.; Melhem-Bertrandt, A.; Gartner, E.M.; Inoue, T.; Akazawa, R.; et al. A phase I study of enfortumab vedotin in Japanese patients with locally advanced or metastatic urothelial carcinoma. Investig. New Drugs 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Evan, Y.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.L.; van der Heijden, M.S.; Loriot, Y.; Balar, A.V. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Powles, T.; Sonpavde, G.; Loriot, Y.; Duran, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 34, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wu, S.; Li, R.; Jiang, Y.; Zheng, J.; Li, Z.; Li, M.; Xin, K.; Guan, X.; Li, S.; et al. Novel ADCs and combination therapy in urothelial carcinoma: Latest updates from the 2023 ASCO-GU Cancers Symposium. J. Hematol. Oncol. 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.S.; Henderson, N.; James, M.; Natesan, D.; Freeman, D.; Nizam, A.; Su, C.T.; Khaki, A.R.; Osterman, C.K.; Glover, M.J.; et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: Analysis from the Urothelial Cancer Network to Investigate Therapeutic Experiences (UNITE) study. Cancer 2021, 128, 1194–1205. [Google Scholar] [CrossRef]
- Minato, A.; Kimuro, R.; Ohno, D.; Tanigawa, K.; Kuretake, K.; Matsukawa, T.; Takaba, T.; Jojima, K.; Harada, M.; Higashijima, K.; et al. Efficacy and Tolerability of Enfortumab Vedotin for Metastatic Urothelial Carcinoma: Early Experience in the Real World. Anticancer Res. 2023, 43, 4055–4060. [Google Scholar] [CrossRef]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2022, 29, 1496–1505. [Google Scholar] [CrossRef]
- Thibodeau, A.; Nallasamy, N. Bilateral Anterior Subcapsular Cataract Development Following Initiation of Enfortumab Vedotin. Int. Med. Case Rep. J. 2021, 14, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, M.E.; Patel, A.B.; Rosenberg, J.E.; O’Donnell, P.H. Management of Dermatologic Events Associated with the Nectin-4-directed Antibody-Drug Conjugate Enfortumab Vedotin. Oncol. 2022, 27, e223–e232. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, N.; Yonese, J.; Kojima, T.; Azuma, H.; Matsumoto, H.; Powles, T.; Rosenberg, J.E.; Petrylak, D.P.; Matsangou, M.; Wu, C.; et al. Japanese subgroup analysis of EV-301: An open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Med. 2022, 12, 2761–2771. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Tanaka, K.; Takeda, N.; Okada, Y.; Torii, S.; Esaki, H.; Sakakibara, T.; Takimoto, N. Two Cases of Exacerbation of Asthma during Treatment with Enfortumab Vedotin. Case Rep. Oncol. 2023, 16, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; de Kouchkovsky, D.; Kalebasty, A.R.; Mar, N. Drug extravasation with Enfortumab vedotin. J. Oncol. Pharm. Pract. 2023, 29, 1789–1792. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Fujimura, T.; Lyu, C.; Aiba, S. Severe eczematoid and lichenoid eruption with full-thickness epidermal necrosis developing from metastatic urothelial cancer treated with enfortumab vedotin. J. Dermatol. 2020, 47, 1436–1438. [Google Scholar] [CrossRef]
- Mimura, Y.; Kobayashi, A.; Utazu, H.; Matsumoto, Y.; Mizusawa, H. Toxic epidermal necrolysis after the administration of enfortumab vedotin for urinary bladder urothelial carcinoma. IJU Case Rep. 2022, 6, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Viscuse, P.V.; Marques-Piubelli, M.L.; Heberton, M.M.; Parra, E.R.; Shah, A.Y.; Siefker-Radtke, A.; Gao, J.; Goswami, S.; Ivan, D.; Curry, J.L.; et al. Case Report: Enfortumab Vedotin for Metastatic Urothelial Carcinoma: A Case Series on the Clinical and Histopathologic Spectrum of Adverse Cutaneous Reactions from Fatal Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis to Dermal Hypersensitivity Reaction. Front. Oncol. 2021, 11, 621591. [Google Scholar] [CrossRef]
- Azizi, A.; Houshyar, R.; Mar, N. Use of enfortumab vedotin in an HIV-positive patient with urothelial carcinoma. J. Oncol. Pharm. Pract. 2022, 28, 107815522210743. [Google Scholar] [CrossRef]
- Giorgio Patelli Zeppellini, A.; Spina, F.; Righetti, E.; Stabile, S.; Alessio Amatu Tosi, F.; Ghezzi, S.; Siena, S.; Sartore-Bianchi, A. The evolving panorama of HER2-targeted treatments in metastatic urothelial cancer: A systematic review and future perspectives. Cancer Treat. Rev. 2022, 104, 102351. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hainsworth, J.; Bose, R.; Iii, H.A.B.; Friedman, C.F.; Kurzrock, R.; Swanton, C.; Wang, Y.; Levy, J.; Schulze, K.; et al. MyPathway HER2 basket study: Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J. Clin. Oncol. 2021, 39 (Suppl. S15), 3004. [Google Scholar] [CrossRef]
- Galsky, M.D.; Ramos, J.; Tan, Q.; Yu, E.Y. SGNTUC-019: Phase 2 basket study of tucatinib and trastuzumab in previously treated solid tumors with HER2 alterations—Urothelial cancer cohort (trial in progress). J. Clin. Oncol. 2022, 40 (Suppl. S6), TPS586. [Google Scholar] [CrossRef]
- Hussain, M.H.; MacVicar, G.R.; Petrylak, D.P.; Dunn, R.L.; Vaishampayan, U.; Lara, P.N.; Chatta, G.S.; Nanus, D.M.; Glode, L.M.; Trump, D.L.; et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human Epidermal Growth factor Receptor-2/NEU–Positive urothelial carcinoma: Results of a multicenter Phase II National Cancer Institute trial. J. Clin. Oncol. 2007, 25, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F.; et al. Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer 2015, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Bordeau, B.M.; Balthasar, J.P. Mechanisms of ADC Toxicity and Strategies to Increase ADC Tolerability. Cancers 2023, 15, 713. [Google Scholar] [CrossRef] [PubMed]
- de Vries, E.G.E.; Rüschoff, J.; Lolkema, M.; Tabernero, J.; Gianni, L.; Voest, E.; de Groot, D.J.A.; Castellano, D.; Erb, G.; Naab, J.; et al. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med. 2023, 12, 12071–12083. [Google Scholar] [CrossRef] [PubMed]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.M.; Tripathi, N.; Agarwal, N.; Swami, U. Current and emerging role of sacituzumab govitecan in the management of urothelial carcinoma. Expert Rev. Anticancer Ther. 2022, 22, 335–341. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Nakano, K.; Kuwahara, K.; Minami, T.; Kato, T.; Hatano, K.; Kawashima, A.; Uemura, M.; Takao, T.; et al. Trop-2 in Upper Tract Urothelial Carcinoma. Curr. Oncol. 2022, 29, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur. Urol. Oncol. 2022, 5, 714–718. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Sharkey, R.M. Antibody-drug conjugates targeting TROP-2 and incorporating SN-38: A case study of anti-TROP-2 sacituzumab govitecan. MAbs 2019, 11, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W.; Kio, E.; Berlin, J.; Vahdat, L.; Masters, G.; Moroose, R.; Santin, A.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Alberti, S. The anti-Trop-2 antibody-drug conjugate Sacituzumab Govitecan—Effectiveness, pitfalls and promises. Ann. Transl. Med. 2022, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Prescott, A.E.; Ravindra, A.; Javed, A. Neutropenic Enterocolitis: A Rare Complication of Sacituzumab Govitecan. Case Rep. Oncol. 2022, 15, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tagawa, S.T.; Jain, R.K.; Bupathi, M.; Balar, A.V.; Rezazadeh, A.; George, S.; Palmbos, P.L.; Nordquist, L.T.; Davis, N.B.; et al. Primary analysis of TROPHY-U-01 cohort 2, a phase 2 study of sacituzumab govitecan (SG) in platinum (PT)-ineligible patients (pts) with metastatic urothelial cancer (mUC) that progressed after prior checkpoint inhibitor (CPI) therapy. J. Clin. Oncol. 2023, 41 (Suppl. S6), 520. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthelemy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Gupta, S.; Flechon, A.; Ramamurthy, C.; et al. Primary analysis of TROPHY-U-01 cohort 3, a phase 2 study of sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) that progressed after platinum (PT)-based therapy. J. Clin. Oncol. 2023, 41 (Suppl. S6), 518. [Google Scholar] [CrossRef]
- Lattanzi, M.; Rosenberg, J.E. The emerging role of antibody-drug conjugates in urothelial carcinoma. Expert Rev. Anticancer. Ther. 2020, 20, 551–561. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.; Sonpavde, G.; Kwak, L.; Regan, M.; Gao, X.; Hvidsten, H.; Mantia, C.; Wei, X.; Berchuck, J.; Berg, S.; et al. The Double Antibody Drug Conjugate (DAD) phase I trial: Sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2024, 35, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Ferrara, F.; Lasala, R.; Zovi, A. Precision Medicine in the Treatment of Locally Advanced or Metastatic Urothelial Cancer: New Molecular Targets and Pharmacological Therapies. Cancers 2022, 14, 5167. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 9674. [Google Scholar] [CrossRef]
- Atiq, S.; Hirshman, N.; Shariff, A.; Zhang, T. The management of toxicities from immune, targeted and ADCs treatments in patients with urothelial cancer. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 410–419. [Google Scholar] [CrossRef] [PubMed]
- de Padua, T.C.; Moschini, M.; Martini, A.; Pederzoli, F.; Nocera, L.; Marandino, L.; Raggi, D.; Briganti, A.; Montorsi, F.; Necchi, A. Efficacy and toxicity of antibody-drug conjugates in the treatment of metastatic urothelial cancer: A scoping review. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Urothelial Carcinoma. Trials Get Standing Ovation. Cancer Discov. 2023, 13, 2496. [Google Scholar] [CrossRef]
- Talukder, R.; Makrakis, D.; Grivas, P.; Khaki, A.R. The Evolving Therapeutic Landscape and Role of Enfortumab Vedotin in Advanced Urothelial Carcinoma: A Systematic Review. Touch Rev. Oncol. Haematol. 2023, 19, 27. [Google Scholar] [CrossRef]
| Agent | Targets of Therapy | Trial Name | Authors | Setting | Phase | Sample Size | Completion Date |
|---|---|---|---|---|---|---|---|
| Enfortumab Vedotin | Nectin-4 | EV-101 | Grivas et al. | Nectin-4-positive patients, including advanced urothelial carcinoma | 1 | 155 patients in the mUC cohort | 23 June 2014 to 25 October 2018 |
| EV-202 cohort 1 | Yu et al. | Patients who had previously received both platinum-based chemotherapy and a PD-1 or PD-L1 inhibitor | 2 | 125 | 8 October 2017 to 2 July 2018 | ||
| EV-202 cohort 2 | Rosenburg et al. | Patients who had received a PD-1 or PD-L1 inhibitor and were ineligible for cisplatin | 2 | 89 | 8 October 2017 to 11 February 2020 | ||
| EV-301 | Powels et al. | Patients who had progressed on platinum-based chemotherapy and immune checkpoint inhibitors | 3 | 301 patients were randomized to EV and 307 patients were randomized to chemotherapy | Date of data cutoff was 15 July 2020 | ||
| EV-302 | Powels et al. | Patients with previously untreated la/mUC who were eligible for cisplatin- or carboplatin-containing chemotherapy | 3 | 886 | |||
| Sacituzumab Govitecan | Trop-2 | IMMU-132-01 | Starodub et al. | Diverse metastatic epithelial cancers | 1 | 25 | |
| TROPHY-U-01 Cohort 1 | Tagawa et al. | Patients with mUC after progression on platinum-based chemotherapy and ICI | 2 | 113 | 17 December 2012 to 22 June 2017 | ||
| TROPHY-U-01 Cohort 2 | Petrylak et al. | Patients considered ineligible for platinum who progressed after ICI | 2 | 28 | Study started 13 August 2018 | ||
| TROPHY-U-01 Cohort 3 | Grivas et al. | Patients with progression on platinum-based chemotherapy | 2 | 41 | Study started 13 August 2018 | ||
| DS-8201a (Trastuzumab Deruxtecan) | HER2 | Hussain et al. | Trastuzumab, carboplatin, gemcitabine, and paclitaxel in advanced urothelial carcinoma patients (1) | 2 | 44 | October 2000 to March 2005 | |
| CVH-CT0 | Oudard et al. | Gemcitabine and platinum salt, with or without trastuzumab, in patients with locally advanced or metastatic urothelial carcinoma (2) | 2 | 61 | February 2004 to October 2009 | ||
| MyPathway | Meric-Bernstam et al. | Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors (3) | 2 | 22 | 14 April 2014 to 15 June 2020 | ||
| DS8201-A-U105 | Glasky et al. | T-DXd in combination with nivolumab in patients with HER2-expressing advanced/metastatic UC | 1b | 34 | July 2021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nathan, P.; Rajeh, A.; Noor, M.; Boldt, G.; Fernandes, R. Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data. Curr. Oncol. 2024, 31, 2316-2327. https://doi.org/10.3390/curroncol31040172
Nathan P, Rajeh A, Noor M, Boldt G, Fernandes R. Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data. Current Oncology. 2024; 31(4):2316-2327. https://doi.org/10.3390/curroncol31040172
Chicago/Turabian StyleNathan, Prathana, Adnan Rajeh, Meh Noor, Gabriel Boldt, and Ricardo Fernandes. 2024. "Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data" Current Oncology 31, no. 4: 2316-2327. https://doi.org/10.3390/curroncol31040172
APA StyleNathan, P., Rajeh, A., Noor, M., Boldt, G., & Fernandes, R. (2024). Antibody–Drug Conjugates in the Treatment of Genitourinary Cancers: An Updated Review of Data. Current Oncology, 31(4), 2316-2327. https://doi.org/10.3390/curroncol31040172






