The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes
Abstract
1. Introduction
2. Methods
2.1. Study Overview and Ethics
2.2. Cohort Identification
2.3. Data Collection
2.4. Data Linkage
2.5. Quality Measures
2.6. Statistical Analysis
3. Results
3.1. Cohort Description
3.2. Changes to Cancer Care Delivery during COVID-19
3.3. Quality Measure Performance
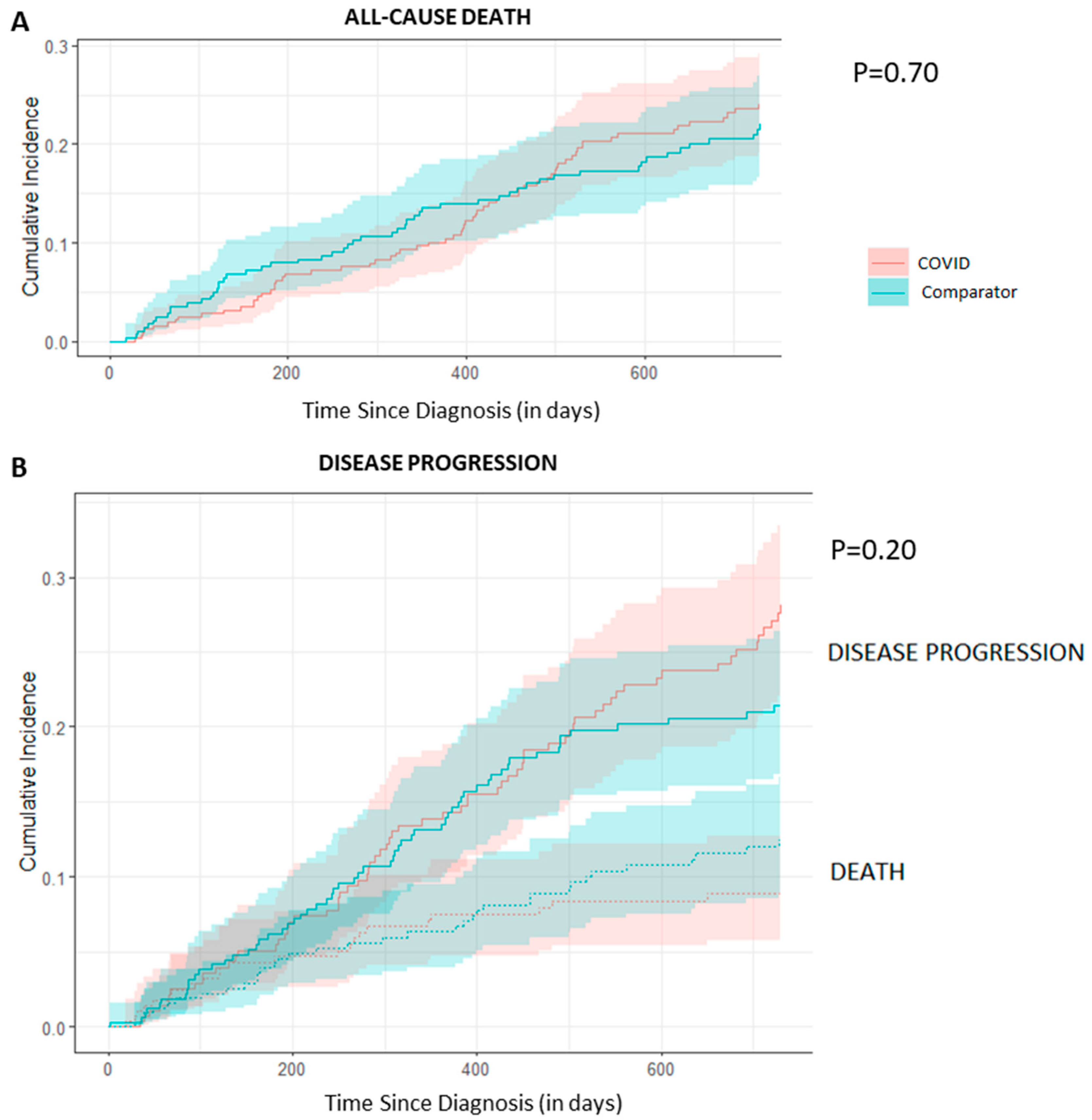
3.4. All-Cause Mortality and Disease Progression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Powis, M.; Milley-Daigle, C.; Hack, S.; Alibhai, S.; Singh, S.; Krzyzanowska, M.K. Impact of the early phase of the COVID pandemic on cancer treatment delivery and the quality of cancer care: A scoping review and conceptual model. Int. J. Qual. Health Care 2021, 33, mzab088. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Wang, J.; Mazuryk, J.; Skinner, S.M.; Meggetto, O.; Ashu, E.; Habbous, S.; Rad, N.N.; Espino-Hernández, G.; Wood, R.; et al. Delivery of Cancer Care in Ontario, Canada, During the First Year of the COVID-19 Pandemic. JAMA Netw Open. 2022, 5, e228855. [Google Scholar] [CrossRef] [PubMed]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Tartarone, A.; Lerose, R. COVID-19 and cancer care: What do international guidelines say? Med. Oncol. 2020, 37, 80. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing Cancer Care During the COVID-19 Pandemic: Agility and Collaboration Toward a Common Goal. J. Natl. Compr. Cancer Netw. 2020, 18, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G. The Treatment of Patients With Cancer and Containment of COVID-19: Experiences From Italy. Available online: https://dailynews.ascopubs.org/do/10.1200/ADN.20.200068/full/ (accessed on 14 July 2023).
- You, B.; Ravaud, A.; Canivet, A.; Ganem, G.; Giraud, P.; Guimbaud, R.; Kaluzinski, L.; Krakowski, I.; Mayeur, D.; Grellety, T.; et al. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020, 21, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.; Mata, E.; Guerra, A.; Jiménez, G.; Montes, M.; Arias, F.; Ciga, M.A.; Ursúa, E.; Ederra, M.; Arín, B.; et al. Impact of the COVID-19 pandemic during Spain’s state of emergency on the diagnosis of colorectal cancer. J. Surg Oncol. 2021, 123, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.A.; Goldacre, R.; Spata, E.; Mafham, M.; Finan, P.J.; Shelton, J.; Richards, M.; Spencer, K.; Emberson, J.; Hollings, S.; et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study. Lancet Gastroenterol. Hepatol. 2021, 6, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.A.; Banerjee, M.; Griggs, J.J.; Iwashyna, T.J.; Davis, M.A. The Effect of Exposure to Disaster on Cancer Survival. J. Gen. Intern. Med. 2020, 35, 380–382. [Google Scholar] [CrossRef]
- Heer, E.; Ruan, Y.; Boyne, D.J.; Jarada, T.N.; Heng, D.; Henning, J.-W.; Morris, D.M.; O’sullivan, D.E.; Cheung, W.Y.; Brenner, D.R. Impact of the COVID-19 pandemic on cancer diagnoses, stage and survival in Alberta. Can. Med. Assoc. J. 2023, 195, E804–E812. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Fernandes, B.; de Lange, M.; Lingsma, H.; Klazinga, N.; Kringos, D. Changes in the quality of cancer care as assessed through performance indicators during the first wave of the COVID-19 pandemic in 2020: A scoping review. BMC Health Serv. Res. 2022, 22, 786. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.I.; Ferguson, J.M.; Castro, E.; Pereira-Estremera, C.D.; Armaiz-Peña, G.N.; Duron, Y.; Hlubocky, F.; Infantado, A.; Nuqui, B.; Julian, D.; et al. Racial and Ethnic Disparities in Cancer Care During the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e2222009. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.J.; Meggetto, O.; Gao, J.; Espino-Hernández, G.; Jembere, N.; Bravo, C.A.; Rey, M.; Aslam, U.; Sheppard, A.J.; Lofters, A.K.; et al. Measuring the impact of the COVID-19 pandemic on organized cancer screening and diagnostic follow-up care in Ontario, Canada: A provincial, population-based study. Prev. Med. 2021, 151, 106586. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- University of Toronto Libraries. Map and Data Library. Postal Code Conversion Files+. Available online: https://mdl.library.utoronto.ca/collections/numeric-data/census-canada/postal-code-conversion-file (accessed on 1 January 2022).
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). 2011 Ontario Marginalization Index: User Guide. Available online: https://www.publichealthontario.ca/-/media/documents/O/2017/on-marg-user-2011.pdf (accessed on 1 November 2022).
- American Society for Clinical Oncology. Quality Oncology Practice Initiative. Available online: https://society.asco.org/practice-patients/quality-improvement/quality-programs/qopi/related-measures (accessed on 17 March 2023).
- Patwardhan, M.; Fisher, D.A.; Mantyh, C.R.; McCrory, D.C.; Morse, M.A.; Prosnitz, R.G.; Cline, K.; Samsa, G.P. Assessing the quality of colorectal cancer care: Do we have appropriate quality measures? (A systematic review of literature). J. Eval. Clin. Pr. 2007, 13, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Keikes, L.; Koopman, M.; Tanis, P.J.; Lemmens, V.E.; Punt, C.J.; van Oijen, M.G. Evaluating the scientific basis of quality indicators in colorectal cancer care: A systematic review. Eur. J. Cancer 2017, 86, 166–177. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative. The impact of surgical delay on resectability of colorectal cancer: An international prospective cohort study. Color. Dis. 2022, 24, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Austin, P.C. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Ob-servational Research. Commun. Stat.-Simul. Comput. 2009, 38, 1228–1234. [Google Scholar] [CrossRef]
- Mamdani, M.; Sykora, K.; Li, P.; Normand, S.L.; Streiner, D.L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ 2005, 330, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Normand, S.L.T.; Landrum, M.B.; Guadagnoli, E.; Ayanian, J.Z.; Ryan, T.J.; Cleary, P.D.; McNeil, B.J. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J. Clin. Epidemiol. 2001, 54, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Academic Press: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Brower, J.V.; Rhodes, S.S.; Remick, J.S.; Russo, A.L.; Dunn, E.F.; Ayala-Peacock, D.N.; Petereit, D.G.; Bradley, K.A.; Taunk, N.K. Effect of COVID-19 on Gynecologic Oncology Care: A Survey of Practicing Gynecologic Radiation Oncologists in the United States. Adv. Radiat. Oncol. 2023, 8, 101188. [Google Scholar] [CrossRef] [PubMed]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Treiman, K.; Kranzler, E.C.; Moultrie, R.; Arena, L.; Mack, N.; Fortune, E.; Garcia, R.; Street, R.L. Patients’ Experiences with Cancer Care: Impact of the COVID-19 Pandemic. J. Patient Exp. 2022, 9, 23743735221092567. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Rev. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Mazidimoradi, A.; Hadavandsiri, F.; Momenimovahed, Z.; Salehiniya, H. Impact of the COVID-19 Pandemic on Colorectal Cancer Diagnosis and Treatment: A Systematic Review. J. Gastrointest. Cancer 2023, 54, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Krautz, C.; Kersting, S.; Weber, G.F.; Stinner, B.; Benz, S.R.; Grützmann, R. Oncological colorectal surgery during the COVID-19 pandemic—A national survey. Br. J. Surg. 2020, 35, 2219–2225. [Google Scholar] [CrossRef]
- Brito, M.; Laranjo, A.; Sabino, J.; Oliveira, C.; Mocanu, I.; Fonseca, J. Digestive Oncology in the COVID-19 Pandemic Era. GE-Port. J. Gastroenterol. 2021, 28, 303–310. [Google Scholar] [CrossRef]
- Gurney, J.K.; Millar, E.; Dunn, A.; Pirie, R.; Mako, M.; Manderson, J.; Hardie, C.; Jackson, C.G.; North, R.; Ruka, M.; et al. The impact of the COVID-19 pandemic on cancer diagnosis and service access in New Zealand–a country pursuing COVID-19 elimination. Lancet Reg. Health-West. Pac. 2021, 10, 100127. [Google Scholar] [CrossRef]
- Lechner, D.; Tschann, P.; Girotti, P.C.N.; Königsrainer, I. Impact of the COVID-19 pandemic on a visceral surgical department in western Austria. Eur. Surg. 2020, 53, 43–47. [Google Scholar] [CrossRef]
- Koczkodaj, P.; Sulkowska, U.; Kamiński, M.F.; Didkowska, J. SARS-CoV-2 as a new possible long-lasting determining factor im-pacting cancer death numbers. Based on the example of breast, colorectal and cervical cancer in Poland. Nowotwory 2021, 71, 42–46. [Google Scholar] [CrossRef]
- Santoro, G.A.; Grossi, U.; Murad-Regadas, S.; Nunoo-Mensah, J.W.; Mellgren, A.; Di Tanna, G.L.; Gallo, G.; Tsang, C.; Wexner, S.D. DElayed COloRectal cancer care during COVID-19 Pandemic (DECOR-19): Global perspective from an international survey. Surgery 2020, 169, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Kamposioras, K.; Saunders, M.; Lim, K.H.J.; Marti, K.; Anderson, D.; Cutting, M.; McCool, D.; Connell, J.; Simpson, L.; Hasan, J.; et al. The Impact of Changes in Service Delivery in Patients With Colorectal Cancer During the Initial Phase of the COVID-19 Pandemic. Clin. Color. Cancer 2020, 20, e120–e128. [Google Scholar] [CrossRef]
- Raj Kumar, B.; Pandey, D. An observational study of the demographic and treatment changes in a tertiary colorectal cancer center during the COVID-19 pandemic. J. Surg. Oncol. 2020, 122, 1271–1275. [Google Scholar] [CrossRef]
- Fu, R.; Sutradhar, R.; Li, Q.; Hanna, T.P.; Chan, K.K.W.; Irish, J.C.; Coburn, N.; Hallet, J.; Dare, A.; Singh, S.; et al. Timeliness and Modality of Treatment for New Cancer Diagnoses During the COVID-19 Pan-demic in Canada. JAMA Netw. Open. 2023, 6, e2250394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV); World Health Organization: Geneva, Switzerland, 2020; Available online: https://covid19.who.int/ (accessed on 10 October 2023).
- Born, K.B.; Levinson, W. Using health care resources wisely during and following the COVID-19 pandemic. Can. J. Health Technol. 2021, 1, 2. [Google Scholar] [CrossRef]
- Frey, M.K.; Ellis, A.E.; Zeligs, K.; Chapman-Davis, E.; Thomas, C.; Christos, P.J.; Kolev, V.; Prasad-Hayes, M.; Cohen, S.; Holcomb, K.; et al. Impact of the coronavirus disease 2019 pandemic on the quality of life for women with ovarian cancer. Am. J. Obstet. Gynecol. 2020, 223, 725.e1–725.e9. [Google Scholar] [CrossRef]
- Satish, T.; Raghunathan, R.; Prigoff, J.G.; Wright, J.D.; Hillyer, G.A.; Trivedi, M.S.; Kalinsky, K.; Crew, K.D.; Hershman, D.L.; Accordino, M.K. Care Delivery Impact of the COVID-19 Pandemic on Breast Cancer Care. JCO Oncol. Pract. 2021, 17, e1215–e1224. [Google Scholar] [CrossRef]
- Bernstein, A.N.; Talwar, R.; Handorf, E.; Syed, K.; Danella, J.; Ginzburg, S.; Belkoff, L.; Reese, A.C.; Tomaszewski, J.; Trabulsi, E.; et al. Assessment of Prostate Cancer Treatment Among Black and White Patients During the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 1467–1473. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef] [PubMed]
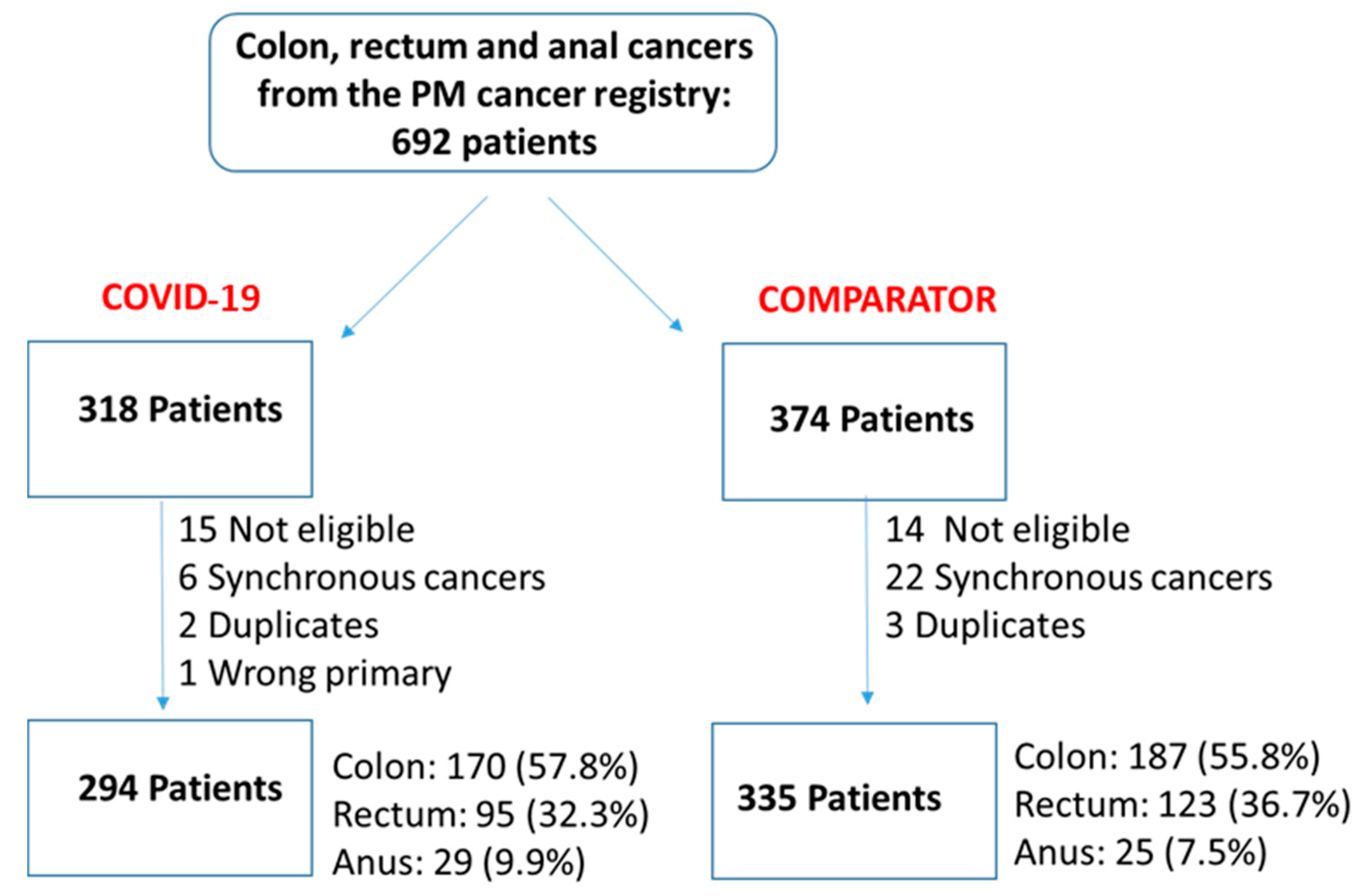
| Variable | COVID-19—2020 (n = 294) | Comparator—2019 (n = 335) | Standardized Difference | |
|---|---|---|---|---|
| Age | Mean (SD) | 66.1 (15.4) | 67.5 (14.9) | 0.14 |
| Sex, n (%) | Male | 151 (51.4) | 174 (51.9) | 0.01 |
| Female | 143 (48.6) | 161 (48.1) | ||
| Marital Status, n (%) | Married/Common-law Partner | 175 (59.5) | 205 (61.2) | 0.09 |
| Divorced | 14 (4.8) | 21 (6.3) | ||
| Single | 49 (16.7) | 52 (15.5) | ||
| Unknown | 56 (19.0) | 57 (17.0) | ||
| Language, n (%) | English as a first language | 252 (85.7) | 286 (85.4) | 0.01 |
| Other | 42 (14.3) | 49 (14.6) | ||
| Highest Level of Education Completed, n (%) | Less than high school | 3 (1.0) | 10 (3.0) | 0.18 |
| High school | 43 (14.6) | 39 (11.6) | ||
| College diploma/Undergraduate degree | 24 (8.2) | 26 (7.8) | ||
| Graduate degree | 11 (3.7) | 14 (4.2) | ||
| Unknown | 213 (72.4) | 245 (73.1) | ||
| Missing | - | 1 | ||
| Socioeconomic Status, n (%) | 1—lowest | 59 (20.1) | 70 (20.9) | 0.12 |
| 2 | 54 (18.4) | 50 (14.9) | ||
| 3 | 52 (17.7) | 61 (18.2) | ||
| 4 | 47 (16.0) | 58 (17.3) | ||
| 5—highest | 64 (21.8) | 79 (23.6) | ||
| Rural | 16 (5.4) | 14 (4.2) | ||
| Missing | 2 (0.7) | 3 (0.9) | ||
| ON-MARG-Households and Dwellings | 1—least marginalized | 44 (15.0) | 44 (13.1) | 0.11 |
| 2 | 31 (10.5) | 42 (12.5) | ||
| 3 | 37 (12.6) | 35 (10.4) | ||
| 4 | 54 (18.4) | 59 (17.6) | ||
| 5—most marginalized | 122 (41.5) | 150 (44.8) | ||
| Missing | 6 (2.0) | 5 (1.5) | ||
| ON-MARG-Material Resources | 1—least marginalized | 81 (27.6) | 85 (25.4) | 0.08 |
| 2 | 62 (21.1) | 74 (22.1) | ||
| 3 | 47 (16.0) | 62 (18.5) | ||
| 4 | 53 (18.0) | 57 (17.0) | ||
| 5—most marginalized | 45 (15.3) | 52 (15.5) | ||
| Missing | 6 (2.0) | 5 (1.5) | ||
| ON-MARG- Age and Labour Force | 1—least marginalized | 77 (26.2) | 83 (24.8) | 0.21 |
| 2 | 81 (27.6) | 80 (23.9) | ||
| 3 | 52 (17.7) | 57 (17.0) | ||
| 4 | 32 (10.9) | 30 (9.0) | ||
| 5—most marginalized | 46 (15.6) | 80 (23.9) | ||
| Missing | 6 (2.0) | 5 (1.5) | ||
| ON-MARG- Racialized and Newcomer Populations | 1—least marginalized | 26 (8.8) | 28 (8.4) | 0.08 |
| 2 | 26 (8.8) | 34 (10.1) | ||
| 3 | 54 (18.4) | 62 (18.5) | ||
| 4 | 84 (28.6) | 103 (30.7) | ||
| 5—most marginalized | 98 (33.3) | 103 (30.7) | ||
| Missing | 6 (2.0) | 5 (1.5) | ||
| Primary, n (%) | Anal | 29 (9.9) | 25 (7.5) | 0.11 |
| Colon | 170 (57.8) | 187 (55.8) | ||
| Rectal | 95 (32.3) | 123 (36.7) | ||
| Stage at Diagnosis, n (%) | I | 46 (15.6) | 53 (15.8) | 0.16 |
| II | 50 (17.0) | 66 (19.7) | ||
| III | 69 (23.5) | 104 (31.0) | ||
| IV | 98 (33.3) | 98 (29.3) | ||
| Unknown | 4 (1.4) | 11 (3.3) | ||
| Missing | 0 (0) | 3 (0.9) | ||
| ECOG Performance Status, n (%) | 0 | 47 (16.0) | 87 (26.0) | 0.14 |
| 1 | 73 (24.8) | 113 (33.7) | ||
| 2 | 12 (4.1) | 28 (6.9) | ||
| 3 | 15 (5.1) | 23 (6.9) | ||
| 4 | 1 (0.3) | 1 (0.3) | ||
| Missing | 146 (49.7) | 83 (24.8) | ||
| Treatment, n (%) a | Surgery | 123 (41.8) | 163 (48.7) | 0.14 |
| Systemic therapy | 125 (42.5) | 121 (36.1) | 0.06 | |
| Radiation therapy | 85 (28.9) | 87 (26.0) | 0.06 | |
| Measure Type | Measure | COVID-19 Cohort Performance; % (Numerator/Denominator) | Comparator Cohort Performance; % (Numerator/Denominator) | Standardized Difference | |
|---|---|---|---|---|---|
| Pandemic-specific Measures | Receipt of neoadjuvant chemotherapy | 16.3 (8/49) | 11.8 (8/68) | 0.13 | |
| Laparoscopic resection | 64.6 (62/96) | 70.8 (85/120) | 0.13 | ||
| Short-course radiotherapy | 32.6 (15/46) | 11.1 (6/54) | 0.54 | ||
| Oral systemic therapy | 31.2 (39/125) | 30.6 (37/121) | 0.01 | ||
| Single-agent regimens | 32.0 (40/125) | 30.6 (37/121) | 0.03 | ||
| Treatment deferral/interruption | Deferral | 12.1 (26/214) | 9.5 (24/252) | 0.09 | |
| Interruption | 32.4 (47/145) | 22.3 (33/148) | 0.23 | ||
| Premature discontinuation | Systemic therapy | 21.6 (27/125) | 19.8 (24/121) | 0.04 | |
| Radiotherapy | 9.4 (8/85) | 1.1 (1/87) | 0.38 | ||
| Trial participation | 2.7 (8/294) | 1.8 (8/335) | 0.06 | ||
| Quality Measures | Receipt of appropriate oncology consultation | Colon: surgical oncologist, with or without a consultation with a medical oncologist | 82.4 (140/170) | 90.9 (170/187) | 0.25 |
| Rectum: surgical oncologist AND medical oncologist AND radiation oncologist | 22.1 (21/95) | 20.3 (25/123) | 0.04 | ||
| Anus: medical oncologist AND radiation oncologist | 65.5 (19/29) | 88.0 (22/25) | 0.55 | ||
| 30-day post-surgical readmission | 10.5 (11/105) | 3.6 (5/139) | 0.27 | ||
| Unplanned acute care visit | 19.7 (58/294) | 23.0 (77/335) | 0.08 | ||
| Receipt of appropriate treatment | Colon: surgery for stage I–III disease | 73.6 (67/91) | 77.6 (83/107) | 0.09 | |
| Rectum: systemic therapy for stage II–IV disease | 24.7 (18/73) | 33.3 (31/93) | 0.19 | ||
| Anus: concurrent chemotherapy and radiation for stage I–III disease | 61.5 (16/26) | 85.7 (18/21) | 0.57 | ||
| Positive margins | 3.3 (3/135) | 2.2 (4/180) | 0.03 | ||
| 30-day mortality | Systemic therapy | 1.0 (1/104) | 13.8 (15/109) | 0.51 | |
| Surgery | 1.9 (2/105) | 0.7 (1/139) | 0.10 | ||
| Timely oncologist consultation: within 2 weeks of referral | 84.9 (73/86) | 72.4 (63/87) | 0.31 | ||
| Upstaging | 33.7 (98/291) | 30.6 (98/320) | 0.07 | ||
| Timely receipt of treatment: within 60 days of the date of diagnosis | 58.2 (85/146) | 55.8 (82/147) | 0.05 | ||
| Diagnosed in emergency department | 30.3 (88/290) | 27.8 (91/327) | 0.19 | ||
| Pathologically confirmed disease | 68.0 (200/294) | 74.6 (250/335) | 0.20 | ||
| Receipt of treatment for advanced disease | 67.3 (66/98) | 70.4 (69/98) | 0.07 | ||
| Receipt of a palliative care consultation | 9.2 (9/98) | 9.2 (9/98) | <0.01 | ||
| Consent to treatment | Surgery | 54.3 (57/105) | 58.3 (81/139) | 0.08 | |
| Systemic therapy | 30.8 (32/104) | 27.5 (30/109) | 0.07 | ||
| Radiotherapy | 14.0 (12/86) | 14.9 (13/87) | 0.03 | ||
| Receipt of psychosocial support | 14.3 (42/294) | 17.9 (60/335) | 0.10 | ||
| Receipt of pain management support | 0.0 (0/98) | 1.0 (1/98) | 0.14 | ||
| Variable | Cox Model: All-Cause Death | Fine-Gray Model: Disease Progression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | Disease Progression | All-Cause Death | ||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |||||
| Groups | Comparator | Ref | - | - | Ref | - | - | Ref | - | - |
| COVID-19 | 0.88 | 0.61–1.27 | 0.50 | 1.16 | 0.82–1.63 | 0.41 | 0.74 | 0.42–1.28 | 0.28 | |
| Age | 5-year increment | 1.22 | 0.81–1.83 | 0.30 | 1.19 | 0.97–1.45 | 0.09 | 1.13 | 0.95–1.35 | 0.18 |
| Primary | Colon | Ref | - | - | Ref | - | - | Ref | - | - |
| Rectal | 0.88 | 0.58–1.34 | 0.50 | 1.14 | 0.76–1.70 | 0.52 | 0.71 | 0.38–1.35 | 0.30 | |
| Anal | 0.79 | 0.34–1.86 | 0.60 | 2.17 | 1.04–5.40 | 0.04 | 0.44 | 0.10–1.95 | 0.28 | |
| Stage | I | Ref | - | - | Ref | - | - | Ref | - | - |
| II | 1.03 | 0.36–2.97 | 1.00 | 1.79 | 0.61–5.24 | 0.29 | 1.28 | 0.31–5.33 | 0.73 | |
| III | 2.71 | 1.13–6.47 | 0.03 | 2.57 | 0.98–6.73 | 0.06 | 3.02 | 0.88–10.4 | 0.08 | |
| IV | 7.25 | 3.11–16.9 | <0.01 | 14.1 | 5.63–35.40 | <0.01 | 4.38 | 1.26–15.3 | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powis, M.; Sutradhar, R.; Singh, S.; Alibhai, S.; Hack, S.; Baiad, A.; Chen, K.; Li, H.; Mohmand, Z.; Krzyzanowska, M.K. The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes. Curr. Oncol. 2024, 31, 2328-2340. https://doi.org/10.3390/curroncol31040173
Powis M, Sutradhar R, Singh S, Alibhai S, Hack S, Baiad A, Chen K, Li H, Mohmand Z, Krzyzanowska MK. The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes. Current Oncology. 2024; 31(4):2328-2340. https://doi.org/10.3390/curroncol31040173
Chicago/Turabian StylePowis, Melanie, Rinku Sutradhar, Simron Singh, Shabbir Alibhai, Saidah Hack, Abed Baiad, Kevin Chen, Huaqi Li, Zuhal Mohmand, and Monika K. Krzyzanowska. 2024. "The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes" Current Oncology 31, no. 4: 2328-2340. https://doi.org/10.3390/curroncol31040173
APA StylePowis, M., Sutradhar, R., Singh, S., Alibhai, S., Hack, S., Baiad, A., Chen, K., Li, H., Mohmand, Z., & Krzyzanowska, M. K. (2024). The Impact of the Pandemic on the Quality of Colorectal and Anal Cancer Care, and 2-Year Clinical Outcomes. Current Oncology, 31(4), 2328-2340. https://doi.org/10.3390/curroncol31040173





