Impact of Pregnancy on Breast Cancer Features and Prognosis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. PABC
3.2. Comparison of PABC with the Other Groups
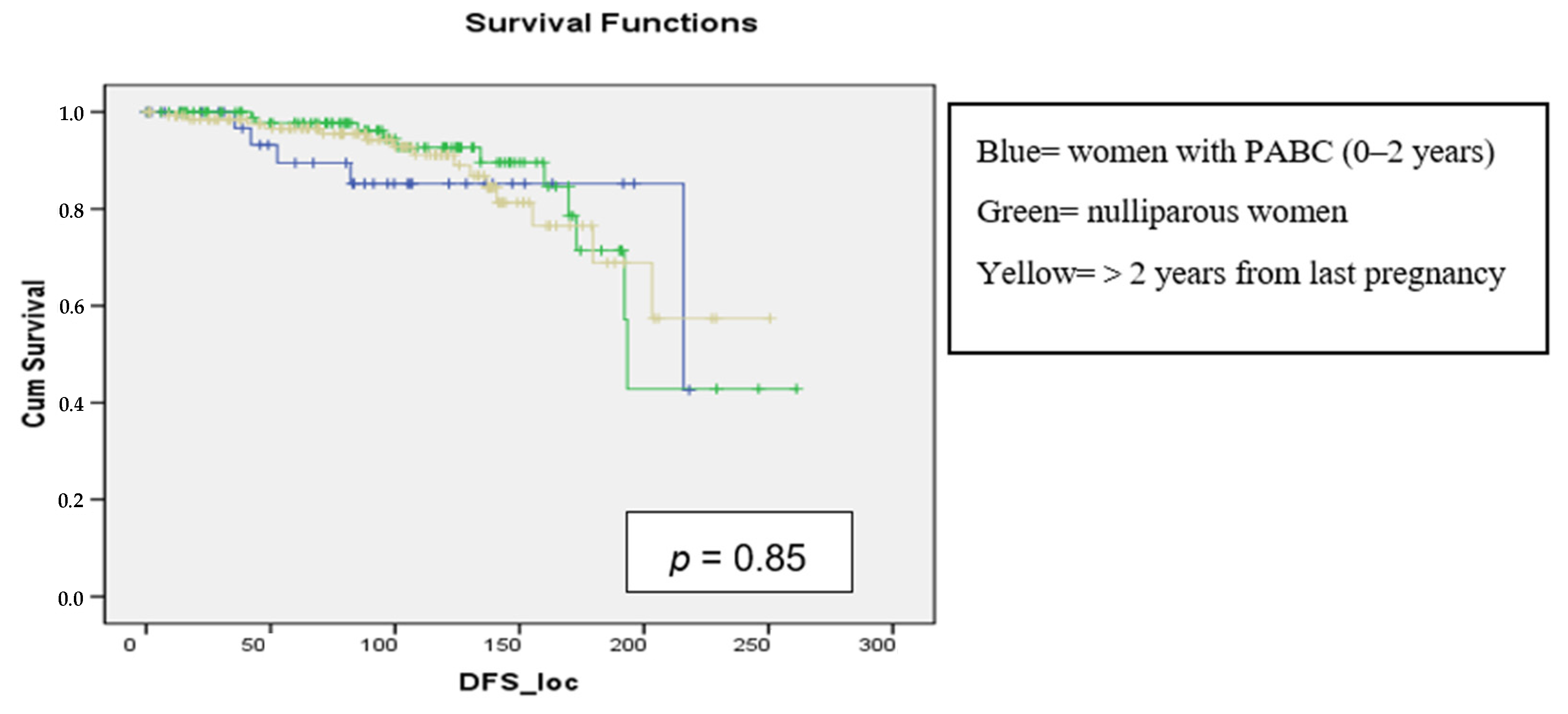
3.3. DFS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galati, F.; Magri, V.; Arias-Cadena, P.A.; Moffa, G.; Rizzo, V.; Pasculli, M.; Botticelli, A.; Pediconi, F. Pregnancy-Associated Breast Cancer: A Diagnostic and Therapeutic Challenge. Diagnostics 2023, 13, 604. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.L.V.; Stensheim, H. Epidemiology of Pregnancy-Associated Breast Cancer. In Diseases of the Breast during Pregnancy and Lactation; Alipour, S., Omranipour, R., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2020; Volume 1252. [Google Scholar]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Update Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; Park, S.; Hwang, H.; Lee, J.S.; Ko, S.M.; Kim, S.I.; Park, B.W. Clinical significance of age at the time of diagnosis among young breast cancer patients. J. Breast Cancer 2011, 14, 314–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Loibl, S.; Azim, H.A., Jr.; Bachelot, T.; Berveiller, P.; Bosch, A.; Cardonick, E.; Denkert, C.; Halaska, M.J.; Hoeltzenbein, M.; Johansson, A.L.V.; et al. ESMO Expert Consensus Statements on the management of breast cancer during pregnancy (PrBC). Ann. Oncol. 2023, 34, 849–866. [Google Scholar] [CrossRef]
- Johansson, A.L.V.; Weibull, C.E.; Fredriksson, I.; Lambe, M. Diagnostic pathways and management in women with pregnancy-associated breast cancer (PABC): No evidence of treatment delays following a first healthcare contact. Breast Cancer Res. Treat. 2019, 174, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Callihan, E.B.; Gao, D.; Jindal, S.; Lyons, T.R.; Manthey, E.; Edgerton, S.; Urquhart, A.; Schedin, P.; Borges, V.F. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res. Treat. 2013, 138, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Yu, Z.; Xiao, J.; Liu, L.; Hong, F.; Zhang, Y.; Jia, H. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer 2020, 20, 746. [Google Scholar] [CrossRef]
- Lee, G.E.; Mayer, E.L.; Partridge, A. Prognosis of pregnancy-associated breast cancer. Breast Cancer Res. Treat. 2017, 163, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Sun, Q.; Shen, S. Clinicopathologic features, treatment, and prognosis of pregnancy-associated breast cancer. Front. Oncol. 2022, 12, 978671. [Google Scholar] [CrossRef]
- Kataoka, A.; Ueno, T.; Yamauchi, H.; Uehiro, N.; Takahata, C.; Takahashi, Y.; Nakashima, E.; Ogiya, A.; Sakai, T.; Kitagawa, D.; et al. Characteristics, treatment trends, and long-term outcomes of Japanese patients with pregnancy-associated breast cancer (PABC). Breast Cancer 2022, 29, 825–834. [Google Scholar] [CrossRef]
- Crown, A.; McCartan, D.; Curry, M.A.; Patil, S.; Kamer, S.; Goldfarb, S.; Gemignani, M.L. Pregnancy-associated breast cancer: Does the timing of presentation affect outcome? Breast Cancer Res. Treat. 2023, 198, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Suelmann, B.B.M.; Bakhuis, C.F.J.; van Dooijeweert, C.; Verloop, J.; Zweemer, R.; Linn, S.; van der Wall, E.; van Diest, P.J. Prognosis of pregnancy-associated breast cancer: Inferior outcome in patients diagnosed during second and third gestational trimesters and lactation. Breast Cancer Res. Treat. 2022, 192, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Liu, Y.; Jemal, A.; Zhou, J.; Weiderpass, E.; Colditz, G.; Boffetta, P.; Weiss, M. Breastfeeding and breast cancer risk by receptor status—A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 2398–2407. [Google Scholar] [CrossRef]
- Lambertini, M.; Santoro, L.; Del Mastro, L.; Nguyen, B.; Livraghi, L.; Ugolini, D.; Peccatori, F.A.; Azim, H.A., Jr. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 2016, 49, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, X.; Huang, W.; Shao, B.; Yan, Y.; Liang, X.; Ran, R.; Song, G.; Di, L.; Jiang, H.; et al. Clinicopathological features and prognosis of patients with pregnancy-associated breast cancer: A matched case-control study. Asia Pac. J. Clin. Oncol. 2021, 17, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.L.V.; Andersson, T.M.; Hsieh, C.C.; Jirström, K.; Cnattingius, S.; Fredriksson, I.; Dickman, P.W.; Lambe, M. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int. J. Cancer 2018, 142, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Pentheroudakis, G.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; ESMO Guidelines Working Group. Cancer, fertility and pregnancy: ESMO Clinical Practice Guidelines for Diagnosis, treatment, and follow-up. Ann. Oncol. 2010, 21, v266–v273. [Google Scholar] [CrossRef]
- Boudy, A.S.; Naoura, I.; Selleret, L.; Zilberman, S.; Gligorov, J.; Richard, S.; Thomassin-Naggara, I.; Chabbert-Buffet, N.; Ballester, M.; Bendifallah, S. Propensity score to evaluate prognosis in pregnancy-associated breast cancer: Analysis from a French cancer network. Breast 2018, 40, 10–15. [Google Scholar] [CrossRef]
- Hartman, E.K.; Eslick, G.D. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: A meta-analysis. Breast Cancer Res. Treat. 2016, 160, 347–360. [Google Scholar] [CrossRef]
- Jo, H.; Park, S.; Kim, H.R.; Kim, H.; Hong, J.; Lee, J.E.; Yu, J.; Chae, B.J.; Lee, S.K.; Ryu, J.M.; et al. Long-Term Breast Cancer Outcomes of Pregnancy-Associated Breast Cancer (PABC) in a Prospective Cohort. Cancers 2022, 14, 4839. [Google Scholar] [CrossRef]
- Gooch, J.C.; Chun, J.; Kaplowitz, E.; Guth, A.; Axelrod, D.; Shapiro, R.; Roses, D.; Schnabel, F. Pregnancy-associated breast cancer in a contemporary cohort of newly diagnosed women. Breast J. 2020, 26, 668–671. [Google Scholar] [CrossRef]
- ASCO/CAP guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 16. [CrossRef]
- ASCO/CAP guideline recommendations for human epidermal growth factor receptor 2 testing in breast Cancer. J. Clin. Oncol. 2007. [CrossRef]
- Nguyen, P.L.; Taghian, A.G.; Katz, M.S.; Niemierko, A.; Abi Raad, R.F.; Boon, W.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Breast cancer subtypes approximated by estrogen receptors, progesterone receptors and HER-2 is associated with local and distant recurrences after breast-conserving therapy. J. Clin. Oncol. 2008, 26, 2373–2378. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Panel members Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Harbeck, N.; St. Thomssen, C. Gallen. Summary of the Consensus Discussion. Breast Care 2011, 6, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Franco, I.; Alshalalfa, M.; Hernandez, A.; Mahal, B.A.; Nguyen, T.; Wang, L.; Punglia, R.; Swami, N.; Goel, N. Genomic Characterization of Aggressive Breast Cancer in Younger Women. Ann. Surg. Oncol. 2023, 30, 7569–7578. [Google Scholar] [CrossRef]
- Asztalos, S.; Gann, P.H.; Hayes, M.K.; Nonn, L.; Beam, C.A.; Dai, Y.; Wiley, E.L.; Tonetti, D.A. Gene expression patterns in the human breast after pregnancy. Cancer Prev. Res. 2010, 3, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Proussaloglou, E.M.; Blanco, L.Z., Jr.; Siziopikou, K.P. Updates in the pathology of Pregnancy Associated Breast Cancer (PABC). Pathol. Res. Pract. 2023, 244, 154413. [Google Scholar] [CrossRef]
- Paris, I.; Di Giorgio, D.; Carbognin, L.; Corrado, G.; Garganese, G.; Franceschini, G.; Sanchez, A.M.; De Vincenzo, R.P.; Accetta, C.; Terribile, D.A.; et al. Pregnancy-Associated Breast Cancer: A Multidisciplinary Approach. Clin. Breast Cancer. 2021, 21, e120–e127. [Google Scholar] [CrossRef]
- Venetis, K.; Sajjadi, E.; Peccatori, F.A.; Guerini-Rocco, E.; Fusco, N. Immune plasticity in pregnancy-associated breast cancer tumorigenesis. Eur. J. Cancer Prev. 2023, 32, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Cardonick, E.; Dougherty, R.; Grana, G.; Gilmandyar, D.; Ghaffar, S.; Usmani, A. Breast Cancer during Pregnancy: Maternal and Fetal Outcomes. Cancer J. 2010, 16, 76–82. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 9 April 2024).
- Lyons, T.R.; Schedin, P.J.; Borges, V.F. Pregnancy and breast cancer: When they collide. J. Mammary Gland Biol. Neoplasia 2009, 14, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Kroman, N.; Wohlfahrt, J.; Andersen, K.W.; Mouridsen, H.T.; Westergaard, T.; Melbye, M. Time since childbirth and prognosis in primary breast cancer: Population-based study. Br. Med. J. 1997, 315, 851–855. [Google Scholar] [CrossRef]
- Zha, N.; Alabousi, M.; Abdullah, P.; Freitas, V.; Linthorst, R.; Muhn, N.; Alabousi, A. Breast Cancer Screening in High-Risk Patients during Pregnancy and Breastfeeding: A Systematic Review of the Literature. J. Breast Imaging 2019, 1, 92–98. [Google Scholar] [CrossRef]
- Pugh, A.M.; Giannini, C.M.; Pinney, S.M.; Hanseman, D.J.; Shaughnessy, E.A.; Lewis, J.D. Characteristics and diagnosis of pregnancy and lactation associated breast cancer: Analysis of a self-reported regional registry. Am. J. Surg. 2018, 216, 809–812. [Google Scholar] [CrossRef]
- AIOM. Guidelines Neoplasie della Mammella; AIOM: Milan, Italy, 2021. [Google Scholar]
- Azim, H.A.; Santoro, L.; Russell-Edu, W.; Pentheroudakis, G.; Pavlidis, N.; Peccatori, F.A. Prognosis of pregnancy-associated breast cancer: A meta-analysis of 30 studies. Cancer Treat Rev. 2012, 38, 834–842. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. JNCCN 2023, 21, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Peccatori, F.A.; Azim, J.A.; Orecchia, R.; Hoekstra, H.J.; Pavlidis, N.; Kesic, V.; Pentheroudakis, G.; ESMO Guidelines Working Group. Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013, 24 (Suppl. 6), vi160–vi170. [Google Scholar] [CrossRef]
- Murphy, C.G.; Mallam, D.; Stein, S.; Patil, S.; Howard, J.; Sklarin, N.; Hudis, C.A.; Gemignani, M.L.; Seidman, A.D. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer 2012, 118, 3254–3259. [Google Scholar] [CrossRef]
- Suelmann, B.B.M.; van Dooijeweert, C.; van der Wall, E.; Linn, S.; van Diest, P.J. Pregnancy-associated breast cancer: A nationwide Dutch study confirms a discriminatory aggressive histopathologic profile. Breast Cancer Res. Treat. 2021, 186, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Deshpande, A.D.; Jeffe, D.B.; Sweet, A.; White, N.; Margenthaler, J.A. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J. Am. Coll. Surg. 2009, 208, 341–347. [Google Scholar] [CrossRef]
- Ejlertsen, B.; Jensen, M.B.; Rank, F.; Rasmussen, B.B.; Christiansen, P.; Kroman, N.; Kvistgaard, M.E.; Overgaard, M.; Toftdahl, D.B.; Mouridsen, H.T.; et al. Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J. Natl. Cancer Inst. 2009, 101, 729–735. [Google Scholar] [CrossRef]
- Mathelin, C.; Annane, K.; Treisser, A.; Chenard, M.P.; Tomasetto, C.; Bellocq, J.P.; Rio, M.C. Pregnancy and post-partum breast cancer: A prospective study. Anticancer Res. 2008, 28, 2447–2452. [Google Scholar]
- Hatem, A.; Botteri, E.; Renne, G.; Dell’orto, P.; Rotmensz, N.; Gentilini, O.; Sangalli, C.; Pruneri, G.; Di Nubila, B.; Locatelli, M.; et al. The biological features and prognosis of breast cancer diagnosed during pregnancy: A case-control study. Acta Oncol. 2012, 51, 653–661. [Google Scholar]



| Nulliparous (%) | PABC (%) | 2–5 Years (%) | 5–10 Years (%) | >10 Years (%) | |
|---|---|---|---|---|---|
| Mean age at diagnosis | 35 (SD 4.58) | 36.4 (SD 3.17) | 36.3 (SD 3.87) | 37.6 (SD 3.08) | 39.2 (SD 1.44) |
| IDC | 87 (80.6) | 34 (85) | 32 (80) | 49 (83.1) | 31 (88.6) |
| DCIS | 8 (7.4) | 4 (10) | 2 (5) | 4 (6.8) | 1 (2.9) |
| ILC | 10 (9.3) | 2 (5) | 5 (12.5) | 6 (10.2) | 1 (2.9) |
| Others | 3 (2.8) | 0 (0) | 1 (2.5) | 0 (0) | 2 (5.7) |
| In situ | 8 (7.4) | 4 (10) | 2 (5) | 4 (6.8) | 1 (2.9) |
| T1 | 66 (61) | 20 (50) | 19 (47.5) | 29 (49.2) | 22 (62.8) |
| T2 | 26 (24) | 13 (32.5) | 17 (42.5) | 25 (42.4) | 11 (31.4) |
| T3-T4 | 8 (7.4) | 3 (7.5) | 2 (5) | 1 (1.7) | 1 (2.9) |
| N0 | 52 (48.1) | 22 (55) | 21 (52.5) | 37 (62.7) | 21 (60) |
| N1 | 41 (38) | 9 (22.5) | 14 (35) | 15 (25.4) | 10 (28.6) |
| N2 | 15 (13.9) | 9 (22.5) | 5 (12.5) | 7 (11.9) | 4 (11.4) |
| G1–G2 | 40 (37) | 10 (25) | 12 (30) | 23 (39) | 16 (45.7) |
| G3 | 68 (63) | 30 (75) | 28 (70) | 36 (61) | 19 (54.3) |
| Luminal A | 27 (25) | 5 (13.2) | 10 (25) | 13 (22) | 10 (28.6) |
| Luminal B | 59 (54.6) | 21 (55.3) | 20 (50) | 36 (61) | 21 (60) |
| HER2 | 1 (0.9) | 4 (10) | 2 (5) | 1 (1.7) | 0 (0.0) |
| TNBC | 21 (19.4) | 8 (20) | 8 (20) | 9 (15.3) | 4 (11.4) |
| Type of Surgery | Nulliparous (%) | PABC (%) | 2–5 Years (%) | 5–10 Years (%) | >10 Years (%) |
|---|---|---|---|---|---|
| BCS | 72 (66.7) | 22 (55) | 22 (55) | 27 (45.8) | 27 (77.1) |
| Mastectomy | 36 (33.3) | 18 (45) | 18 (45) | 8 (54.2) | 8 (22.9) |
| Variables | Odds Ratio | IC 95% | p | |
|---|---|---|---|---|
| T Stage > 2 | 1.5 | 1 | 1.3 | 0.05 |
| Node involvement | 2.4 | 1.6 | 3.5 | 0.0001 |
| High grade | 2.6 | 1.2 | 5.8 | 0.01 |
| Lymphatic invasion | 2.3 | 1.2 | 4.3 | 0.009 |
| Vascular invasion | 2.3 | 1.2 | 4.5 | 0.007 |
| ER positivity | 0.8 | 0.4 | 1.6 | 0.48 |
| HER2 molecular subtype | 5.2 | 1.4 | 18.8 | 0.01 |
| PABC | 0.8 | 0.5 | 1.2 | 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounous, V.E.; Minella, C.; Fuso, L.; Actis, S.; Petroni, G.; Sgrò, L.G.; Borghese, M.; Tomasi Cont, N.; Ponzone, R.; Ferrero, A. Impact of Pregnancy on Breast Cancer Features and Prognosis. Curr. Oncol. 2024, 31, 2305-2315. https://doi.org/10.3390/curroncol31040171
Bounous VE, Minella C, Fuso L, Actis S, Petroni G, Sgrò LG, Borghese M, Tomasi Cont N, Ponzone R, Ferrero A. Impact of Pregnancy on Breast Cancer Features and Prognosis. Current Oncology. 2024; 31(4):2305-2315. https://doi.org/10.3390/curroncol31040171
Chicago/Turabian StyleBounous, Valentina E., Carola Minella, Luca Fuso, Silvia Actis, Greta Petroni, Luca G. Sgrò, Martina Borghese, Nicoletta Tomasi Cont, Riccardo Ponzone, and Annamaria Ferrero. 2024. "Impact of Pregnancy on Breast Cancer Features and Prognosis" Current Oncology 31, no. 4: 2305-2315. https://doi.org/10.3390/curroncol31040171
APA StyleBounous, V. E., Minella, C., Fuso, L., Actis, S., Petroni, G., Sgrò, L. G., Borghese, M., Tomasi Cont, N., Ponzone, R., & Ferrero, A. (2024). Impact of Pregnancy on Breast Cancer Features and Prognosis. Current Oncology, 31(4), 2305-2315. https://doi.org/10.3390/curroncol31040171






