Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review
Abstract
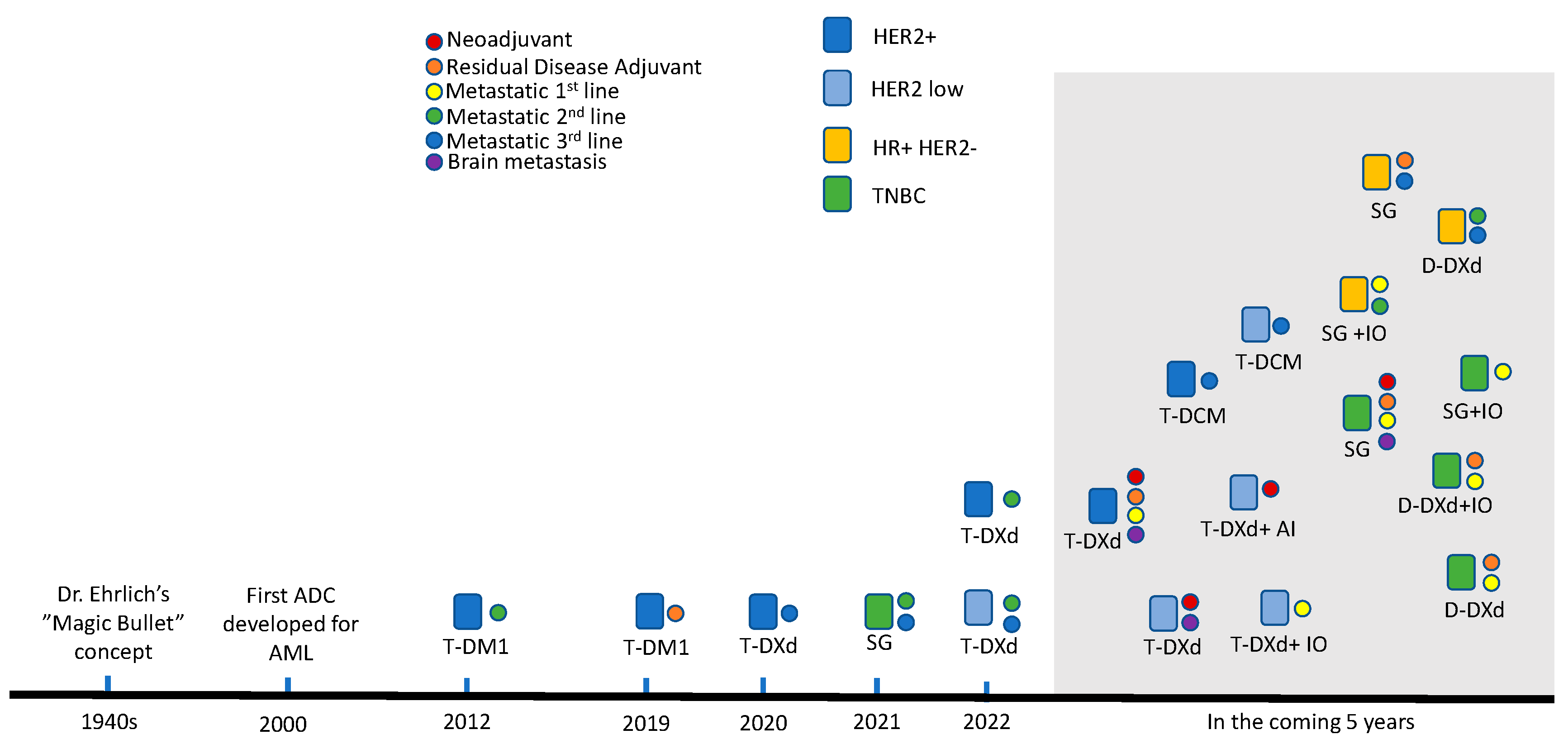
1. Introduction: Development and History of ADCs in Breast Cancer
2. Current Landscape of ADCs in Breast Cancer
2.1. Trastuzumab Emtansine (T-DM1)
2.2. Trastuzumab Deruxtecan (T-DXd)
2.3. Sacituzumab Govitecan (SG)
3. Emerging ADC Development
3.1. Datopotamab Deruxtecan (Dato-DXd)
3.2. Trastuzumab Duocarmazine
4. Discussion
4.1. Future Directions for Current ADC Therapy
4.2. Toxicities of ADCs
4.3. Hormone Receptor (HR)-Positive and HER2-Low/HER2-Negative Breast Cancer
4.4. Resistance Mechanisms and Drug Design
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Maadi, H.; Soheilifar, M.H.; Choi, W.-S.; Moshtaghian, A.; Wang, Z. Trastuzumab Mechanism of Action; 20 Years of Research to Unravel a Dilemma. Cancers 2021, 13, 3540. [Google Scholar] [CrossRef] [PubMed]
- Petersdorf, S.H.; Kopecky, K.J.; Slovak, M.; Willman, C.; Nevill, T.; Brandwein, J.; Larson, R.A.; Erba, H.P.; Stiff, P.J.; Stuart, R.K.; et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013, 121, 4854–4860. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Sig. Transduct Target Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Peddi, P.F.; Hurvitz, S.A. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: Latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2014, 6, 202–209. [Google Scholar] [CrossRef]
- Peddi, P.F.; Hurvitz, S.A. Trastuzumab emtansine: The first targeted chemotherapy for treatment of breast cancer. Future Oncol. 2013, 9, 319–326. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Perez, E.A.; Barrios, C.; Eiermann, W.; Toi, M.; Im, Y.-H.; Conte, P.; Martin, M.; Pienkowski, T.; Pivot, X.B.; Burris III, H.A.; et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab with taxane for human epidermal growth factor receptor 2–positive advanced breast cancer: Final results from MARIANNE. Cancer 2019, 125, 3974–3984. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Wedam, S.; Fashoyin-Aje, L.; Gao, X.; Bloomquist, E.; Tang, S.; Sridhara, R.; Goldberg, K.B.; King-Kallimanis, B.L.; Theoret, M.R.; Ibrahim, A.; et al. FDA Approval Summary: Ado-Trastuzumab Emtansine for the Adjuvant Treatment of HER2-positive Early Breast Cancer. Clin. Cancer Res. 2020, 26, 4180–4185. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- André, F.; Park, Y.H.; Kim, S.-B.; Takano, T.; Im, S.-A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gregori, J.G.; Laurentiis, M.D.; et al. Trastuzumab deruxtecan versus treatment of physician’s choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.-P.; Im, S.-A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef]
- Jacobson, A. Trastuzumab Deruxtecan Improves Progression-Free Survival and Intracranial Response in Patients with HER2-Positive Metastatic Breast Cancer and Brain Metastases. Oncologist 2022, 27, S3–S4. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for HER2-Low Breast Cancer. FDA. 5 August 2022. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer (accessed on 14 May 2023).
- AstraZeneca. A Phase 3 Open-Label Trial of Neoadjuvant Trastuzumab Deruxtecan (T-DXd) Monotherapy or T-DXd Followed by THP Compared to DdAC-THP in Participants with High-Risk HER2-Positive Early-Stage Breast Cancer (DESTINY-Breast11). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05113251 (accessed on 9 April 2023).
- Daiichi Sankyo, Inc. A Phase 3, Multicenter, Randomized, Open-Label, Active-Controlled Study of Trastuzumab Deruxtecan (T-DXd) Versus Trastuzumab Emtansine (T-DM1) in Participants with High-Risk HER2-Positive Primary Breast Cancer Who Have Residual Invasive Disease in Breast or Axillary Lymph Nodes Following Neoadjuvant Therapy (DESTINY-Breast05). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04622319 (accessed on 9 April 2023).
- AstraZeneca. Phase III Study of Trastuzumab Deruxtecan (T-DXd) with or without Pertuzumab Versus Taxane, Trastuzumab and Pertuzumab in HER2-Positive, First-Line Metastatic Breast Cancer (DESTINY-Breast09). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04784715 (accessed on 9 April 2023).
- AstraZeneca. An Open-Label, Multinational, Multicenter, Phase 3b/4 Study of Trastuzumab Deruxtecan in Patients with or without Baseline Brain Metastasis with Previously Treated Advanced/Metastatic HER2-Positive Breast Cancer (DESTINY-Breast12). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04739761 (accessed on 9 April 2023).
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro-Oncology 2023, 25, 157–166. [Google Scholar] [CrossRef]
- AstraZeneca. A Phase 3, Randomized, Multi-Center, Open-Label Study of Trastuzumab Deruxtecan (T-DXd) versus Investigator’s Choice Chemotherapy in HER2-Low, Hormone Receptor Positive Breast Cancer Patients Whose Disease Has Progressed on Endocrine Therapy in the Metastatic Setting (DESTINY-Breast06). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04494425 (accessed on 9 April 2023).
- SABCS 2021: Trastuzumab Deruxtecan (T-DXd) for Advanced Breast Cancer Patients (ABC), Regardless HER2 Status: A Phase II Study with Biomarkers Analysis (DAISY). Available online: https://clin.larvol.com/abstract-detail/SABCS%202021/52081167 (accessed on 15 May 2023).
- Jeon, Y.; Jo, U.; Hong, J.; Gong, G.; Lee, H.J. Trophoblast cell-surface antigen 2 (TROP2) expression in triple-negative breast cancer. BMC Cancer 2022, 22, 1014. [Google Scholar] [CrossRef]
- Kopp, A.; Hofsess, S.; Cardillo, T.M.; Govindan, S.V.; Donnell, J.; Thurber, G.M. Antibody–Drug Conjugate Sacituzumab Govitecan Drives Efficient Tissue Penetration and Rapid Intracellular Drug Release. Mol. Cancer Ther. 2023, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; McShane, L.M.; Dowsett, M. HER2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update Summary. JOP 2018, 14, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Wahby, S.; Fashoyin-Aje, L.; Osgood, C.L.; Cheng, J.; Fiero, M.H.; Zhang, L.; Tang, S.; Hamed, S.S.; Song, P.; Charlab, R.; et al. FDA Approval Summary: Accelerated Approval of Sacituzumab Govitecan-hziy for Third-line Treatment of Metastatic Triple-negative Breast Cancer. Clin. Cancer Res. 2021, 27, 1850–1854. [Google Scholar] [CrossRef]
- Gilead Sciences. A Randomized, Open-Label, Phase 3 Study of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Patients with Previously Untreated, Locally Advanced, Inoperable or Metastatic Triple-Negative Breast Cancer Whose Tumors Do Not Express PD-L1 or in Patients Previously Treated with Anti-PD-(L)1 Agents in the Early Setting Whose Tumors Do Express PD-L1. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05382299 (accessed on 9 April 2023).
- Gilead Sciences. A Randomized, Open-Label, Phase 3 Study of Sacituzumab Govitecan and Pembrolizumab versus Treatment of Physician’s Choice and Pembrolizumab in Patients with Previously Untreated, Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer, Whose Tumors Express PD-L1. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05382286 (accessed on 9 April 2023).
- Holthuis, E.I.; Vondeling, G.T.; Kuiper, J.G.; Dezentjé, V.; Rosenlund, M.; Overbeek, J.A.; van Deurzen, C.H.M. Real-world data of HER2-low metastatic breast cancer: A population based cohort study. Breast 2022, 66, 278–284. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021, 23, 112. [Google Scholar] [CrossRef]
- Gilead Sciences. Phase 3 Study of Sacituzumab Govitecan (IMMU-132) Versus Treatment of Physician’s Choice (TPC) in Subjects with Hormonal Receptor-Positive (HR+) Human Epidermal Growth Factor Receptor 2 (HER2) Negative Metastatic Breast Cancer (MBC) Who Have Failed at Least Two Prior Chemotherapy Regimens. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03901339 (accessed on 9 April 2023).
- Helwick, C. Is Efficacy of Sacituzumab Govitecan-Hziy in the TROPiCS-02 Trial Dependent on Trop-2 Expression?—The ASCO Post. Available online: https://ascopost.com/issues/february-10-2023/is-efficacy-of-sacituzumab-govitecan-hziy-in-the-tropics-02-trial-dependent-on-trop-2-expression/ (accessed on 12 April 2023).
- German Breast Group. Phase III Postneoadjuvant Study Evaluating Sacituzumab Govitecan, an Antibody Drug Conjugate in Primary HER2-Negative Breast Cancer Patients with High Relapse Risk After Standard Neoadjuvant Treatment—SASCIA. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04595565 (accessed on 9 April 2023).
- Daiichi Sankyo Co., Ltd. Phase I/II, Two-Part, Multicenter First-in-Human Study of DS-7300a in Subjects with Advanced Solid Malignant Tumors. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04145622 (accessed on 14 May 2023).
- Mersana Therapeutics. A Phase 1, First-in-Human, Dose Escalation and Expansion, Multicenter Study of XMT-1660 in Participants with Solid Tumors. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05377996 (accessed on 14 May 2023).
- CytomX Therapeutics. A Phase 2, Open-Label Study to Evaluate the Safety and Antitumor Activity of Praluzatamab Ravtansine (CX-2009) in Advanced HR-Positive/HER2-Negative Breast Cancer and of Praluzatamab Ravtansine as Monotherapy and in Combination with Pacmilimab (CX-072) in Advanced Triple-Negative Breast Cancer (CTMX-2009-002). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04596150 (accessed on 14 May 2023).
- Daiichi Sankyo Co., Ltd. Phase 1/2, Multicenter, Open-Label, Multiple-Dose First-in-Human Study of U3-1402, in Subjects with HER3 Positive Metastatic Breast Cancer. clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02980341 (accessed on 14 May 2023).
- Seagen Inc. A Phase 1, Open-Label, Dose-Escalation Study to Evaluate the Safety and Tolerability of SGN-LIV1A in Patients with Metastatic Breast Cancer. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT01969643 (accessed on 14 May 2023).
- Astellas Pharma Global Development, Inc. An Open-Label, Multicenter, Multicohort, Phase 2 Study to Evaluate Enfortumab Vedotin in Subjects with Previously Treated Locally Advanced or Metastatic Malignant Solid Tumors (EV-202). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04225117 (accessed on 10 April 2023).
- VelosBio Inc. A Phase 2 Study of VLS-101 in Patients with Solid Tumors. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04504916 (accessed on 14 May 2023).
- BioAtla, Inc. A Phase 1/2 Safety and Efficacy Dose Escalation / Dose Expansion Study of a CAB-ROR2-ADC, Alone and in Combination with a PD-1 Inhibitor, in Patients with Advanced Solid Tumors (Ph1) and Melanoma and NSCLC Patients (Ph2). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03504488 (accessed on 14 May 2023).
- Daiichi Sankyo Co., Ltd. Phase 1, Two-Part, Multicenter, Open-Label, Multiple Dose, First-in-Human Study of DS-1062a in Subjects with Advanced Solid Tumors (TROPION-PanTumor01). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03401385 (accessed on 9 April 2023).
- AstraZeneca. A Phase-3, Open-Label, Randomized Study of Dato-DXd versus Investigator’s Choice of Chemotherapy (ICC) in Participants with Inoperable or Metastatic HR-Positive, HER2-Negative Breast Cancer Who Have Been Treated with One or Two Prior Lines of Systemic Chemotherapy (TROPION-Breast01). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05104866 (accessed on 10 April 2023).
- AstraZeneca. A Phase 3, Open-Label, Randomised Study of Datopotamab Deruxtecan (Dato-DXd) Versus Investigator’s Choice of Chemotherapy in Patients Who Are Not Candidates for PD-1/PD-L1 Inhibitor Therapy in First-Line Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (TROPION Breast02). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05374512 (accessed on 10 April 2023).
- Schmid, P.; Im, S.-A.; Armstrong, A.; Park, Y.H.; Chung, W.-P.; Nowecki, Z.; Lord, S.; Wysocki, P.J.; Lu, Y.-S.; Dry, H.; et al. BEGONIA: Phase 1b/2 study of durvalumab (D) combinations in locally advanced/metastatic triple-negative breast cancer (TNBC)—Initial results from arm 1, d+paclitaxel (P), and arm 6, d+trastuzumab deruxtecan (T-DXd). JCO 2021, 39, 1023. [Google Scholar] [CrossRef]
- AstraZeneca. A Phase 3 Open-Label, Randomised Study of Datopotamab Deruxtecan (DatoDXd) with or without Durvalumab Versus Investigator’s Choice of Therapy in Patients with Stage I-III Triple-Negative Breast Cancer Who Have Residual Invasive Disease in the Breast and/or Axillary Lymph Nodes at Surgical Resection Following Neoadjuvant Systemic Therapy (TROPION-Breast03). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05629585 (accessed on 10 April 2023).
- Yao, H.-P.; Zhao, H.; Hudson, R.; Tong, X.-M.; Wang, M.-H. Duocarmycin-based antibody–drug conjugates as an emerging biotherapeutic entity for targeted cancer therapy: Pharmaceutical strategy and clinical progress. Drug Discov. Today 2021, 26, 1857–1874. [Google Scholar] [CrossRef]
- Manich, C.S.; O’Shaughnessy, J.; Aftimos, P.G.; van den Tweel, E.; Oesterholt, M.; Escrivá-de-Romaní, S.I.; Tueux, N.Q.; Tan, T.J.; Lim, J.S.; Ladoire, S.; et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021, 32, S1288. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.; Tolaney, S.M.; Desai, N.V.; Fell, G.; Trippa, L.; Comander, A.H.; Mulvey, T.M.; McLaughlin, S.; Ryan, P.; Rosenstock, A.S.; et al. Phase 2 study of response-guided neoadjuvant sacituzumab govitecan (IMMU-132) in patients with localized triple-negative breast cancer: Results from the NeoSTAR trial. JCO 2022, 40, 512. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- M.D. Anderson Cancer Center. A Phase II Study of Neoadjuvant Sacituzumab Govitecan and Pembrolizumab Therapy for Immunochemotherapy-Resistant Early-Stage Triple-Negative Breast Cancer (TNBC). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05675579 (accessed on 12 May 2023).
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Metzger Filho, O.; Viale, G.; Trippa, L.; Li, T.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.; Waks, A.G.; et al. HER2 heterogeneity as a predictor of response to neoadjuvant T-DM1 plus pertuzumab: Results from a prospective clinical trial. JCO 2019, 37, 502. [Google Scholar] [CrossRef]
- Seagen Inc. A Single Arm, Open Label Phase 2 Study of Tucatinib in Combination with Trastuzumab Deruxtecan in Subjects with Previously Treated Unresectable Locally-Advanced or Metastatic HER2+ Breast Cancer. clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04539938 (accessed on 10 April 2023).
- AstraZeneca. A Phase 1b/2 Multicentre, Open-Label, Modular, Dose-Finding and Dose-Expansion Study to Explore the Safety, Tolerability, and Anti-Tumour Activity of Trastuzumab Deruxtecan (T-DXd) in Combination with Other Anti-Cancer Agents in Patients with HER2-Positive Metastatic Breast Cancer (DESTINY-Breast07). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT04538742 (accessed on 10 April 2023).
- Jensen, S.G.; Thomas, P.E.; Christensen, I.J.; Balslev, E.; Hansen, A.; Høgdall, E. Evaluation of analytical accuracy of HER2 status in patients with breast cancer. APMIS 2020, 128, 573–582. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Thanopoulou, E.; Peggs, K.S.; Quezada, S.A.; Swanton, C. Tumour heterogeneity and immune-modulation. Curr. Opin. Pharmacol. 2013, 13, 497–503. [Google Scholar] [CrossRef]
- Morganti, S.; Ivanova, M.; Ferraro, E.; Ascione, L.; Vivanet, G.; Bonizzi, G.; Curigliano, G.; Fusco, N.; Criscitiello, C. Loss of HER2 in breast cancer: Biological mechanisms and technical pitfalls. Cancer Drug Resist. 2022, 5, 971–980. [Google Scholar] [CrossRef]
- Guidi, L.; Pellizzari, G.; Tarantino, P.; Valenza, C.; Curigliano, G. Resistance to Antibody-Drug Conjugates Targeting HER2 in Breast Cancer: Molecular Landscape and Future Challenges. Cancers 2023, 15, 1130. [Google Scholar] [CrossRef]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef]
- Coates, J.T.; Sun, S.; Leshchiner, I.; Thimmiah, N.; Martin, E.E.; McLoughlin, D.; Danysh, B.P.; Slowik, K.; Jacobs, R.A.; Rhrissorrakrai, K.; et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021, 11, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Fernanda, M.; Deluche, E.; Lusque, A.; Le-Bescond, L.; Filleron, T.; Pradat, Y.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; et al. Mechanism of Action and Resistance to Trastuzumab Deruxtecan in Patients with Metastatic Breast Cancer: The DAISY Trial. Nat. Portf. 2022; in review. [Google Scholar] [CrossRef]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Sapra, P. Bioengineering and Mechanism of Action of Antibody Drug Conjugates. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 7 December 2022. [Google Scholar]
| ADC | Target | Antibody | Linker | Payload | DAR |
|---|---|---|---|---|---|
| Trastuzumab emtansine (T-DM1) | HER2 | Trastuzumab | Non-Cleavable | Emtansine (Microtubule Inhibitor) | 3.5 |
| Trastuzumab deruxtecan (T-DXd) | Cleavable | Deruxtecan (Topoisomerase I Inhibitor) | 8 | ||
| Trastuzumab duocarmazine | Cleavable | Duocarmazine (Alkylating Agent) | 2.8 | ||
| Datopotamab deruxtecan | Trop-2 | Datopotamab | Cleavable | Deruxtecan (Topoisomerase I Inhibitor) | 4 |
| Sacituzumab govitecan | Sacituzumab | Cleavable | Govitecan (Topoisomerase I Inhibitor) | 7.6 |
| ADC | Study | Setting | Phase | Treatment | Recurrence Outcome (Hazard Ratio, 95% CI; p Value) | OS (Hazard Ratio, 95% CI; p Value) |
|---|---|---|---|---|---|---|
| HER2-positive | ||||||
| T-DM1 | KATHERINE (2019) | Adjuvant (residual disease post-neoadjuvant) | 3 | T-DM1 vs. trastuzumab | 3-year IDFS: 88.3% vs. 77.02% (HR = 0.50, 95% CI: 0.39–0.64; p < 0.001) | Data not mature |
| EMILIA (2012) | 2nd line metastatic | 3 | T-DM1 vs. lapatinib and capecitabine | PFS: 9.6 vs. 6.4 months (HR = 0.65, 95% CI: 0.55–0.77; p < 0.001) | 29.9 vs. 25.9 months HR = 0·75 (95% CI: 0.64–0.88) | |
| T-DXd | Destiny-Breast03 (2022) | 2nd line metastatic | 3 | T-DXd vs. T-DM1 | PFS: 28.8 vs. 6.8 months (HR = 0.33, 95% CI: 0.26–0.43; p < 0.0001) | Data not mature |
| Destiny-Breast02 (2023) | 3rd line metastatic | 3 | T-DXd vs. physician’s choice of therapy | PFS: 17.8 vs. 6.9 months (HR = 0.36, 95% CI: 0.28–0.45; p < 0.0001) | 39.2 vs. 26.5 months (HR = 0.66, 95% CI: 0.50–0.86; p = 0.0021) | |
| HER2-low, Hormone receptor-positive and negative | ||||||
| T-DXd | Destiny-Breast04 (2022) | 2nd or 3rd line metastatic | 3 | T-DXd vs. physician’s choice of therapy | PFS: 9.9 vs. 5.1 months (HR = 0.50, 95% CI: 0.40–0.63; p < 0.001) | 23.4 vs. 16.8 months (HR = 0.64, 95% CI: 0.49–0.84; p = 0.001) |
| HER2-negative, Hormone receptor-positive | ||||||
| SG | TROPiCS-02 (2022) * | 3rd or later line metastatic | 3 | SG vs. physician’s choice of therapy | PFS: 5.5 vs. 4.0 months (HR = 0.66, 95% CI: 0.53–0.83; p = 0.0003) | 14.4 vs. 11.2 months (HR = 0.79, 95% CI: 0.65–0.96; p = 0.02) |
| Triple Negative (TNBC) | ||||||
| SG | ASCENT (2022) | 2nd or 3rd line metastatic | 3 | SG vs. physician’s choice of therapy | PFS: 5.6 vs. 1.7 months (HR = 0.41, 95% CI: 0.32–0.52; p < 0.001) | 12.1 vs. 6.7 months (HR = 0.48, 95% CI: 0.38–0.59; p < 0.001) |
| ADC | Receptor Status | Study | Setting | Phase | Intervention | Primary End Point |
|---|---|---|---|---|---|---|
| HER2-positive | ||||||
| T-DXd | DESTINY-Breast05 | Adjuvant (residual disease post-neoadjuvant chemotherapy) | 3 | T-DXd vs. T-DM1 | IDFS | |
| DESTINY-Breast09 | 1st line metastatic | 3 | T-DXd ± pertuzumab vs. pertuzumab, trastuzumab and taxane chemotherapy | PFS | ||
| DESTINY-Breast11 | Neoadjuvant | 3 | T-DXd alone vs. T-DXd followed by taxane, pertuzumab, trastuzumab vs. anthracycline, taxane chemotherapy and pertuzumab, trastuzumab | pCR | ||
| DESTINY-Breast12 | 2nd or 3rd line with CNS metastasis | 3 | T-DXd | ORR (cohort 1) | ||
| PFS (cohort 2) | ||||||
| DEBBRAH (Cohort 1) | ≥2-line with CNS metastasis | 2 | T-DXd | PFS | ||
| Trastuzumab Duocarmazine | TULIP | ≥3rd line metastatic | 3 | Trastuzumab duocarmazine vs. physician’s choice of therapy | PFS | |
| HER2-low, Hormone receptor-positive (HR+) | ||||||
| T-DXd | HER2-low (HR+) | TRIO-US B-12/TALENT | Neoadjuvant | 2 | T-DXd vs. T-DXd plus anastrozole | pCR |
| Includes HER2+/HER2-low/HER2− (HR+ and HR−) | DAISY | ≥1 chemotherapy in the metastatic setting | 2 | T-DXd monotherapy | ORR | |
| HER2-low/HER2− (HR+) | DESTINY Breast-06 | 2nd or 3rd line metastatic (no prior chemotherapy in metastatic setting) | 3 | T-DXd vs. physician’s choice of therapy | PFS | |
| Trastuzumab Duocarmazine | HER2-low (HR+) | NCT02277717 | Metastatic | 1 | Trastuzumab duocarmazine | Safety, Pharmacokinetics and Efficacy |
| HER2-negative | ||||||
| SG | HER2− (HR+/HR−) | SASCIA | Adjuvant setting (residual disease post-neoadjuvant chemotherapy) | 3 | SG vs. physician’s choice of therapy | IDFS |
| HER2− (HR+) | NCT04448886 | 1st or 2nd line metastatic | 2 | SG plus pembrolizumab vs. SG alone | PFS | |
| Dato-DXd | HER2− (HR+) | TROPION Breast-01 | 2nd or 3rd line metastatic | 3 | Dato-DXd vs. physician’s choice of therapy | PFS |
| OS | ||||||
| Triple Negative (TNBC) | ||||||
| SG | NeoSTAR | Neoadjuvant | 2 | SG vs. SG plus pembrolizumab | pCR | |
| SASCIA | Adjuvant setting | 3 | SG vs. physician’s choice of therapy | IDFS | ||
| (residual disease post-neoadjuvant chemotherapy) | ||||||
| ASCENT-04 | 1st line metastatic | 3 | SG and pembrolizumab vs. physician’s choice of therapy and pembrolizumab | PFS | ||
| (PD-L1-positive) | ||||||
| ASCENT-03 | 1st line metastatic | 3 | SG vs. physician’s choice of therapy | PFS | ||
| (PD-L1-negative) | ||||||
| Dato-DXd | TROPION Breast-03 | Adjuvant (residual disease post-neoadjuvant chemotherapy) | 3 | Dato-DXd ± durvalumab vs. physician’s choice of therapy | IDFS | |
| BEGONIA | 1st line metastatic | 1–2 | Durvalumab plus novel therapies (including Dato-DXd) | Safety, Pharmacokinetics and Efficacy | ||
| TROPION Breast-02 | 1st line metastatic | 3 | Dato-DXd vs. physician’s choice of chemotherapy of therapy | PFS | ||
| (PD-L1-negative) | OS | |||||
| TROPION Breast-05 | 1st line metastatic | 3 | Dato-DXd plus durvalumab | PFS | ||
| (PD-L1-positive) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, T.; Ali, S.; Mata, D.G.M.M.; Lohmann, A.E.; Blanchette, P.S. Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review. Curr. Oncol. 2023, 30, 6447-6461. https://doi.org/10.3390/curroncol30070474
Xiao T, Ali S, Mata DGMM, Lohmann AE, Blanchette PS. Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review. Current Oncology. 2023; 30(7):6447-6461. https://doi.org/10.3390/curroncol30070474
Chicago/Turabian StyleXiao, Tian, Sanji Ali, Danilo Giffoni M. M. Mata, Ana Elisa Lohmann, and Phillip S. Blanchette. 2023. "Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review" Current Oncology 30, no. 7: 6447-6461. https://doi.org/10.3390/curroncol30070474
APA StyleXiao, T., Ali, S., Mata, D. G. M. M., Lohmann, A. E., & Blanchette, P. S. (2023). Antibody–Drug Conjugates in Breast Cancer: Ascent to Destiny and Beyond—A 2023 Review. Current Oncology, 30(7), 6447-6461. https://doi.org/10.3390/curroncol30070474





