The Level of Agreement between Self-Assessments and Examiner Assessments of Melanocytic Nevus Counts: Findings from an Evaluation of 4548 Double Assessments
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Nevus Counting Procedure
2.3. Instructions for Nevus Self-Counting
2.4. Standardization of Nevus Counting by the Examiners
2.5. Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Distribution of Nevus Counts
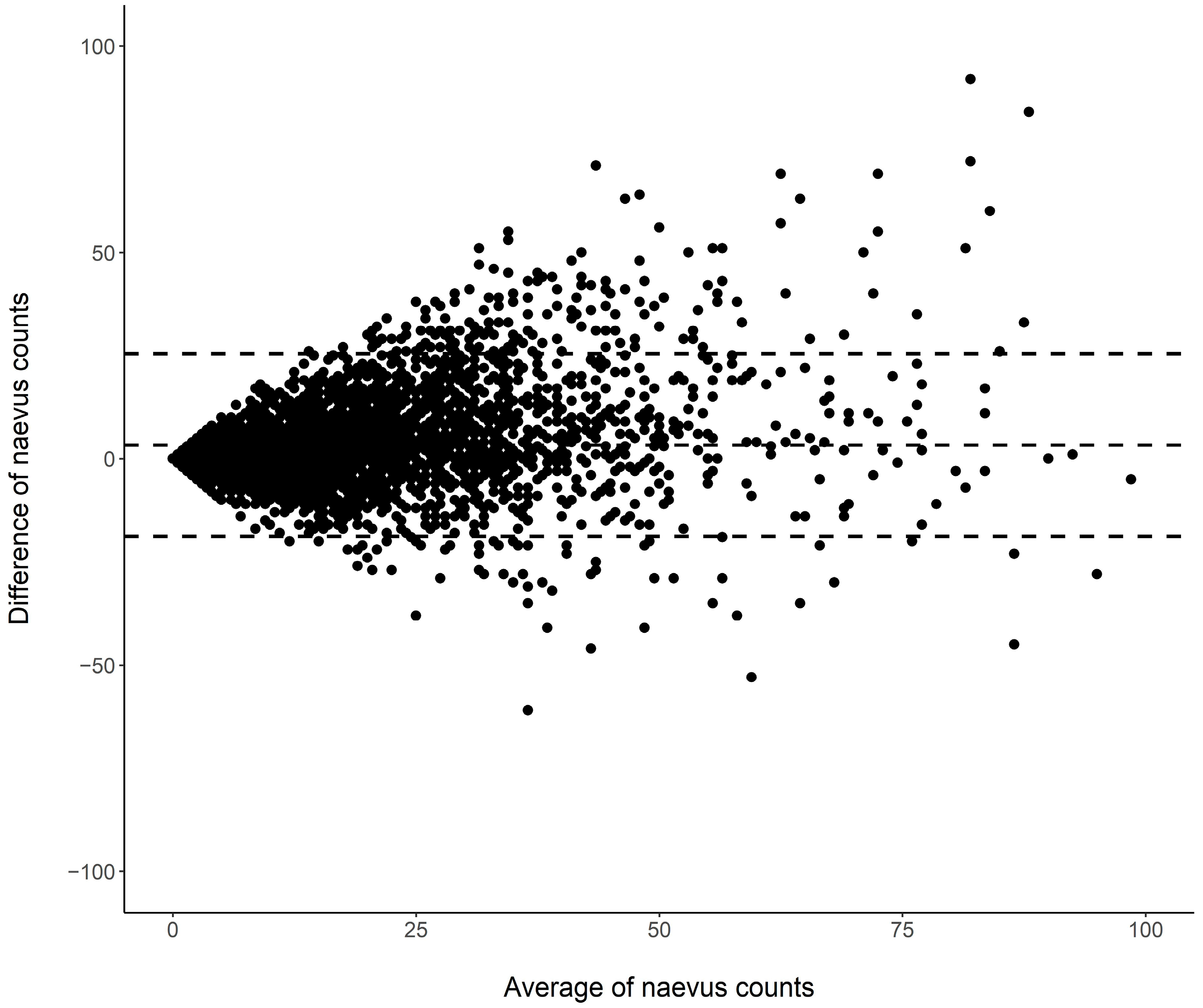
3.2. Nevus Counts: Differences between Assessments
3.3. Nevus Score: Agreement between Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garbe, C.; Keim, U.; Gandini, S.; Amaral, T.; Katalinic, A.; Hollezcek, B.; Martus, P.; Flatz, L.; Leiter, U.; Whiteman, D. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943–2036. Eur. J. Cancer 2021, 152, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.; Flannery, J.; Viola, M.V. Malignant melanoma in Connecticut and Denmark. Int. J. Cancer 1980, 25, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bulliard, J.L.; Cox, B.; Elwood, J.M. Latitude gradients in melanoma incidence and mortality in the non-Maori population of New Zealand. Cancer Causes Control 1994, 5, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Moser, J.C.; Weaver, A.L.; Markovic, S.N.; Brewer, J.D.; Leontovich, A.A.; Hieken, T.J.; Shuster, L.; Kottschade, L.A.; Olariu, A.; et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017, 6, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, H.; Utjes, D.; Olofsson Bagge, R.; Gillgren, P.; Isaksson, K.; Lapins, J.; Schultz, I.L.; Lyth, J.; Andersson, T.M. The Proportion Cured of Patients with Resected Stage II-III Cutaneous Melanoma in Sweden. Cancers 2021, 13, 2456. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin Cancer Screening: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2023, 329, 1296–1307. [Google Scholar] [CrossRef]
- Bell, K.J.L.; Nijsten, T. Melanoma overdiagnosis: Why it matters and what can be done about it. Br. J. Dermatol. 2022, 187, 459–460. [Google Scholar] [CrossRef]
- Ebell, M.H.; Thai, T.N.; Royalty, K.J. Cancer screening recommendations: An international comparison of high income countries. Public Health Rev. 2018, 39, 7. [Google Scholar] [CrossRef]
- Breitbart, E.W.; Waldmann, A.; Nolte, S.; Capellaro, M.; Greinert, R.; Volkmer, B.; Katalinic, A. Systematic skin cancer screening in Northern Germany. J. Am. Acad. Dermatol. 2012, 66, 201–211. [Google Scholar] [CrossRef]
- Rat, C.; Blachier, L.; Hild, S.; Molinie, F.; Gaultier, A.; Dreno, B.; Nguyen, J.-M. Targeted screening for melanoma after a 5-year follow-up: Comparison of melanoma incidence and lesion thickness at diagnosis in screened (versus unscreened) patients. La Presse Médicale Open 2021, 2, 100013. [Google Scholar] [CrossRef]
- Bobrowska, A.; Murton, M.; Seedat, F.; Visintin, C.; Mackie, A.; Steele, R.; Marshall, J. Targeted screening in the UK: A narrow concept with broad application. Lancet Reg. Health Eur. 2022, 16, 100353. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. J. Am. Acad. Dermatol. 2017, 77, 938–945.e934. [Google Scholar] [CrossRef]
- Kaiser, I.; Pfahlberg, A.B.; Uter, W.; Heppt, M.V.; Veierod, M.B.; Gefeller, O. Risk Prediction Models for Melanoma: A Systematic Review on the Heterogeneity in Model Development and Validation. Int. J. Environ. Res. Public Health 2020, 17, 7919. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Epidemiological Studies on Melanocytic Naevi: Protocol for Identifying and Recording Naevi; IARC Internal Report No. 90/002; International Agency for Research on Cancer: Lyon, France, 1990. [Google Scholar]
- Farinas-Alvarez, C.; Rodenas, J.M.; Herranz, M.T.; Delgado-Rodriguez, M. The naevus count on the arms as a predictor of the number of melanocytic naevi on the whole body. Br. J. Dermatol. 1999, 140, 457–462. [Google Scholar] [CrossRef][Green Version]
- Carli, P.; Giorgi, V.d.; Nardini, P.; Mannone, F.; Palli, D.; Giannotti, B. Melanoma detection rate and concordance between self-skin examination and clinical evaluation in patients attending a pigmented lesion clinic in Italy. Br. J. Dermatol. 2002, 146, 261–266. [Google Scholar] [CrossRef]
- Gefeller, O.; Tarantino, J.; Lederer, P.; Uter, W.; Pfahlberg, A.B. The relation between patterns of vacation sun exposure and the development of acquired melanocytic nevi in German children 6–7 years of age. Am. J. Epidemiol. 2007, 165, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The validity and practicality of sun-reactive skin types I through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Carrasco, J.L. A generalized concordance correlation coefficient based on the variance components generalized linear mixed models for overdispersed count data. Biometrics 2010, 66, 897–904. [Google Scholar] [CrossRef]
- Cohen, J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.V.; Allison, T. A New Procedure for Assessing Reliability of Scoring EEG Sleep Recording. Am. J. EEG Technol. 1971, 11, 101–110. [Google Scholar] [CrossRef]
- Bowker, A.H. A test for symmetry in contingency tables. J. Am. Stat. Assoc. 1948, 43, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L.; Levin, B.; Paik, M.C. The Measurement of Interrater Agreement. In Statistical Methods for Rates and Proportions; John Wiley & Sons, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Walter, S.D.; Marrett, L.D.; Hertzman, C. Reliability of interviewer and subject assessments of nevus counts in a study of melanoma. J. Clin. Epidemiol. 1991, 44, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.B.; Roush, G.C.; Barnhill, R.L. Sensitivity and Specificity of Self-examination for Cutaneous Malignant Melanoma Risk Factors. Am. J. Prev. Med. 1993, 9, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lawson, D.D.; Schneider, J.S.; Sagebiel, R.W. Nevus counting as a risk factor for melanoma: Comparison of self-count with count by physician. J. Am. Acad. Dermatol. 1994, 31, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Little, P.; Keefe, M.; White, J. Self screening for risk of melanoma: Validity of self mole counting by patients in a single general practice. BMJ 1995, 310, 912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, A.; Wilkinson, C.; Ranger, M.; Pill, R.; August, P. Can primary prevention or selective screening for melanoma be more precisely targeted through general practice? A prospective study to validate a self administered risk score. BMJ 1998, 316, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Buettner, P.G.; Garbe, C. Agreement between self-assessment of melanocytic nevi by patients and dermatologic examination. Am. J. Epidemiol. 2000, 151, 72–77. [Google Scholar] [CrossRef][Green Version]
- Melia, J.; Harland, C.; Moss, S.; Eiser, J.R.; Pendry, L. Feasibility of targeted early detection for melanoma: A population-based screening study. Br. J. Cancer 2000, 82, 1605–1609. [Google Scholar] [CrossRef]
- Harrison, S.L.; Buettner, P.G.; MacLennan, R.; Kelly, J.W.; Rivers, J.K. How good are parents at assessing melanocytic nevi on their children? A study comparing parental counts, dermatologist counts, and counts obtained from photographs. Am. J. Epidemiol. 2002, 155, 1128–1136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harbauer, A.; Binder, M.; Pehamberger, H.; Wolff, K.; Kittler, H. Validity of an unsupervised self-administered questionnaire for self-assessment of melanoma risk. Melanoma Res. 2003, 13, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Richtig, E.; Santigli, E.; Fink-Puches, R.; Weger, W.; Hofmann-Wellenhof, R. Assessing melanoma risk factors: How closely do patients and doctors agree? Public Health 2008, 122, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.L.; Stapleton, J.; Turrisi, R.; Ortiz, S.; Robinson, J.K.; Mallett, K.A. Thoroughness of skin examination by melanoma patients: Influence of age, sex and partner. Australas. J. Dermatol. 2009, 50, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Richtig, E.; Jung, E.; Asback, K.; Trapp, M.; Hofmann-Wellenhof, R. Knowledge and Perception of Melanocytic Nevi and Sunburn in Young Children. Pediatr. Dermatol. 2009, 26, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Quereux, G.; Nguyen, J.M.; Volteau, C.; Lequeux, Y.; Dreno, B. Creation and test of a questionnaire for self-assessment of melanoma risk factors. Eur. J. Cancer Prev. 2010, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Morze, C.J.; Olsen, C.M.; Perry, S.L.; Jackman, L.M.; Ranieri, B.A.; O’Brien, S.M.; Cicero, R.A.; Whiteman, D.C. Good test-retest reproducibility for an instrument to capture self-reported melanoma risk factors. J. Clin. Epidemiol. 2012, 65, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Fiessler, C.; Pfahlberg, A.; Li, J.; Uter, W.; Gefeller, O. Accuracy and reliability of naevus self-counts. Melanoma Res. 2014, 24, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E.; Pickles, K.M.; Goumas, C.; Vu, T.; Schmid, H.; Nagore, E.; Kelly, J.; Aitken, J.F.; Giles, G.G.; Hopper, J.L.; et al. Accuracy of self-reported nevus and pigmentation phenotype compared with clinical assessment in a population-based study of young Australian Adults. Cancer Epidemiol. Biomark. Prev. 2015, 24, 736–743. [Google Scholar] [CrossRef]
- Stapleton, J.L.; Turrisi, R.; Mallett, K.A.; Robinson, J.K. Correspondence between pigmented lesions identified by melanoma patients trained to perform partner-assisted skin self-examination and dermatological examination. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1247–1253. [Google Scholar] [CrossRef]
- Winkler, A.; Plugfelder, A.; Weide, B.; Leiter, U.; Held, L.; Garbe, C.; Eigentler, T. Screening for skin cancer in bank and insurance employees: Risk profile and correlation of self and physician’s assessment. Int. J. Dermatol. 2015, 54, 419–423. [Google Scholar] [CrossRef]
- Betz-Stablein, B.; Koh, U.; Plasmeijer, E.I.; Janda, M.; Aitken, J.F.; Soyer, H.P.; Green, A.C. Self-reported naevus density may lead to misclassification of melanoma risk. Br. J. Dermatol. 2020, 182, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.; Sollena, P.; Esposito, M.; Fargnoli, M.C.; Peris, K.; Nagore, E. Self-Assessment Questionnaire on Patient-Physician Concordance on Nevus Self-Count and Models Development to Predict High-Risk Phenotype > 50 Nevi. Dermatology 2022, 238, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E.; Goumas, C.; Vuong, K.; Davies, J.R.; Barrett, J.H.; Holland, E.A.; Schmid, H.; Agha-Hamilton, C.; Armstrong, B.K.; Kefford, R.F.; et al. MC1R genotype as a predictor of early-onset melanoma, compared with self-reported and physician-measured traditional risk factors: An Australian case-control-family study. BMC Cancer 2013, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Vuong, K.; Armstrong, B.K.; Weiderpass, E.; Lund, E.; Adami, H.O.; Veierod, M.B.; Barrett, J.H.; Davies, J.R.; Bishop, D.T.; Whiteman, D.C.; et al. Development and External Validation of a Melanoma Risk Prediction Model Based on Self-assessed Risk Factors. JAMA Dermatol. 2016, 152, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E.; Drummond, M.; Kanetsky, P.A.; Australian Melanoma Family Study, I.; Leeds Case-Control Study, I.; Goldstein, A.M.; Barrett, J.H.; MacGregor, S.; Law, M.H.; Iles, M.M.; et al. Assessing the Incremental Contribution of Common Genomic Variants to Melanoma Risk Prediction in Two Population-Based Studies. J. Investig. Dermatol. 2018, 138, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Vuong, K.; Armstrong, B.K.; Drummond, M.; Hopper, J.L.; Barrett, J.H.; Davies, J.R.; Bishop, D.T.; Newton-Bishop, J.; Aitken, J.F.; Giles, G.G.; et al. Development and external validation study of a melanoma risk prediction model incorporating clinically assessed naevi and solar lentigines. Br. J. Dermatol. 2020, 182, 1262–1268. [Google Scholar] [CrossRef]
- Cust, A.E.; Schmid, H.; Maskiell, J.A.; Jetann, J.; Ferguson, M.; Holland, E.A.; Agha-Hamilton, C.; Jenkins, M.A.; Kelly, J.; Kefford, R.F.; et al. Population-based, case-control-family design to investigate genetic and environmental influences on melanoma risk: Australian Melanoma Family Study. Am. J. Epidemiol. 2009, 170, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Newton-Bishop, J.A.; Chang, Y.M.; Iles, M.M.; Taylor, J.C.; Bakker, B.; Chan, M.; Leake, S.; Karpavicius, B.; Haynes, S.; Fitzgibbon, E.; et al. Melanocytic nevi, nevus genes, and melanoma risk in a large case-control study in the United Kingdom. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2043–2054. [Google Scholar] [CrossRef]
- De, A.; Sarda, A.; Gupta, S.; Das, S. Use of Artificial Intelligence in Dermatology. Indian J. Dermatol. 2020, 65, 352–357. [Google Scholar] [CrossRef]
- Young, A.T.; Xiong, M.; Pfau, J.; Keiser, M.J.; Wei, M.L. Artificial Intelligence in Dermatology: A Primer. J. Investig. Dermatol. 2020, 140, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, S.; Rao, B.K. Usefulness of Smartphones in Dermatology: A US-Based Review. Int. J. Environ. Res. Public Health 2022, 19, 3553. [Google Scholar] [CrossRef] [PubMed]
| Phenotype Variable | Absolute Number (n 1) | Proportion (%) |
|---|---|---|
| Fitzpatrick skin type | ||
| Type I | 234 | 5.18 |
| Type II | 1524 | 33.70 |
| Type III | 2352 | 52.01 |
| Type IV | 412 | 9.11 |
| Freckling | ||
| None | 2632 | 58.02 |
| Few | 1546 | 34.08 |
| Many | 358 | 7.89 |
| Hair color | ||
| Red | 61 | 1.34 |
| Blonde | 1214 | 26.75 |
| Brown | 2971 | 65.47 |
| Black | 292 | 6.44 |
| Eye color | ||
| Dark blue | 496 | 10.94 |
| Light blue/gray | 1003 | 22.12 |
| Green | 847 | 18.68 |
| Green/brown | 1060 | 23.37 |
| Light brown | 610 | 13.45 |
| Dark brown | 519 | 11.44 |
| Nevus score | ||
| [0, 5] | 1082 | 23.79 |
| (5, 10] | 940 | 20.67 |
| (10, 15] | 796 | 17.50 |
| (15, 20] | 561 | 12.34 |
| (20, 30] | 628 | 13.81 |
| (30, 50] | 415 | 9.12 |
| >50 | 126 | 2.77 |
| Examiner Assessment | ||||||
|---|---|---|---|---|---|---|
| [0, 5] | (5, 10] | (10, 16] | (16, 26] | >26 | ||
| Self-assessment | [0, 5] | 695 | 152 | 37 | 12 | 0 |
| (5, 10] | 251 | 380 | 166 | 51 | 12 | |
| (10, 16] | 87 | 218 | 285 | 165 | 21 | |
| (16, 26] | 38 | 135 | 292 | 368 | 115 | |
| >26 | 11 | 55 | 132 | 311 | 559 | |
| Subgroup | Observed Agreement in % (95% CI) | Weighted Kappa (95% CI) | p-Value |
|---|---|---|---|
| Sex | 0.08 | ||
| Male | 47.90 (45.52–50.28) | 0.579 (0.554–0.604) | |
| Female | 51.70 (49.87–53.54) | 0.607 (0.588–0.626) | |
| Degree course | 0.76 | ||
| Clinical medicine | 50.14 (48.62–51.66) | 0.596 (0.580–0.611) | |
| Other | 51.79 (46.84–56.73) | 0.605 (0.554–0.655) | |
| Time | 0.54 | ||
| Summer term | 50.25 (48.18–52.32) | 0.601 (0.580–0.622) | |
| Winter term | 50.33 (48.28–52.37) | 0.592 (0.570–0.613) | |
| Fitzpatrick skin type | 0.72 | ||
| Type I | 53.42 (47.03–59.81) | 0.609 (0.542–0.677) | |
| Type II | 49.87 (47.36–52.38) | 0.585 (0.558–0.612) | |
| Type III | 49.53 (47.51–51.55) | 0.581 (0.560–0.603) | |
| Type IV | 55.10 (50.29–59.90) | 0.607 (0.556–0.658) | |
| Freckling | 0.89 | ||
| None | 50.27 (48.36–52.18) | 0.589 (0.569–0.609) | |
| Few | 49.74 (47.25–52.23) | 0.588 (0.561–0.614) | |
| Many | 52.79 (47.62–57.97) | 0.574 (0.514–0.633) | |
| Hair color | 0.15 | ||
| Red | 52.46 (39.93–64.99) | 0.561 (0.412–0.711) | |
| Blonde | 48.11 (45.29–50.92) | 0.565 (0.534–0.595) | |
| Brown | 50.32 (48.52–52.12) | 0.599 (0.581–0.618) | |
| Black | 58.90 (53.26–64.55) | 0.631 (0.571–0.691) | |
| Eye color | 0.01 | ||
| Dark blue | 43.55 (39.18–47.91) | 0.512 (0.462–0.561) | |
| Light blue/gray | 48.75 (45.66–51.85) | 0.590 (0.558–0.622) | |
| Green | 51.71 (48.35–55.08) | 0.606 (0.570–0.641) | |
| Green/brown | 49.91 (46.90–52.92) | 0.581 (0.548–0.613) | |
| Light brown | 51.48 (47.51–55.44) | 0.595 (0.553–0.638) | |
| Dark rown | 56.84 (52.58–61.10) | 0.642 (0.598–0.685) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gefeller, O.; Kaiser, I.; Brockmann, E.M.; Uter, W.; Pfahlberg, A.B. The Level of Agreement between Self-Assessments and Examiner Assessments of Melanocytic Nevus Counts: Findings from an Evaluation of 4548 Double Assessments. Curr. Oncol. 2024, 31, 2221-2232. https://doi.org/10.3390/curroncol31040164
Gefeller O, Kaiser I, Brockmann EM, Uter W, Pfahlberg AB. The Level of Agreement between Self-Assessments and Examiner Assessments of Melanocytic Nevus Counts: Findings from an Evaluation of 4548 Double Assessments. Current Oncology. 2024; 31(4):2221-2232. https://doi.org/10.3390/curroncol31040164
Chicago/Turabian StyleGefeller, Olaf, Isabelle Kaiser, Emily M. Brockmann, Wolfgang Uter, and Annette B. Pfahlberg. 2024. "The Level of Agreement between Self-Assessments and Examiner Assessments of Melanocytic Nevus Counts: Findings from an Evaluation of 4548 Double Assessments" Current Oncology 31, no. 4: 2221-2232. https://doi.org/10.3390/curroncol31040164
APA StyleGefeller, O., Kaiser, I., Brockmann, E. M., Uter, W., & Pfahlberg, A. B. (2024). The Level of Agreement between Self-Assessments and Examiner Assessments of Melanocytic Nevus Counts: Findings from an Evaluation of 4548 Double Assessments. Current Oncology, 31(4), 2221-2232. https://doi.org/10.3390/curroncol31040164






