Prioritizing Melanoma Surgeries to Prevent Wait Time Delays and Upstaging of Melanoma during the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
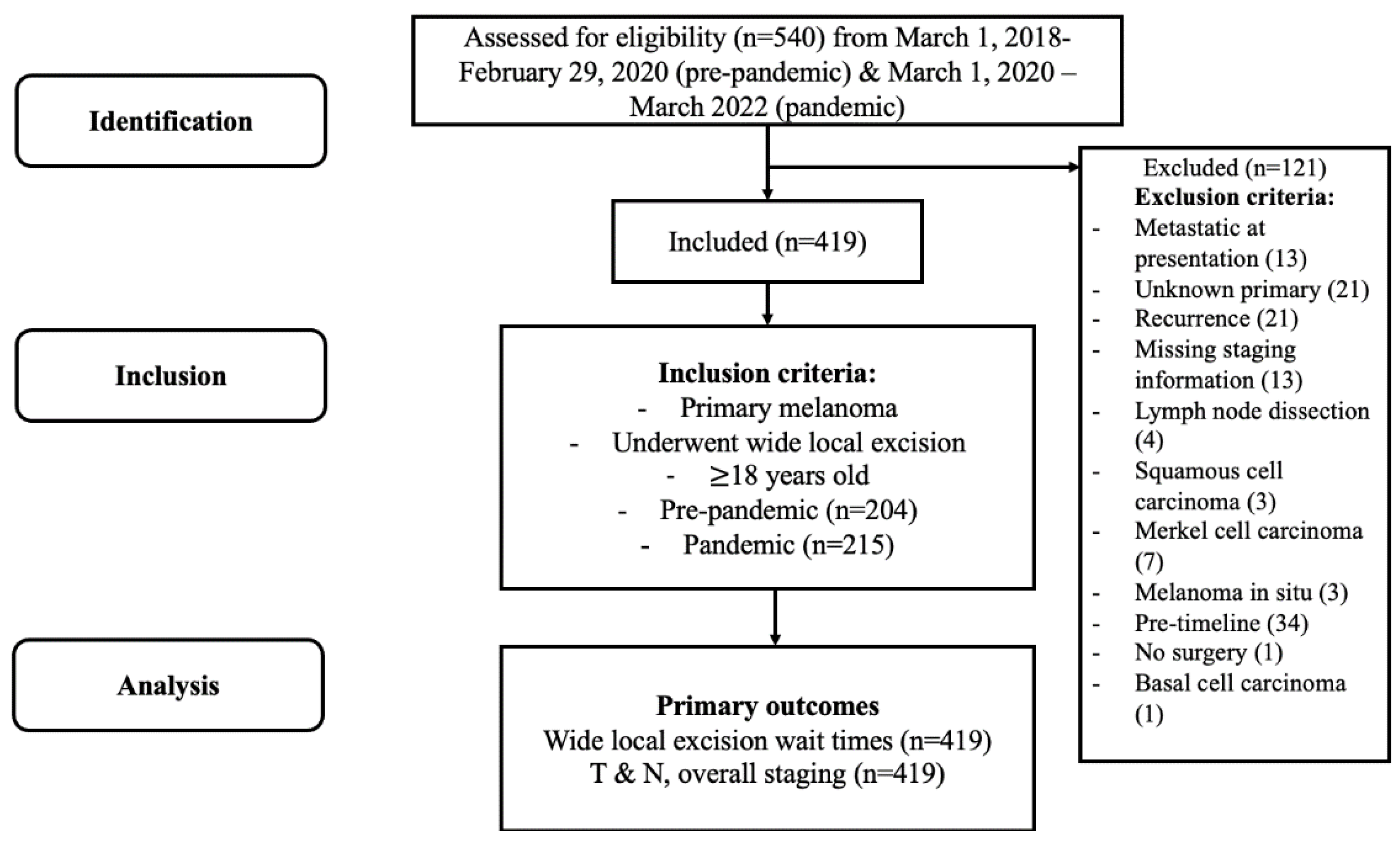
3.1. Study Population
3.2. Wait Times
3.3. Tumour, Nodal, and Overall Staging
4. Discussion
4.1. Key Findings
4.2. Study Limitations
4.3. Implications and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M.; et al. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: An international, prospective, cohort study. Lancet Oncol. 2021, 22, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Melanoma Skin Cancer Statistics. Canadian Cancer Society. Available online: https://cancer.ca/en/cancer-information/cancer-types/skin-melanoma/statistics (accessed on 5 May 2023).
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- von Schuckmann, L.A.; Hughes, M.C.B.; Ghiasvand, R.; Malt, M.; van der Pols, J.C.; Beesley, V.L.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019, 155, 688–693. [Google Scholar] [CrossRef]
- Measuring Wait Times for Cancer Surgeries. Health Quality Ontario. Available online: https://www.hqontario.ca/System-Performance/Measuring-System-Performance/Measuring-Wait-Times-for-Cancer-Surgeries (accessed on 5 January 2023).
- Heer, E.; Ruan, Y.; Boyne, D.J.; Jarada, T.N.; Heng, D.; Henning, J.W.; Morris, D.M.; O’Sullivan, D.E.; Cheung, W.Y.; Brenner, D.R. Impact of the COVID-19 pandemic on cancer diagnoses, stage and survival in Alberta. CMAJ 2023, 195, E804–E812. [Google Scholar] [CrossRef]
- Fu, R.; Sutradhar, R.; Li, Q.; Kamalraj, P.; Dare, A.; Hanna, T.P.; Chan, K.K.W.; Irish, J.C.; Coburn, N.; Hallet, J.; et al. Early survival for patients newly diagnosed with cancer during COVID-19 in Ontario, Canada: A population-based cohort study. Cancer Med. 2023, 12, 11849–11859. [Google Scholar] [CrossRef]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.M.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systematic review and meta-analysis. BMJ 2020, 371, m4087. [Google Scholar] [CrossRef]
- CCO Pandemic Plan Review Group. Pandemic Planning Clinical Guideline for Patients with Cancer; Cancer Care Ontario: Toronto, ON, Canada, 2021; Available online: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/64736 (accessed on 5 January 2023).
- McClean, A.; Matteucci, P.; Totty, J. The impact of COVID19 on the presentation, diagnosis and management of cutaneous melanoma and squamous cell carcinoma in a single tertiary referral centre. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2831–2870. [Google Scholar] [CrossRef]
- Balakirski, G.; Hofmann, S.C.; Isselmann, N.; Giordano, A.; Dippel, E.; Löser, C.R. Effects of the COVID-19 pandemic on inpatient skin surgery in Germany: Results of the DESSI-survey. J. Dtsch. Dermatol. Ges. 2023, 21, 727–738. [Google Scholar] [CrossRef]
- Davis, C.H.; Ho, J.; Greco, S.H.; Koshenkov, V.P.; Vidri, R.J.; Farma, J.M.; Berger, A.C. COVID-19 is Affecting the presentation and treatment of melanoma patients in the northeastern United States. Ann. Surg. Oncol. 2022, 29, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Weston, G.K.; Jeong, H.S.; Mu, E.W.; Polsky, D.; Meehan, S.A. Impact of COVID-19 on melanoma diagnosis. Melanoma Res. 2021, 31, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, L.; Apostu, A.P.; Vesa, Ș.C.; Cășeriu, A.E.; Frățilă, S.; Iancu, G.; Bejinariu, N.; Munteanu, M.; Șenilă, S.C.; Vasilovici, A. Impact of the COVID-19 pandemic on melanoma diagnosis in romania-data from two university centers. Int. J. Environ. Res. Public Health 2022, 19, 15129. [Google Scholar] [CrossRef]
- Barcaui, C.B.; Machado, C.J.; Piñeiro-Maceira, J. Impact of the SARS-CoV-2 pandemic on the diagnosis of primary cutaneous melanoma at a University Hospital in Rio de Janeiro. An. Bras. Dermatol. 2022, 97, 801–803. [Google Scholar] [CrossRef]
- Kostner, L.; Cerminara, S.E.; Pamplona, G.S.P.; Maul, J.T.; Dummer, R.; Ramelyte, E.; Mangana, J.; Wagner, N.B.; Cozzio, A.; Kreiter, S.; et al. Effects of COVID-19 Lockdown on melanoma diagnosis in switzerland: Increased tumor thickness in elderly females and shift towards stage IV melanoma during lockdown. Cancers 2022, 14, 2360. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Porreca, A.; Amoruso, G.F.; Atzori, L.; Calzavara-Pinton, P.; De Tursi, M.; Buduo, A.D.; Marino, P.D.; Fabroncini, G.; Fantini, F.; et al. The effect of the COVID-19 lockdown on melanoma diagnosis in italy. Clin. Dermatol. 2021, 39, 911–919. [Google Scholar] [CrossRef]
- Aabed, H.; Bloanca, V.; Crainiceanu, Z.; Bratosin, F.; Citu, C.; Diaconu, M.M.; Ciorica, O.; Bratu, T. The impact of sars-cov-2 pandemic on patients with malignant melanoma at a Romanian academic center: A four-year retrospective analysis. Int. J. Environ. Res. Public Health 2022, 19, 8499. [Google Scholar] [CrossRef]
- Demaerel, P.G.; Leloup, A.; Brochez, L.; Van Eycken, L.; Garmyn, M. Impact of the COVID-19 pandemic on the incidence and thickness of cutaneous melanoma in Belgium. Biomedicines 2023, 11, 1645. [Google Scholar] [CrossRef]
- Lo Bello, G.; Pini, G.M.; Ferguglia, G.; Regazzini, R.; Locatelli, A.; Patriarca, C. Effects of COVID-19 restriction measures and clinical resetting on delayed melanoma diagnosis: A single-institution experience. Ital. J. Dermatol. Venerol. 2021, 156, 497–498. [Google Scholar] [CrossRef]
- Gedeah, C.; Damsin, T.; Absil, G.; Somja, J.; Collins, P.; Rorive, A.; Marchal, N.; Seidel, L.; Nikkels, A.F. The impact of COVID-19 on the new diagnoses of melanoma. Eur. J. Dermatol. 2021, 31, 565–567. [Google Scholar] [CrossRef]
- Rich, H.; Jones, B.; Malin, I.; Hemington-Gorse, S.J.; Cubitt, J.J. Plastic surgical management of skin cancer patients during the COVID-19 pandemic. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 644–710. [Google Scholar] [CrossRef] [PubMed]
- Venables, Z.C.; Ahmed, S.; Bleiker, T.O.; Broggio, J.; Kwiatkowska, M.; Levell, N.J.; Millington, G.W.M.; Paley, L.; Payne, E.; M Proby, C.M.; et al. The impact of the COVID-19 pandemic on skin cancer incidence and treatment in England, 2020. Br. J. Dermatol. 2021, 185, 460–462. [Google Scholar] [CrossRef]
- Ferrara, G.; De Vincentiis, L.; Ambrosini-Spaltro, A.; Barbareschi, M.; Bertolini, V.; Contato, E.; Crivelli, F.; Feyles, E.; Mariani, M.P.; Morelli, L.; et al. Cancer diagnostic delay in northern and central Italy during the 2020 lockdown due to the coronavirus disease 2019 pandemic. Am. J. Clin. Pathol. 2021, 155, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Kyrgidis, A.; Manoli, S.M.; Papageorgiou, C.; Lallas, K.; Sotiriou, E.; Vakirlis, E.; Sidiropoulos, T.; Ioannides, D.; Apalla, Z. Delayed skin cancer diagnosis in 2020 because of the COVID-19-related restrictions: Data from an institutional registry. J. Am. Acad. Dermatol. 2021, 85, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Cocuz, I.G.; Cocuz, M.E.; Niculescu, R.; Șincu, M.C.; Tinca, A.C.; Sabău, A.H.; Chiorean, D.M.; Morariu, S.H.; Cotoi, O.S. The impact of and adaptations due to the COVID-19 pandemic on the histopathological diagnosis of skin pathologies, including non-melanocyte and melanoma skin cancers-a single-center study in Romania. Medicina 2021, 57, 533. [Google Scholar] [CrossRef] [PubMed]
- Hoellwerth, M.; Kaiser, A.; Emberger, M.; Brandlmaier, M.; Laimer, M.; Egger, A.; Bauer, J.W.; Koelblinger, P. COVID-19-induced reduction in primary melanoma diagnoses: Experience from a dermatopathology referral center. J. Clin. Med. 2021, 10, 4059. [Google Scholar] [CrossRef]
- Ibrahim, L.S.; Venables, Z.C.; McPhail, S.; Levell, N.J. Missing melanomas in England during the COVID-19 pandemic: 2485 fewer melanoma diagnoses in 2020 than in 2019. Br. J. Dermatol. 2023, 189, 345–347. [Google Scholar] [CrossRef]
- Asai, Y.; Nguyen, P.; Hanna, T.P. Impact of the COVID-19 pandemic on skin cancer diagnosis: A population-based study. PLoS ONE 2021, 16, e0248492. [Google Scholar] [CrossRef]
- DP17: Impact of the COVID-19 pandemic on melanoma staging in a Scottish health board. Br. J. Dermatol. 2022, 187 (Suppl. S1), 152. [CrossRef]
- Lamblin, G.; Chene, G.; Leaune, E.; Philip, C.A.; Moret, S.; Nohuz, E.; Golfier, F.; Cortet, M. The psychological impact of therapeutic changes during the COVID-19-lockdown for gynaecological and breast cancer patients. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102311. [Google Scholar] [CrossRef]
- Wang, X.; Li, M.; Shi, Q.; Ji, H.; Kong, S.; Zhu, L.; Zhang, H.M. Fear of Progression, Anxiety, and Depression in Patients With Advanced Melanoma in the COVID-19 and Post-COVID-19 Era. Front. Psychiatry 2022, 13, 880978. [Google Scholar] [CrossRef] [PubMed]
- Crealey, G.E.; Hackett, C.; Harkin, K.; Heckmann, P.; Kelleher, F.; Lyng, Á.; McCarthy, T.; McEnery, M.; Meaney, C.; Roche, D. Melanoma-related costs by disease stage and phase of management in Ireland. J. Public Health 2023, 45, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Lyth, J.; Carstensen, J.; Synnerstad, I.; Lindholm, C. Stage-specific direct health care costs in patients with cutaneous malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Guy, G.P., Jr.; Ekwueme, D.U.; Tangka, F.K.; Richardson, L.C. Melanoma treatment costs: A systematic review of the literature, 1990–2011. Am. J. Prev. Med. 2012, 43, 537–545. [Google Scholar] [CrossRef]
| Characteristic | Pre-Pandemic (n = 204) | Pandemic (n = 215) | p-Value |
|---|---|---|---|
| Age, years, median (IQR) | 68.0 (60.0–76.5) | 64.0 (55.0–74.0) | 0.017 |
| Sex, n (%) | 0.624 | ||
| Male | 117 (57.4) | 118 (54.9) | |
| Female | 87 (42.6) | 97 (45.1) | |
| CCS, median (IQR) | 3 (3-4) | 3 (3-3) | 0.007 |
| CCS, n (%) | |||
| 0–2 | 28 (13.7) | 43 (20.0) | |
| 3–5 | 166 (81.4) | 160 (74.4) | |
| 6–8 | 10 (4.9) | 12 (5.6) | |
| Palpable disease at presentation, n (%) | 14 (6.9) | 11 (5.1) | 0.538 |
| SLNB performed, n (%) | 183 (89.7) | 205 (95.3) | 0.039 |
| LND performed, n (%) | 26 (12.7) | 17 (7.9) | 0.110 |
| Characteristic | Pre-Pandemic (n = 204) | Pandemic (n = 215) | p-Value |
|---|---|---|---|
| Histological type, n (%) | 0.094 | ||
| Superficial spreading | 78 (38.2) | 97 (45.1) | |
| Nodular | 57 (27.9) | 59 (27.4) | |
| Not otherwise specified | 35 (16.6) | 31 (14.4) | |
| Lentigo maligna | 12 (5.9) * | 3 (1.4) * | |
| Desmoplastic | 5 (2.5) | 7 (3.3) | |
| Acral lentiginous | 3 (1.5) | 3 (1.4) | |
| Spitzoid | 0 | 2 (0.9) | |
| Mixed | 5 (2.5) | 3 (1.4) | |
| Not reported | 5 (2.5) | 1 (0.5) | |
| Other | 4 (2.0) | 9 (4.2) | |
| Location, n (%) | 0.271 | ||
| Head and neck | 40 (19.6) * | 25 (11.6) * | |
| Back | 43 (21.1) | 52 (24.2) | |
| Trunk | 21 (10.3) | 20 (9.3) | |
| Arm | 40 (19.6) | 43 (20.0) | |
| Leg | 29 (14.2) | 39 (18.1) | |
| Shoulder | 12 (5.9) | 15 (7.0) | |
| Finger | 0 | 2 (0.9) | |
| Scalp | 10 (4.9) | 9 (4.2) | |
| Foot | 4 (2.0) | 8 (3.7) | |
| Toes | 3 (1.5) | 1 (0.5) | |
| Vulva/Vagina | 0 | 1 (0.5) | |
| Other | 2 (1.0) | 0 | |
| Breslow thickness, mm, median (IQR) | 1.7 (1.0–3.1) n = 202 | 1.7 (1.0–3.0) n = 213 | 0.968 |
| Ulceration, n (%) | 55 (27.8) n = 198 | 52 (24.5) n = 212 | 0.459 |
| Mitotic index, mitoses/mm2, median (IQR) | 2.0 (1.0–5.0) n = 197 | 2.0 (1.0–5.0) n = 209 | 0.453 |
| Characteristic | Pre-Pandemic (n = 204) 1 | Pandemic (n = 215) 2 | p-Value |
|---|---|---|---|
| Wait time 3, days, median (IQR) | 35.5 (22.0–48.0) | 35.0 (24.0–49.0) | 0.888 |
| Wait time by stage, days, median (IQR) | |||
| IA | 38.5 (23.0–49.5) | 43.0 (30.0–51.5) | 0.270 |
| IB | 29.0 (19.0–43.0) | 32.0 (20.0–43.0) | 0.818 |
| IIA | 25.0 (13.0–40.0) | 34.0 (25.0–48.0) | 0.048 |
| IIB | 40.0 (24.0–44.0) | 41.0 (31.0–59.0) | 0.169 |
| IIC | 43.0 (30.0–57.0) | 32.0 (23.0–46.0) | 0.201 |
| IIIA | 29.0 (18.0–35.0) | 35.0 (30.0–49.0) | 0.297 |
| IIIB | 43.0 (28.0–47.0) | 36.0 (26.0–61.0) | 0.926 |
| IIIC | 39.0 (29.0–60.0) | 30.0 (21.5–40.0) | 0.139 |
| IIID | 31.0 (23.0–48.0) | 29.0 (14.0–43.0) | 0.624 |
| Characteristic | Pre-Pandemic (n = 204) | Pandemic (n = 215) | p-Value |
|---|---|---|---|
| T stage 1, n (%) | 0.060 | ||
| T1a | 10 (4.9) | 18 (8.4) | |
| T1b | 42 (20.6) | 33 (15.3) | |
| T2a | 47 (23.0) | 64 (29.8) | |
| T2b | 2 (1.0) * | 10 (4.7) * | |
| T3a | 27 (13.2) | 30 (14.0) | |
| T3b | 19 (9.3) | 12 (5.6) | |
| T4a | 17 (8.3) | 15 (7.0) | |
| T4b | 40 (19.6) | 33 (15.3) | |
| N stage 1, n (%) | 0.214 | ||
| N0 | 144 (70.6) | 159 (74.0) | |
| N1a | 25 (12.3) | 21 (9.8) | |
| N1b | 2 (1.0) | 3 (1.4) | |
| N1c | 3 (1.5) | 8 (3.7) | |
| N2a | 10 (4.9) | 9 (4.2) | |
| N2b | 2 (1.0) | 0 | |
| N2c | 2 (1.0) | 6 (2.8) | |
| N3a | 1 (0.5) | 2 (0.9) | |
| N3b | 3 (1.5) | 0 | |
| N3c | 12 (5.9) | 7 (3.3) | |
| Stage 1, n (%) | 0.192 | ||
| IA | 48 (23.5) | 48 (22.3) | |
| IB | 41 (20.1) | 57 (26.5) | |
| IIA | 19 (9.3) | 25 (11.6) | |
| IIB | 21 (10.3) | 13 (6.0) | |
| IIC | 15 (7.4) | 13 (6.0) | |
| IIIA | 9 (4.4) | 9 (4.2) | |
| IIIB | 9 (4.4) | 14 (6.5) | |
| IIIC | 29 (14.2) | 32 (14.9) | |
| IIID | 13 (6.4) * | 4 (1.9) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aw, K.; Lau, R.; Nessim, C. Prioritizing Melanoma Surgeries to Prevent Wait Time Delays and Upstaging of Melanoma during the COVID-19 Pandemic. Curr. Oncol. 2023, 30, 8328-8337. https://doi.org/10.3390/curroncol30090604
Aw K, Lau R, Nessim C. Prioritizing Melanoma Surgeries to Prevent Wait Time Delays and Upstaging of Melanoma during the COVID-19 Pandemic. Current Oncology. 2023; 30(9):8328-8337. https://doi.org/10.3390/curroncol30090604
Chicago/Turabian StyleAw, Katherine, Rebecca Lau, and Carolyn Nessim. 2023. "Prioritizing Melanoma Surgeries to Prevent Wait Time Delays and Upstaging of Melanoma during the COVID-19 Pandemic" Current Oncology 30, no. 9: 8328-8337. https://doi.org/10.3390/curroncol30090604
APA StyleAw, K., Lau, R., & Nessim, C. (2023). Prioritizing Melanoma Surgeries to Prevent Wait Time Delays and Upstaging of Melanoma during the COVID-19 Pandemic. Current Oncology, 30(9), 8328-8337. https://doi.org/10.3390/curroncol30090604






