Antibody–Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases
Abstract
1. Introduction
2. Antibody–Drug Conjugates
2.1. Antibodies and Antigens
2.2. Linkers
2.3. Cytotoxic Payloads
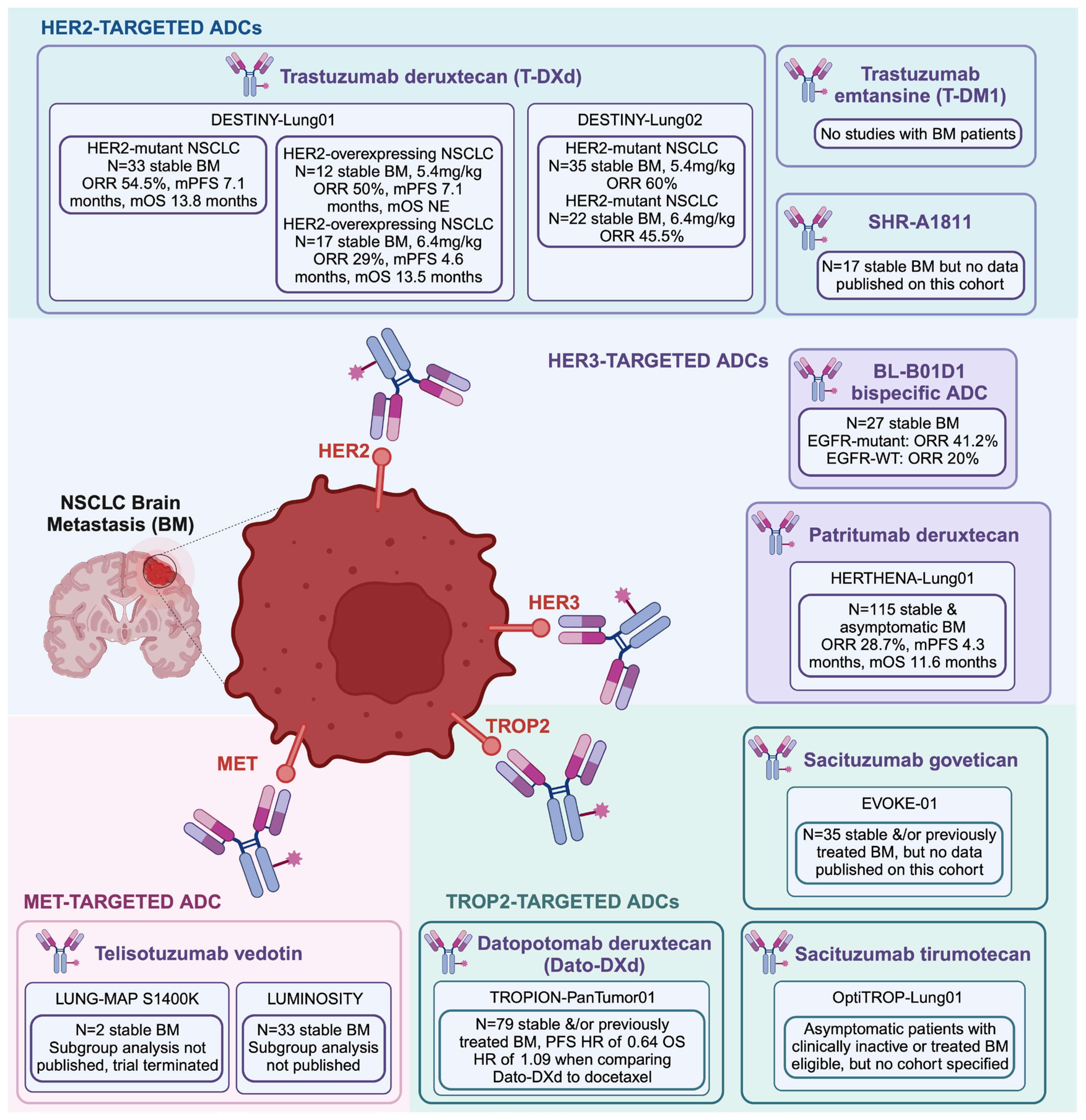
3. Antibody–Drug Conjugates and Brain Metastases
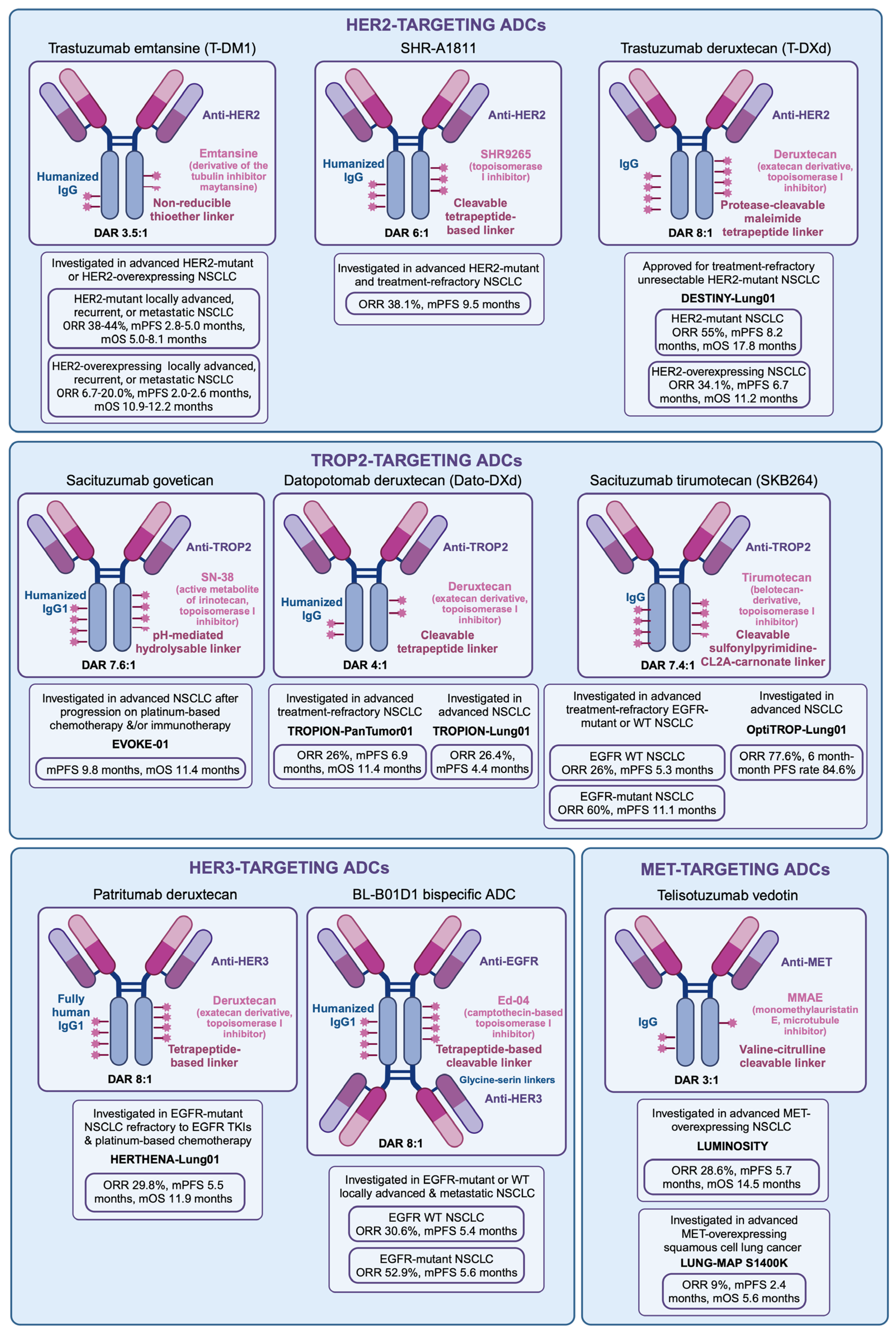
4. HER2 Antibody–Drug Conjugates
4.1. Trastuzumab Emtansine
4.2. Trastuzumab Deruxtecan
| Trial | PMID | Patient Population | Treatment Arms | CNS Metastasis Eligibility | ALL PATIENTS | PATIENTS WITH BM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population Size (N) | ORR | DCR | Median DOR | Median PFS | Median OS | Population Size (N) | ORR | DCR | Median DOR | Median PFS | Median OS | |||||
| HERTHENA-Lung01 | PMID: 37689979 [93] | Previously treated patients (EGFR-TKI or platinum-based chemotherapy) with locally advanced or metastatic NSCLC with EGFR-activating mutations (exon 19 deletion or L858R) | Patritumab deruxtecan 5.6 mg/kg | Stable BM | 225 | 29.8% | 73.8% | 6.4 months | 5.5 months | 11.9 months | 115 | 28.7% | 70.4% | 5.5 months | 4.3 months | 11.6 months |
| U31402-A-U102 | PMID: 34548309 [94] | Previously treated patients (EGFR-TKI) with locally advanced or metastatic NSCLC with EGFR-activating mutations | Patritumab deruxtecan 5.6 mg/kg | Stable BM | 57 | 39.0% | 72.0% | 6.9 months | 8.2 months | NA | 25 | 32.0% | NA | NA | NA | NA |
| BL-B01D1 | PMID: 38823410 [95] | Locally advanced or metastatic patients with solid tumors, including NSCLC for which no standard treatment was available | BL-B01D1 dose escalation to 3.0 mg/kg, 3.5 mg/kg, or 6.0 mg/kg | Stable BM | mEGFR: 40; wt EGFR: 62 | mEGFR: 52.9%; wtEGFR: 30.6% | mEGFR: 87.5%; wtEGFR: 87.1% | mEGFR: 8.5 months; wtEGFR: NE | mEGFR: 5.6 months; wtEGFR: 5.4 months | NA | mEGFR: 17; wtEGFR: 10 | mEGFR: 41.2%; wtEGFR: 20.0% | mEGFR: 100.0%; wtEGFR: 100.0% | NA | NA | NA |
| DESTINY Lung-01 | PMID: 38547891 [91] | Unresectable or metastatic NSCLC patients that was refractory to standard treatment with HER2 overexpression | Trastuzumab deruxtecan 5.4 mg/kg versus 6.4 mg/kg | Stable BM | 5.4 mg/kg 41; 6.4 mg/kg: 49 | 5.4 mg/kg: 34.1%; 6.4 mg/kg: 26.5% | 5.4 mg/kg: 78.0%; 6.4 mg/kg: 69.4% | 5.4 mg/kg: 6.2 months; 6.4 mg/kg: 5.8 months | 5.4 mg/kg: 6.7 months; 6.4 mg/kg: 5.7 months | 5.4 mg/kg: 11.2 months; 6.4 mg/kg: 12.4 months | 5.4 mg/kg 12; 6.4 mg/kg: 17 | 5.4 mg/kg: 50.0%; 6.4 mg/kg: 29.0% | NA | NA | 5.4 mg/kg: 7.1 months; 6.4 mg/kg: 4.6 months | 5.4 mg/kg: NE; 6.4 mg/kg: 13.5 months |
| DESTINY Lung-01 | PMID: 34534430 [89] | Unresectable or metastatic NSCLC patients that was refractory to standard treatment with HER2 mutations | Trastuzumab deruxtecan 6.4 mg/kg | Stable BM | 91 | 55.0% | 92.0% | 9.3 months | 8.2 months | 17.8 months | 33 | 54.5% | NA | NA | 7.1 months | 13.8 months |
| DESTINY Lung-02 | PMID: 37694347 [92] | Unresectable or metastatic NSCLC patients that was previously treated with standard treatment with HER2 mutations | Trastuzumab deruxtecan 5.4 mg/kg versus 6.4 mg/kg | Stable BM | 5.4 mg/kg: 102, 6.4 mg/kg: 50 | 5.4 mg/kg: 49%, 6.4 mg/kg: 56% | 5.4 mg/kg: 93.1%, 6.4 mg/kg: 92.0% | 5.4 mg/kg: 16.8 months, 6.4 mg/kg: NE | 5.4 mg/kg: 9.9 months, 6.4 mg/kg: 15.4 months | 5.4 mg/kg: 19.5 months, 6.4 mg/kg: NE | 5.4 mg/kg: 35, 6.4 mg/kg: 22 | 5.4 mg/kg: 60%, 6.4 mg/kg: 45.5% | NA | NA | NA | NA |
4.3. Other HER2-Targeting ADCs
5. HER3 Antibody–Drug Conjugates
5.1. Patritumab Deruxtecan
5.2. Other HER3-Targeting Antibody–Drug Conjugates
6. TROP2 Antibody–Drug Conjugates
6.1. Sacituzumab Govitecan
6.2. Datopotamab Deruxtecan
6.3. Sacituzumab Tirumotecan
7. MET Antibody–Drug Conjugates
Telisotuzumab Vedotin
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society. Cancer Statistics at a Glance. Cancer Statistics. 2024. Available online: https://cancer.ca/en/research/cancer-statistics/cancer-statistics-at-a-glance (accessed on 1 September 2024).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Y.; Gaebe, K.; Zulfiqar, A.; Lee, G.; Jerzak, K.J.; Sahgal, A.; Habbous, S.; Erickson, A.W.; Das, S. Association of Brain Metastases with Survival in Patients with Limited or Stable Extracranial Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2023, 6, e230475. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Contessa, J.N.; Omay, S.B.; Chiang, V. Lung Cancer Brain Metastases. Cancer J. 2015, 21, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.S.; Mustafa, M.A.; Richardson, G.E.; Alam, A.M.; Lee, K.S.; Hughes, D.M.; Escriu, C.; Zakaria, R. Genomic Alterations and the Incidence of Brain Metastases in Advanced and Metastatic NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2023, 18, 1703–1713. [Google Scholar] [CrossRef]
- Dankner, M.; Lam, S.; Degenhard, T.; Garzia, L.; Guiot, M.C.; Petrecca, K.; Siegel, P.M. The Underlying Biology and Therapeutic Vulnerabilities of Leptomeningeal Metastases in Adult Solid Cancers. Cancers 2021, 13, 732. [Google Scholar] [CrossRef]
- Fecci, P.E.; Champion, C.D.; Hoj, J.; McKernan, C.M.; Goodwin, C.R.; Kirkpatrick, J.P.; Anders, C.K.; Pendergast, A.M.; Sampson, J.H. The Evolving Modern Management of Brain Metastasis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6570–6580. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2021, 40, 492–516. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Golden, P.L.; Pollack, G.M. Blood-Brain Barrier Efflux Transport. J. Pharm. Sci. 2003, 92, 1739–1753. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2019, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.J.; Yang, J.C.H.; Han, J.Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Garcia Campelo, M.R.; Kim, D.W.; et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J. Thorac. Oncol. 2021, 16, 2091–2108. [Google Scholar] [CrossRef]
- Solomon, B.J.; Liu, G.; Felip, E.; Mok, T.S.K.; Soo, R.A.; Mazieres, J.; Shaw, A.T.; de Marinis, F.; Goto, Y.; Wu, Y.L.; et al. Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes From the Phase III CROWN Study. J. Clin. Oncol. 2024, 42, 3400–3409. [Google Scholar] [CrossRef]
- Bian, D.J.H.; Lazaratos, A.-M.; Maritan, S.M.; Quaiattini, A.; Zeng, Z.; Zhu, Z.; Sener, U.; Malani, R.; Kim, Y.J.; Ichihara, E.; et al. Osimertinib is associated with improved outcomes in pre-treated non-small cell lung cancer leptomeningeal metastases: A systematic review and meta-analysis. Heliyon 2024, 10, e29668. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Krebs, M.G.; Braud, F.D.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion–Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef]
- Garon, E.B.; Heist, R.S.; Seto, T.; Han, J.-Y.; Reguart, N.; Groen, H.J.; Tan, D.S.; Hida, T.; de Jonge, M.J.; Orlov, S.V.; et al. Abstract CT082: Capmatinib in METex14-mutated (mut) advanced non-small cell lung cancer (NSCLC): Results from the phase II GEOMETRY mono-1 study, including efficacy in patients (pts) with brain metastases (BM). Cancer Res. 2020, 80 (Suppl. S16), CT082. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Meerbeeck, J.V.; et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. New Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Subbiah, V.; Gainor, J.F.; Oxnard, G.R.; Tan, D.S.W.; Owen, D.H.; Cho, B.C.; Loong, H.H.; McCoach, C.E.; Weiss, J.; Kim, Y.J.; et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin. Cancer Res. 2021, 27, 4160–4167. [Google Scholar] [CrossRef]
- Yamamoto, G.; Sakakibara-Konishi, J.; Ikari, T.; Kitai, H.; Mizugaki, H.; Asahina, H.; Kikuchi, E.; Shinagawa, N. Response of BRAFV600E-Mutant Lung Adenocarcinoma with Brain Metastasis and Leptomeningeal Dissemination to Dabrafenib Plus Trametinib Treatment. J. Thorac. Oncol. 2019, 14, e97–e99. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.-M.C.; Syrigos, K.; Livi, L.; Paulus, A.; Kim, S.-W.; Chen, Y.; Felip, E.; Griesinger, F.; Ohashi, K.; Zalcman, G.; et al. Intracranial efficacy of sotorasib versus docetaxel in pretreated KRAS G12C-mutated advanced non-small cell lung cancer (NSCLC): Practice-informing data from a global, phase 3, randomized, controlled trial (RCT). J. Clin. Oncol. 2023, 41 (Suppl. S17), LBA9016. [Google Scholar] [CrossRef]
- Doebele, R.; Paz-Ares, L.; Farago, A.F.; Liu, S.V.; Chawla, S.P.; Tosi, D.; Blakely, C.M.; Krauss, J.C.; Sigal, D.; Bazhenova, L.; et al. Abstract CT131: Entrectinib in NTRK-fusion positive (NTRK-FP) non-small cell lung cancer (NSCLC): Integrated analysis of patients enrolled in three trials (STARTRK-2, STARTRK-1 and ALKA-372-001). Cancer Res. 2019, 79 (Suppl. S13), CT131. [Google Scholar] [CrossRef]
- Drilon, A.E.; DuBois, S.G.; Farago, A.F.; Geoerger, B.; Grilley-Olson, J.E.; Hong, D.S.; Sohal, D.; Tilburg, C.M.v.; Ziegler, D.S.; Ku, N.; et al. Activity of larotrectinib in TRK fusion cancer patients with brain metastases or primary central nervous system tumors. J. Clin. Oncol. 2019, 37 (Suppl. S15), 2006. [Google Scholar] [CrossRef]
- Schapira, E.; Hubbeling, H.; Yeap, B.Y.; Mehan, W.A., Jr.; Shaw, A.T.; Oh, K.; Gainor, J.F.; Shih, H.A. Improved Overall Survival and Locoregional Disease Control with Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients with Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 624–629. [Google Scholar] [CrossRef]
- Taggart, D.; Andreou, T.; Scott, K.J.; Williams, J.; Rippaus, N.; Brownlie, R.J.; Ilett, E.J.; Salmond, R.J.; Melcher, A.; Lorger, M. Anti-PD-1/anti-CTLA-4 efficacy in melanoma brain metastases depends on extracranial disease and augmentation of CD8(+) T cell trafficking. Proc. Natl. Acad. Sci. USA 2018, 115, E1540. [Google Scholar] [CrossRef]
- Coleman, N.; Yap, T.A.; Heymach, J.V.; Meric-Bernstam, F.; Le, X. Antibody-drug conjugates in lung cancer: Dawn of a new era? npj Precis. Oncol. 2023, 7, 5. [Google Scholar] [CrossRef]
- Mair, M.J.; Bartsch, R.; Le Rhun, E.; Berghoff, A.S.; Brastianos, P.K.; Cortes, J.; Gan, H.K.; Lin, N.U.; Lassman, A.B.; Wen, P.Y.; et al. Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours. Nat. Rev. Clin. Oncol. 2023, 20, 372–389. [Google Scholar] [CrossRef]
- Flynn, P.; Suryaprakash, S.; Grossman, D.; Panier, V.; Wu, J. The antibody-drug conjugate landscape. Nat. Rev. Drug Discov. 2024, 23, 577–578. [Google Scholar] [CrossRef]
- De Cecco, M.; Galbraith, D.N.; McDermott, L.L. What makes a good antibody-drug conjugate? Expert. Opin. Biol. Ther. 2021, 21, 841–847. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, Y.; Liu, W.; Wang, S.; Wang, L.; Zheng, P.; Zi, G.; Liu, H.; Liu, W.; Wei, S. Drug conjugates for the treatment of lung cancer: From drug discovery to clinical practice. Exp. Hematol. Oncol. 2024, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Saunus, J.M.; Quinn, M.C.; Patch, A.-M.; Pearson, J.V.; Bailey, P.J.; Nones, K.; McCart Reed, A.E.; Miller, D.; Wilson, P.J.; Al-Ejeh, F.; et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J. Pathol. 2015, 237, 363–378. [Google Scholar] [CrossRef]
- Da Silva, L.; Simpson, P.T.; Smart, C.E.; Cocciardi, S.; Waddell, N.; Lane, A.; Morrison, B.J.; Vargas, A.C.; Healey, S.; Beesley, J.; et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010, 12, R46. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Bartsch, R.; Preusser, M.; Ricken, G.; Steger, G.G.; Bago-Horvath, Z.; Rudas, M.; Streubel, B.; Dubsky, P.; Gnant, M.; et al. Co-overexpression of HER2/HER3 is a predictor of impaired survival in breast cancer patients. Breast 2014, 23, 637–643. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Magerle, M.; Ilhan-Mutlu, A.; Dinhof, C.; Widhalm, G.; Dieckman, K.; Marosi, C.; Wöhrer, A.; Hackl, M.; Zöchbauer-Müller, S.; et al. Frequent overexpression of ErbB—Receptor family members in brain metastases of non-small cell lung cancer patients. APMIS 2013, 121, 1144–1152. [Google Scholar] [CrossRef]
- Kodack, D.P.; Askoxylakis, V.; Ferraro, G.B.; Sheng, Q.; Badeaux, M.; Goel, S.; Qi, X.; Shankaraiah, R.; Cao, Z.A.; Ramjiawan, R.R.; et al. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci. Transl. Med. 2017, 9, eaal4682. [Google Scholar] [CrossRef] [PubMed]
- Tomasich, E.; Steindl, A.; Paiato, C.; Hatziioannou, T.; Kleinberger, M.; Berchtold, L.; Puhr, R.; Hainfellner, J.A.; Müllauer, L.; Widhalm, G.; et al. Frequent Overexpression of HER3 in Brain Metastases from Breast and Lung Cancer. Clin. Cancer Res. 2023, 29, 3225–3236. [Google Scholar] [CrossRef]
- Preusser, M.; Streubel, B.; Berghoff, A.S.; Hainfellner, J.A.; von Deimling, A.; Widhalm, G.; Dieckmann, K.; Wöhrer, A.; Hackl, M.; Zielinski, C.; et al. Amplification and overexpression of is a common event in brain metastases of non-small cell lung cancer. Histopathology 2014, 65, 684–692. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Smith, M.R.; Wang, Y.; D’Agostino, R.; Ruiz, J.; Lycan, T.; Kucera, G.L.; Miller, L.D.; Li, W.; Chan, M.D.; et al. c-Met Mediated Cytokine Network Promotes Brain Metastasis of Breast Cancer by Remodeling Neutrophil Activities. Cancers 2023, 15, 2626. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Seol, H.J.; Lee, H.W.; Kang, W.Y.; Kang, B.G.; Jin, J.; Jo, M.-Y.; Jin, Y.; Lee, J.-I.; Joo, K.M.; et al. Gene silencing of c-Met leads to brain metastasis inhibitory effects. Clin. Exp. Metastasis 2013, 30, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2016, 9, 33–46. [Google Scholar] [CrossRef]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef]
- Nolting, B. Linker Technologies for Antibody–Drug Conjugates. In Antibody-Drug Conjugates; Ducry, L., Ed.; Humana Press: Totowa, NJ, USA, 2013; pp. 71–100. [Google Scholar]
- Oflazoglu, E.; Stone, I.J.; Gordon, K.; Wood, C.G.; Repasky, E.A.; Grewal, I.S.; Law, C.-L.; Gerber, H.-P. Potent Anticarcinoma Activity of the Humanized Anti-CD70 Antibody h1F6 Conjugated to the Tubulin Inhibitor Auristatin via an Uncleavable Linker. Clin. Cancer Res. 2008, 14, 6171–6180. [Google Scholar] [CrossRef] [PubMed]
- Baah, S.; Laws, M.; Rahman, K.M. Antibody–Drug Conjugates—A Tutorial Review. Molecules 2021, 26, 2943. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Mckertish, C.M.; Kayser, V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines 2021, 9, 872. [Google Scholar] [CrossRef]
- Nagayama, A.; Ellisen, L.W.; Chabner, B.; Bardia, A. Antibody–Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments. Target. Oncol. 2017, 12, 719–739. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Alsaab, H.O.; Kashaw, S.K.; Tatiparti, K.; Iyer, A.K. Advances in antibody–drug conjugates: A new era of targeted cancer therapy. Drug Discov. Today 2017, 22, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.E.; Pearson, C.I.; Gregorio, J.D.; Gonzalez, J.C.; Kenkel, J.A.; Hartmann, F.J.; Luo, A.; Ho, P.Y.; LeBlanc, H.; Blum, L.K.; et al. Immune-stimulating antibody conjugates elicit robust myeloid activation and durable antitumor immunity. Nat. Cancer 2021, 2, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody–drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Gogia, P.; Ashraf, H.; Bhasin, S.; Xu, Y. Antibody–Drug Conjugates: A Review of Approved Drugs and Their Clinical Level of Evidence. Cancers 2023, 15, 3886. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Mair, M.J.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; et al. Final outcome analysis from the phase II Tuxedo-1 Trial of Trastuzumab-Deruxtecan in HER2-positive breast cancer patients with active brain metastases. Neuro-Oncol. 2024, noae123. [Google Scholar] [CrossRef]
- Bartsch, R.; Batista, M.V.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Oberndorfer, F.; Garde, J.; Ruiz-Borrego, M.; Greil, R.; Raimondi, G.; et al. Patritumab deruxtecan (HER3-DXd) in active brain metastases from metastatic breast and non-small cell lung cancers, and leptomeningeal disease from advanced solid tumors: The TUXEDO-3 phase II trial. J. Clin. Oncol. 2024, 42 (Suppl. S16), TPS2091. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm, phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef]
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro-Oncol. 2022, 25, 157–166. [Google Scholar] [CrossRef]
- Vaz Batista, M.; Pérez-García, J.M.; Garrigós, L.; García-Sáenz, J.Á.; Cortez, P.; Racca, F.; Blanch, S.; Ruiz-Borrego, M.; Fernández-Ortega, A.; Fernández-Abad, M.; et al. The DEBBRAH trial: Trastuzumab deruxtecan in HER2-positive and HER2-low breast cancer patients with leptomeningeal carcinomatosis. Med. 2024. [Google Scholar] [CrossRef]
- Harbeck, N.; Ciruelos, E.; Jerusalem, G.; Müller, V.; Niikura, N.; Viale, G.; Bartsch, R.; Kurzeder, C.; Higgins, M.J.; Connolly, R.M.; et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: A phase 3b/4 trial. Nat. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, J.; Ying, J.; Mitsudomi, T.; Lee, D.H.; Wang, Z.; Chu, Q.; Mack, P.C.; Cheng, Y.; Duan, J.; et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef]
- Mazières, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung Cancer That Harbors an HER2 Mutation: Epidemiologic Characteristics and Therapeutic Perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Berry, L.D.; Rossi, M.R.; Kris, M.G.; Johnson, B.E.; Bunn, P.A.; Ramalingam, S.S.; Khuri, F.R. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer 2017, 123, 4099–4105. [Google Scholar] [CrossRef]
- Ahn, B.C.; Han, Y.J.; Kim, H.R.; Hong, M.H.; Cho, B.C.; Lim, S.M. Real World Characteristics and Clinical Outcomes of HER2-Mutant Non-Small Cell Lung Cancer Patients Detected by Next-Generation Sequencing. Cancer Res. Treat. 2023, 55, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Bu, S.; Wang, R.; Pan, Y.; Yu, S.; Shen, X.; Li, Y.; Sun, Y.; Chen, H. Clinicopathologic Characteristics of Patients with HER2 Insertions in Non-small Cell Lung Cancer. Ann. Surg. Oncol. 2017, 24, 291–297. [Google Scholar] [CrossRef]
- Zhuo, X.; Guo, H.; Ma, J.; Lai, J.; Liu, L.; Yin, K.; Zhao, J.; Wang, J.; Jiang, F.; Xu, W.; et al. Clinical characteristics and prognostic factors of patients with non-small cell lung cancer having HER2 alterations. J. Cancer Res. Clin. Oncol. 2023, 149, 2029–2039. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Su, C.; Ren, S.; Chen, X.; Zhou, C. Epidemiological study of HER-2 mutations among EGFR wild-type lung adenocarcinoma patients in China. BMC Cancer 2016, 16, 828. [Google Scholar] [CrossRef]
- Kim, E.K.; Kim, K.A.; Lee, C.Y.; Shim, H.S. The frequency and clinical impact of HER2 alterations in lung adenocarcinoma. PLoS ONE 2017, 12, e0171280. [Google Scholar] [CrossRef] [PubMed]
- Offin, M.; Feldman, D.; Ni, A.; Myers, M.L.; Lai, W.V.; Pentsova, E.; Boire, A.; Daras, M.; Jordan, E.J.; Solit, D.B.; et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer 2019, 125, 4380–4387. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, Y.; Zhao, C.; Li, X.; Liu, Q.; Mao, S.; Liu, Y.; Yu, X.; Wang, W.; Tian, Q.; et al. Exon 20 YVMA insertion is associated with high incidence of brain metastasis and inferior outcome of chemotherapy in advanced non-small cell lung cancer patients with HER2 kinase domain mutations. Transl. Lung Cancer Res. 2021, 10, 753–765. [Google Scholar] [CrossRef]
- Pellegrini, C.; Falleni, M.; Marchetti, A.; Cassani, B.; Miozzo, M.; Buttitta, F.; Roncalli, M.; Coggi, G.; Bosari, S. HER-2/Neu Alterations in Non-Small Cell Lung Cancer: A Comprehensive Evaluation by Real Time Reverse Transcription-PCR, Fluorescence in Situ Hybridization, and Immunohistochemistry1. Clin. Cancer Res. 2003, 9, 3645–3652. [Google Scholar] [PubMed]
- Heinmöller, P.; Gross, C.; Beyser, K.; Schmidtgen, C.; Maass, G.; Pedrocchi, M.; Rüschoff, J. HER2 status in non-small cell lung cancer: Results from patient screening for enrollment to a phase II study of herceptin. Clin. Cancer Res. 2003, 9, 5238–5243. [Google Scholar] [CrossRef]
- Lee, K.; Jung, H.A.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; Ahn, M.J. Clinical Characteristics and Outcomes of Non-small Cell Lung Cancer Patients with HER2 Alterations in Korea. Cancer Res. Treat. 2020, 52, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Krop, I.E.; Kim, S.-B.; Martin, A.G.; LoRusso, P.M.; Ferrero, J.-M.; Badovinac-Crnjevic, T.; Hoersch, S.; Smitt, M.; Wildiers, H. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017, 18, 743–754. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer patients with brain metastases from the randomized DESTINY-Breast03 trial. ESMO Open 2024, 9, 102924. [Google Scholar] [CrossRef]
- Montemurro, F.; Delaloge, S.; Barrios, C.H.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Iwama, E.; Zenke, Y.; Sugawara, S.; Daga, H.; Morise, M.; Yanagitani, N.; Sakamoto, T.; Murakami, H.; Kishimoto, J.; Matsumoto, S.; et al. Trastuzumab emtansine for patients with non–small cell lung cancer positive for human epidermal growth factor receptor 2 exon-20 insertion mutations. Eur. J. Cancer 2022, 162, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients with HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.D.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non–Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef]
- Hotta, K.; Aoe, K.; Kozuki, T.; Ohashi, K.; Ninomiya, K.; Ichihara, E.; Kubo, T.; Ninomiya, T.; Chikamori, K.; Harada, D.; et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 273–279. [Google Scholar] [CrossRef]
- Li, B.T.; Michelini, F.; Misale, S.; Cocco, E.; Baldino, L.; Cai, Y.; Shifman, S.; Tu, H.-Y.; Myers, M.L.; Xu, C.; et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020, 10, 674–687. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. New Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Phillips, C. FDA Approves Trastuzumab Deruxtecan for Any HER2-Positive Solid Cancer. In Cancer Currents: An NCI Cancer Research Blog; National Cancer Institute: Bethesda, MA, USA, 2024. [Google Scholar]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Paz-Ares Rodríguez, L.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; Langen, A.J.d.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients with HER2-Mutant Metastatic Non-Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef]
- Yu, H.A.; Goto, Y.; Hayashi, H.; Felip, E.; Yang, J.C.-H.; Reck, M.; Yoh, K.; Lee, S.-H.; Paz-Ares, L.; Besse, B.; et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor–Mutated Non–Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J. Clin. Oncol. 2023, 41, 5363–5375. [Google Scholar] [CrossRef]
- Jänne, P.A.; Baik, C.; Su, W.-C.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Kim, D.-W.; Koczywas, M.; Gold, K.A.; Steuer, C.E.; et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor–Resistant, EGFR-Mutated Non–Small Cell Lung Cancer. Cancer Discov. 2022, 12, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, Y.; Zhao, Y.; Zhao, S.; Xue, J.; Yang, Y.; Fang, W.; Guo, Y.; Han, Y.; Yang, K.; et al. BL-B01D1, a first-in-class EGFR–HER3 bispecific antibody–drug conjugate, in patients with locally advanced or metastatic solid tumours: A first-in-human, open-label, multicentre, phase 1 study. Lancet Oncol. 2024, 25, 901–911. [Google Scholar] [CrossRef]
- Niikura, N.; Yamanaka, T.; Nomura, H.; Shiraishi, K.; Kusama, H.; Yamamoto, M.; Matsuura, K.; Inoue, K.; Takahara, S.; Kita, S.; et al. Treatment with trastuzumab deruxtecan in patients with HER2-positive breast cancer and brain metastases and/or leptomeningeal disease (ROSET-BM). npj Breast Cancer 2023, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Lazaratos, A.-M.; Maritan, S.M.; Quaiattini, A.; Darlix, A.; Ratosa, I.; Ferraro, E.; Griguolo, G.; Guarneri, V.; Pellerino, A.; Hofer, S.; et al. Intrathecal trastuzumab versus alternate routes of delivery for HER2-targeted therapies in patients with HER2+ breast cancer leptomeningeal metastases. Breast 2023, 69, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yuan, B.; Li, C.; Wang, Z.; Song, Y.; Liu, H. A narrative review of advances in treatment and survival prognosis of HER2-positive malignant lung cancers. J. Thorac. Dis. 2021, 13, 3708–3720. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Hong, W.; Yang, N.; Wang, Y.; Jian, H.; Liang, Z.; Hu, S.; Peng, M.; Yu, Y.; et al. SHR-A1811 (antibody-drug conjugate) in advanced HER2-mutant non-small cell lung cancer: A multicenter, open-label, phase 1/2 study. Signal Transduct. Target. Ther. 2024, 9, 182. [Google Scholar] [CrossRef]
- Gandullo-Sánchez, L.; Ocaña, A.; Pandiella, A. HER3 in cancer: From the bench to the bedside. J. Exp. Clin. Cancer Res. 2022, 41, 310. [Google Scholar] [CrossRef]
- Roskoski, R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.-M.; Zhao, X.; Christensen, J.; et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Sun, M.; Behrens, C.; Feng, L.; Ozburn, N.; Tang, X.; Yin, G.; Komaki, R.; Varella-Garcia, M.; Hong, W.K.; Aldape, K.D.; et al. HER Family Receptor Abnormalities in Lung Cancer Brain Metastases and Corresponding Primary Tumors. Clin. Cancer Res. 2009, 15, 4829–4837. [Google Scholar] [CrossRef]
- Scharpenseel, H.; Hanssen, A.; Loges, S.; Mohme, M.; Bernreuther, C.; Peine, S.; Lamszus, K.; Goy, Y.; Petersen, C.; Westphal, M.; et al. EGFR and HER3 expression in circulating tumor cells and tumor tissue from non-small cell lung cancer patients. Sci. Rep. 2019, 9, 7406. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody–Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Baik, C.; Kim, D.W.; Johnson, M.L.; Hayashi, H.; Nishio, M.; Yang, J.C.H.; Su, W.C.; Gold, K.A.; Koczywas, M.; et al. Translational insights and overall survival in the U31402-A-U102 study of patritumab deruxtecan (HER3-DXd) in EGFR-mutated NSCLC. Ann. Oncol. 2024, 35, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Yamamoto, Y.; Alvarez, R.H.; Toyama, T.; et al. Patritumab Deruxtecan (HER3-DXd), a Human Epidermal Growth Factor Receptor 3–Directed Antibody-Drug Conjugate, in Patients with Previously Treated Human Epidermal Growth Factor Receptor 3–Expressing Metastatic Breast Cancer: A Multicenter, Phase I/II Trial. J. Clin. Oncol. 2023, 41, 5550–5560. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.; Jänne, P.A.; Nishio, M.; Novello, S.; Reck, M.; Steuer, C.; Wu, Y.-L.; Fougeray, R.; Fan, P.-D.; Meng, J.; et al. HERTHENA-Lung02: Phase III study of patritumab deruxtecan in advanced EGFR-mutated NSCLC after a third-generation EGFR TKI. Future Oncol. 2024, 20, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Odintsov, I.; Sholl, L.M. Prognostic and predictive biomarkers in non-small cell lung carcinoma. Pathology 2024, 56, 192–204. [Google Scholar] [CrossRef]
- Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Zhang, W. TROP2 overexpression promotes proliferation and invasion of lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2016, 470, 197–204. [Google Scholar] [CrossRef]
- Zheng, Z.; Dong, X.-J. Clinical value of serum trophoblast cell surface protein 2 (TROP2) antibody in non-small-cell lung cancer patients. Biomarkers 2016, 21, 739–742. [Google Scholar] [CrossRef]
- Jiang, A.; Gao, X.; Zhang, D.; Zhang, L.; Lu, H. Expression and clinical significance of the Trop-2 gene in advanced non-small cell lung carcinoma. Oncol. Lett. 2013, 6, 375–380. [Google Scholar] [CrossRef]
- Mito, R.; Matsubara, E.; Komohara, Y.; Shinchi, Y.; Sato, K.; Yoshii, D.; Ohnishi, K.; Fujiwara, Y.; Tomita, Y.; Ikeda, K.; et al. Clinical impact of TROP2 in non-small lung cancers and its correlation with abnormal p53 nuclear accumulation. Pathol. Int. 2020, 70, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Ninomiya, H.; Sakakibara, R.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 2017, 8, 28725–28735. [Google Scholar] [CrossRef] [PubMed]
- Bessede, A.; Peyraud, F.; Besse, B.; Cousin, S.; Cabart, M.; Chomy, F.; Rey, C.; Lara, O.; Odin, O.; Nafia, I.; et al. TROP2 Is Associated with Primary Resistance to Immune Checkpoint Inhibition in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hijazo-Pechero, S.; Alay Badosa, A.; Vilariño, N.; Brenes Castro, J.; Moliner, L.; Mosteiro, M.A.; Vidal, N.; Muñoz-Pinedo, C.; Bruna, J.; Nadal, E. 1419P TACSTD2 (Trop-2) constitutes a promising antibody-drug conjugate target for patients with non-small cell lung cancer brain metastases. Ann. Oncol. 2023, 34, S810–S811. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. New Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Bardia, A.; Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Kalinsky, K.; Cortés, J.; Shaughnessy, J.O.; et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression. J. Clin. Oncol. 2024, 42, 1738–1744. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Juan-Vidal, O.; Mountzios, G.S.; Felip, E.; Reinmuth, N.; Marinis, F.d.; Girard, N.; Patel, V.M.; Takahama, T.; Owen, S.P.; et al. Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study. J. Clin. Oncol. 2024, 42, 2860–2872. [Google Scholar] [CrossRef]
- Balinda, H.U.; Kelly, W.J.; Kaklamani, V.G.; Lathrop, K.I.; Canola, M.M.; Ghamasaee, P.; Sareddy, G.R.; Michalek, J.; Gilbert, A.R.; Surapaneni, P.; et al. Sacituzumab Govitecan in patients with breast cancer brain metastases and recurrent glioblastoma: A phase 0 window-of-opportunity trial. Nat. Commun. 2024, 15, 6707. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Shimizu, T.; Sands, J.; Yoh, K.; Spira, A.; Garon, E.B.; Kitazono, S.; Johnson, M.L.; Meric-Bernstam, F.; Tolcher, A.W.; Yamamoto, N.; et al. First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2-Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non-Small-Cell Lung Cancer: TROPION-PanTumor01. J. Clin. Oncol. 2023, 41, 4678–4687. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Vicente Baz, D.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2024, JCO-24-01544. [Google Scholar] [CrossRef]
- Levy, B.P.; Felip, E.; Reck, M.; Yang, J.C.; Cappuzzo, F.; Yoneshima, Y.; Zhou, C.; Rawat, S.; Xie, J.; Basak, P.; et al. TROPION-Lung08: Phase III study of datopotamab deruxtecan plus pembrolizumab as first-line therapy for advanced NSCLC. Future Oncol. 2023, 19, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yuan, X.; Tian, Q.; Huang, X.; Chen, Y.; Pu, Y.; Long, H.; Xu, M.; Ji, Y.; Xie, J.; et al. Preclinical profiles of SKB264, a novel anti-TROP2 antibody conjugated to topoisomerase inhibitor, demonstrated promising antitumor efficacy compared to IMMU-132. Front. Oncol. 2022, 12, 951589. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Cheng, Y.; Chen, Z.; Wang, W.; Yin, Y.; Li, Y.; Xu, H.; Li, X.; Wainberg, Z.A.; Yu, G.; et al. SKB264 (TROP2-ADC) for the treatment of patients with advanced NSCLC: Efficacy and safety data from a phase 2 study. J. Clin. Oncol. 2023, 41 (Suppl. S16), 9114. [Google Scholar] [CrossRef]
- Fang, W.; Wang, Q.; Cheng, Y.; Luo, Y.; Qu, X.; Zhu, H.; Ding, Z.; Li, X.; Wu, L.; Wang, Y.; et al. Sacituzumab tirumotecan (SKB264/MK-2870) in combination with KL-A167 (anti-PD-L1) as first-line treatment for patients with advanced NSCLC from the phase II OptiTROP-Lung01 study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 8502. [Google Scholar] [CrossRef]
- Olivero, M.; Rizzo, M.; Madeddu, R.; Casadio, C.; Pennacchietti, S.; Nicotra, M.R.; Prat, M.; Maggi, G.; Arena, N.; Natali, P.G.; et al. Overexpression and activation of hepatocyte growth factor/scatter factor in human non-small-cell lung carcinomas. Br. J. Cancer 1996, 74, 1862–1868. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W.; Gherardi, E.; Vande Woude, G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003, 4, 915–925. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Sharma, S.; Wu, K.; Chan, M.D.; Lo, H.-W.; Carpenter, R.L.; Metheny-Barlow, L.J.; Zhou, X.; Qasem, S.A.; et al. Activation of the c-Met Pathway Mobilizes an Inflammatory Network in the Brain Microenvironment to Promote Brain Metastasis of Breast Cancer. Cancer Res. 2016, 76, 4970–4980. [Google Scholar] [CrossRef]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef]
- Yang, M.; Mandal, E.; Liu, F.X.; O’Hara, R.M.; Lesher, B.; Sanborn, R.E. Non-small cell lung cancer with MET amplification: Review of epidemiology, associated disease characteristics, testing procedures, burden, and treatments. Front. Oncol. 2024, 13, 1241402. [Google Scholar] [CrossRef]
- Huang, R.S.P.; Harries, L.; Decker, B.; Hiemenz, M.C.; Murugesan, K.; Creeden, J.; Tolba, K.; Stabile, L.P.; Ramkissoon, S.H.; Burns, T.F.; et al. Clinicopathologic and Genomic Landscape of Non-Small Cell Lung Cancer Brain Metastases. Oncologist 2022, 27, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Vioix, H.; Pfeiffer, B.M.; Campden, R.I.; Chen, Z.; Heeg, B.; Cortot, A.B. MET Exon 14 Skipping in NSCLC: A Systematic Literature Review of Epidemiology, Clinical Characteristics, and Outcomes. Clin. Lung Cancer 2023, 24, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.L.; Sung, C.O.; An, J.; Seo, J.; Ahn, M.J.; Ahn, J.S.; Park, K.; Shin, Y.K.; Erkin, O.C.; et al. High MET copy number and MET overexpression: Poor outcome in non-small cell lung cancer patients. Histol. Histopathol. 2012, 27, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Dafni, U.; Schöbel, M.; Finn, S.P.; Tischler, V.; Sejda, A.; Marchetti, A.; Thunnissen, E.; Verbeken, E.K.; Warth, A.; et al. Prevalence and clinical association of MET gene overexpression and amplification in patients with NSCLC: Results from the European Thoracic Oncology Platform (ETOP) Lungscape project. Lung Cancer 2017, 111, 143–149. [Google Scholar] [CrossRef]
- Bar, J.; Cai, M.H.; Choi, Y.C.; Baijal, S.; Zhao, W.; Liede, A.; Raskin, L.; Vasilopoulos, A.; Li, M.; Roberts-Rapp, L.; et al. 1397P Prevalence, molecular characterization, and prognosis of MET-overexpressing non-small cell lung cancer (NSCLC) in a real-world patient cohort. Ann. Oncol. 2023, 34, S799–S800. [Google Scholar] [CrossRef]
- Wang, J.; Anderson, M.G.; Oleksijew, A.; Vaidya, K.S.; Boghaert, E.R.; Tucker, L.; Zhang, Q.; Han, E.K.; Palma, J.P.; Naumovski, L.; et al. ABBV-399, a c-Met Antibody–Drug Conjugate that Targets Both MET–Amplified and c-Met–Overexpressing Tumors, Irrespective of MET Pathway Dependence. Clin. Cancer Res. 2017, 23, 992–1000. [Google Scholar] [CrossRef]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.; Moiseenko, F.; Filippova, E.; Cicin, I.; Ciuleanu, T.; Daaboul, N.; Liu, C.; et al. Telisotuzumab Vedotin Monotherapy in Patients with Previously Treated c-Met Protein–Overexpressing Advanced Nonsquamous EGFR-Wildtype Non–Small Cell Lung Cancer in the Phase II LUMINOSITY Trial. J. Clin. Oncol. 2024, 42, 3000–3011. [Google Scholar] [CrossRef]
- Waqar, S.N.; Redman, M.W.; Arnold, S.M.; Hirsch, F.R.; Mack, P.C.; Schwartz, L.H.; Gandara, D.R.; Stinchcombe, T.E.; Leighl, N.B.; Ramalingam, S.S.; et al. A Phase II Study of Telisotuzumab Vedotin in Patients with c-MET-positive Stage IV or Recurrent Squamous Cell Lung Cancer (LUNG-MAP Sub-study S1400K, NCT03574753). Clin. Lung Cancer 2021, 22, 170–177. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primers 2019, 5, 5. [Google Scholar] [CrossRef]
- Jänne, P.A.; Planchard, D.; Kobayashi, K.; Cheng, Y.; Lee, C.K.; Valdiviezo, N.; Laktionov, K.; Yang, T.-Y.; Yu, Y.; Kato, T.; et al. CNS Efficacy of Osimertinib with or Without Chemotherapy in Epidermal Growth Factor Receptor–Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 808–820. [Google Scholar] [CrossRef]
- Peled, N.; Kian, W.; Inbar, E.; Goldstein, I.M.; Zemel, M.; Rotem, O.; Rozenblum, A.B.; Nechushtan, H.; Dudnik, E.; Levin, D.; et al. Osimertinib in advanced EGFR-mutant lung adenocarcinoma with asymptomatic brain metastases: An open-label, 3-arm, phase II pilot study. Neuro-Oncol. Adv. 2021, 4, vdab188. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baldry, R.; Jung, H.A.; Sun, J.-M.; Lee, S.-H.; Ahn, J.S.; Kim, Y.J.; Lee, Y.; Kim, D.-W.; Kim, S.-W.; et al. Phase II Efficacy and Safety of 80 mg Osimertinib in Patients with Leptomeningeal Metastases Associated with Epidermal Growth Factor Receptor Mutation–Positive Non–Small Cell Lung Cancer (BLOSSOM). J. Clin. Oncol. 2024, 42, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yuan, J.Q.; Wang, K.F.; Fu, X.H.; Han, X.R.; Threapleton, D.; Yang, Z.Y.; Mao, C.; Tang, J.L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed]
| Trial | Trial Type | National Clinical Trial Number (NCT) | Patient Population | Antibody–Drug Conjugate | Antibody Target | Payload Type | CNS Metastasis Eligibility | Status | |
|---|---|---|---|---|---|---|---|---|---|
| CNS-specific NSCLC studies | Efficacy and Safety of T-DXd in HER2-mutant Advanced Lung Cancer Patients with Asymptomatic Brain Metastases (ELPIS) | Phase II | NCT06250777 | Locally advanced and unresectable NSCLC with activating HER2 mutation and untreated asymptomatic BM at baseline | Trastuzumab deruxtecan | HER2 | Topoisomerase I inhibitor | CNS metastasis-specific study | Not yet recruiting |
| HER3-DXd in Breast Cancer and NSCLC Brain Metastases and Solid Tumor Leptomeningeal Disease (TUXEDO-3) | Phase II | NCT05865990 | Previously treated metastatic NSCLC with newly diagnosed or progressive BM | Patritumab deruxtecan | HER3 | Topoisomerase I inhibitor | CNS metastasis-specific study | Recruiting | |
| Non-CNS-specific NSCLC studies | CAB-AXL-ADC Safety and Efficacy Study in Adults with NSCLC | Phase II | NCT04681131 | Metastatic NSCLC | CAB-AXL-ADC | AXL | Auristatin microtubule inhibitor | Uncontrolled CNS disease excluded | Recruiting |
| A Study of MORAb-202 in Participants with Previously Treated Metastatic Non-Small Cell Lung Cancer (NSCLC) Adenocarcinoma (AC) | Phase II | NCT05577715 | Metastatic NSCLC with treatment-refractory progressive disease while on PD-1/L1-, EGFR-, or ALK- targeting therapies | Mecbotamab vedotin, no dosing available; mecbotamab vedotin and a PD-1 inhibitor | AXL | Auristatin microtubule inhibitor | No mention of CNS disease in the eligibility criteria | Recruiting | |
| A Study of BL-B01D1 and BL-B01D1 in Combination with Osimertinib Mesylate Tablets in Patients with Locally Advanced or Metastatic Non-small Cell Lung Cancer | Phase II | NCT05880706 | Locally advanced, unresectable, or metastatic NSCLC | BL-B01D1 | EGFRxHER3 | Topoisomerase I inhibitor | Active CNS disease excluded | Recruiting | |
| Phase 2 Study to Investigate Luveltamab Tazevibulin in Adults with Advanced or Metastatic Non-small Cell Lung Cancer | Phase II | NCT06555263 | Locally advanced, unresectable, or metastatic NSCLC with positive FOLR1 expression and treatment-refractory disease | Luveltamab tazevibulin | FOLR1 | Tubulin inhibitor | Previously treated BM included; untreated CNS disease excluded | Recruiting | |
| Study to Investigate Luveltamab Tazevibulin in Adults with Advanced or Metastatic Non-small Cell Lung Cancer | Phase II | NCT06555263 | Locally advanced, unresectable or metastatic NSCLC that is refractory to systemic therapy with positive FOLR1 expression | Luveltamab tazevibulin | FOLR1 | Tubulin inhibitor | Untreated CNS disease excluded | Recruiting | |
| Phase Ib Study of the Safety of T-DXd and Immunotherapy Agents with and Without Chemotherapy in Advanced or Metastatic HER2+, Non-squamous NSCLC (DL03) | Phase I | NCT04686305 | Locally advanced or metastatic non-squamous NSCLC that are refractory to systemic therapy or treatment-naïve with HER2 overexpression | Trastuzumab deruxtecan | HER2 | Topoisomerase I inhibitor | Untreated an symptomatic CNS disease excluded | Recruiting | |
| A Clinical Trial of TQB2102 for Injection in Non-small Cell Lung Cancer with HER2 Gene Abnormality | Phase II | NCT06496490 | Locally advanced, unresectable or metastatic NSCLC that is refractory to standard of care therapy | TQB2102 | HER2 | Topoisomerase I inhibitor | Stable BM included; symptomatic and progressive CNS disease, or LMD excluded | Recruiting | |
| HER3-DXd in Metastatic or Unresectable Non-Small Cell Lung Cancer | Phase I | NCT03260491 | Locally advanced, unresectable or metastatic NSCLC with EGFR-activating mutations with progression on previously responsive EGFR-TKI treatment | Patritumab deruxtecan | HER3 | Topoisomerase I inhibitor | Clinically inactive or treated and asymptomatic BM included; untreated and symptomatic CNS disease excluded | Recruiting | |
| HERTHENA-Lung02: A Study of Patritumab Deruxtecan Versus Platinum-based Chemotherapy in Metastatic or Locally Advanced EGFRm NSCLC After Failure of EGFR TKI Therapy | Phase III | NCT05338970 | Locally advanced, or metastatic nonsquamous NSCLC with EGFR TKI treatment-refractory disease and EGFR mutations | Patritumab deruxtecan | HER3 | Topoisomerase I inhibitor | Untreated and symptomatic BM, and history or presence of LMD excluded | Active, not recruiting | |
| A Study of SGN-B6A Versus Docetaxel in Previously Treated Non-small Cell Lung Cancer | Phase III | NCT06012435 | Locally advanced, unresectable, or metastatic NSCLC with non-squamous histology and with progression on previous chemotherapy or targeted therapy | Sigvotatug vedotin | IB6 | Auristatin microtubule inhibitor | Stable and treated BM included; active CNS disease and LMD excluded | Recruiting | |
| Clinical Study of Antibody-Drug Conjugate MYTX-011 in Subjects with Non-Small Cell Lung Cancer | Phase I | NCT05652868 | Locally advanced, recurrent, or metastatic NSCLC with MET alterations that is refractory to the standard of care therapy | MYTX-011 | MET | Auristatin microtubule inhibitor | Uncontrolled and untreated CNS disease excluded | Recruiting | |
| A Study to Assess Disease Activity and Adverse Events of Intravenous (IV) Telisotuzumab Vedotin Compared to IV Docetaxel in Adult Participants with Previously Treated Non-Squamous Non-Small Cell Lung Cancer (NSCLC) | Phase III | NCT04928846 | Locally advanced, unresectable, or metastatic nonsquamous NSCLC with treatment-refractory disease and MET overexpression | Telisotuzumab Vedotin | MET | Microtubule inhibitor | Stable CNS disease included; new and untreated CNS disease, and LMD excluded | Recruiting | |
| Study of REGN5093-M114 (METxMET Antibody-Drug Conjugate) in Adult Patients with Mesenchymal Epithelial Transition Factor (MET) Overexpressing Advanced Cancer | Phase I/II | NCT04982224 | Locally advanced, unresectable or metastatic NSCLC with MET overexpression | REGN5093-M114 | METxMET | Microtubule inhibitor | Untreated and active CNS disease and LMD excluded | Recruiting | |
| First-in-Human Study of XMT-1536 in Cancers Likely to Express NaPi2b | Phase I/II | NCT03319628 | Metastatic NSCLC adenocarcinoma | Upifitamab rilsodotin | NaPi2b | Auristatin microtubule inhibitor | Untreated CNS disease, such as new and progressive BM, and history of LMD excluded | Active, not recruiting | |
| An Efficacy and Safety Study of Cofetuzumab Pelidotin in Participants with PTK7-Expressing, Recurrent Non-Small Cell Lung Cancer | Phase I | NCT04189614 | Recurrent and treatment-refractory NSCLC with PTK7 expression | Cofetuzumab pelidotin | PTK7 | Auristatin microtubule inhibitor | Asymptomatic and treated CNS disease included; active CNS disease excluded | Active, not recruiting | |
| Datopotamab Deruxtecan (Dato-DXd) in Combination with Pembrolizumab with or Without Platinum Chemotherapy in Subjects with Advanced or Metastatic Non-Small Cell Lung Cancer (TROPION-Lung02) | Phase I | NCT04526691 | Locally advanced, unresectable, or metastatic NSCLC without actionable genomic alterations (e.g., ROS1, MET, EGFR, ALK, etc.) and with treatment-refractory progressive disase | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | Active and untreated CNS disease excluded | Active, not recruiting | |
| Phase 1b Study of Dato-DXd in Combination with Immunotherapy with or Without Carboplatin in Advanced or Metastatic Non-Small Cell Lung Cancer (TROPION-Lung04) | Phase I | NCT04612751 | Locally advanced, unresectable, or metastatic NSCLC without EGFR- nor ALK-mutations | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | Clinically active CNS disease excluded | Recruiting | |
| A Study of Dato-DXd in Chinese Patients with Advanced Non-Small Cell Lung Cancer, Triple-negative Breast Cancer and Other Solid Tumors (TROPION-PanTumor02) | Phase I/II | NCT05460273 | Locally advanced, unresectable, or metastatic NSCLC with treatment-refractory progressive disease | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | LMD excluded | Active, not recruiting | |
| SKB264 Combination Therapy in Patients with Advanced or Metastatic Non-small Cell Lung Cancer. | Phase II | NCT05351788 | Locally advanced, unresectable, or metastatic NSCLC | SKB264 | TROP2 | Topoisomerase I inhibitor | Active CNS metastasis, LMD, and metastases to the brainstem and spinal cord excluded | Recruiting | |
| Study of DS-1062a in Advanced or Metastatic Non-small Cell Lung Cancer with Actionable Genomic Alterations (TROPION-Lung05) | Phase II | NCT04484142 | Locally advanced, unresectable, or metastatic NSCLC with actionable genomic alterations (e.g., ROS1, MET, EGFR, ALK, etc.) and with treatment-refractory progressive disase | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | Inactive BM included; untreated and symptomatic CNS disease, and LMD excluded | Active, not recruiting with results (no survival data available) | |
| Study of Dato-DXd Plus Pembrolizumab vs. Pembrolizumab Alone in the First-line Treatment of Subjects with Advanced or Metastatic NSCLC Without Actionable Genomic Alterations (TROPION-Lung08) | Phase III | NCT05215340 | Locally advanced, unresectable, or metastatic NSCLC without actionable genomic alterations (e.g., ROS1, MET, EGFR, ALK, etc.) and with PD-L1 expression of 50% or more | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | Previously treated and stable BM included; active and untreated CNS disease, and LMD excluded | Recruiting | |
| Phase III, Open-label, First-line Study of Dato-DXd in Combination with Durvalumab and Carboplatin for Advanced NSCLC Without Actionable Genomic Alterations (AVANZAR) | Phase III | NCT05687266 | Locally advanced, unresectable, or metastatic NSCLC that is not amenable to chemoradiation and without actionable genomic alterations (e.g., ALK, ROS1, MET, etc.) | Datopotamab deruxtecan | TROP2 | Topoisomerase I inhibitor | Active BM and history of LMD excluded | Recruiting | |
| Sacituzumab Tirumotecan (MK-2870) Versus Chemotherapy in Previously Treated Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer (NSCLC) with EGFR Mutations or Other Genomic Alterations (MK-2870-004) | Phase III | NCT06074588 | Locally advanced, unresectable, or metastatic non-squamous NSCLC with treatment-refractory progressive disease | Sacituzumab tirumotecan | TROP2 | Topoisomerase I inhibitor | Previously treated BM included; active CNS disease and LMD excluded | Recruiting | |
| Study of Pembrolizumab (MK-3475) Monotherapy Versus Sacituzumab Govitecan in Combination with Pembrolizumab for Participants with Metastatic Non-small Cell Lung Cancer (NSCLC) with Programmed Cell Death Ligand 1 (PD-L1) Tumor Proportion Score (TPS) ≥50% (MK-3475-D46) | Phase III | NCT05609968 | Metastatic NSCLC without an indication of EGFR-, ALK-1, or ROS-1 targeted therapies and with a PD-L1 tumor proportion score of 50% or more | Sacituzumab govitecan | TROP2 | Topoisomerase I inhibitor | Active CNS disease and LMD excluded | Recruiting | |
| Solid tumor studies with NSCLC cohort | A Study to Assess the Safety, Pharmacokinetics, and Antitumor Activity of BC3195 in Patients with Advanced or Metastatic Cancer | Phase I | NCT06548672 | Locally advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | BC3195 | CDH3 | Auristatin microtubule inhibitor | Previously treated and stable BM included; active CNS disease and LMD excluded | Recruiting |
| A Study of PF-08046050 (SGN-CEACAM5C) in Adults with Advanced Solid Tumors | Phase I | NCT06131840 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | PF-08046050 | CEACAM5 | Topoisomerase I inhibitor | Stable and treated BM included | Recruiting | |
| A Phase 1 Study of CPO301 in Adult Patients with Advanced or Metastatic Solid Tumors | Phase I | NCT05948865 | Advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | CPO301 | EGFR | NA | Known, active, or uncontrolled CNS disease and LMD excluded | Recruiting | |
| First in Human Study of AZD9592 in Solid Tumors (EGRET) | Phase I | NCT05647122 | Locally advanced or metastatic solid tumors, including metastatic NSCLC in subgroups of EGFR-mutant or EGFR wild-type disease, that is refractory or not amenable to the standard of care therapy | AZD9592 | EGFRxMET | Topoisomerase I inhibitor | Stable and treated BM included; active and untreated BM and history of LMD excluded | Recruiting | |
| AMT-151 in Patients with Selected Advanced Solid Tumours | Phase I | NCT05498597 | Advanced solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | AMT-151 | FOLR1 | NA | Untreated CNS disease excluded | Recruiting | |
| A Study of LY4170156 in Participants with Selected Advanced Solid Tumors | Phase I | NCT06400472 | Advanced solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | LY4170156 | FOLR1 | Topoisomerase I inhibitor | Active and untreated BM, and history of LMD excluded | Recruiting | |
| A Study to Evaluate the Safety, Tolerability, and Efficacy of MORAb-202 (Herein Referred to as Farletuzumab Ecteribulin), a Folate Receptor Alpha (FRα)-Targeting Antibody-drug Conjugate (ADC) in Participants with Selected Tumor Types | Phase I/II | NCT04300556 | Metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | Farletuzumab ecteribulin | FOLR1 | Tubulin inhibitor | Treated and stable BM included; untreated BM or subdural disease excluded | Recruiting | |
| PRO1184 for Advanced Solid Tumors (PRO1184-001) | Phase I/II | NCT05579366 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | PRO1184 | FOLR1 | Topoisomerase I inhibitor | Previously treated and stable BM included; active CNS disease excluded | Recruiting | |
| Safety of GQ1001 in Adult Patients with HER2-Positive Advanced Solid Tumors | Phase I | NCT04450732 | Locally advanced or metastatic HER2-expressing solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | GQ1001 | HER2 | Pyrotinib, HER-TKI | Treated and asymptomatic BM included; untreated and symptomatic BM excluded | Recruiting | |
| DS8201a and Pembrolizumab in Participants with Locally Advanced/Metastatic Breast or Non-Small Cell Lung Cancer | Phase I | NCT04042701 | Locally advanced or metastatic breast cancer or NSCLC with HER2-overexpression or HER2-mutant disease | Trastuzumab deruxtecan | HER2 | Topoisomerase I inhibitor | Active CNS disease excluded | Recruiting | |
| A Study of Disitamab Vedotin in Previously Treated Solid Tumors That Express HER2 | Phase II | NCT06003231 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy, has received prior PDL-(L)1, and has a HER2 overexpression | Disitamab vedotin | HER2 | Auristatin microtubule inhibitor | Active and untreated CNS disease and LMD excluded | Recruiting | |
| A Study of SGN-B6A in Chinese Participants with Advanced Solid Tumors | Phase I | NCT06549816 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | Sigvotatug vedotin | IB6 | Auristatin microtubule inhibitor | Stable and treated BM included; active CNS disease excluded | Not yet recruiting | |
| A Study of SGN-MesoC2 in Advanced Solid Tumors | Phase I | NCT06466187 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | SGN-MesoC2 | MSLN | N/A | Stable and treated BM included; untreated BM and LMD excluded | Recruiting | |
| A Study of PHN-010 in Patients with Advanced Solid Tumors | Phase I | NCT06457997 | Advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | PHN-010 | NA | NA | Untreated CNS disease excluded | Recruiting | |
| A Study of LY4101174 in Participants with Recurrent, Advanced or Metastatic Solid Tumors | Phase I | NCT06238479 | Locally advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | LY4101174 | NECTIN4 | Auristatin microtubule inhibitor | Known or suspected uncontrolled CNS disease excluded | Recruiting | |
| A Study of LY4052031 in Participants with Advanced or Metastatic Urothelial Cancer or Other Solid Tumors (NEXUS-01) | Phase I | NCT06465069 | Locally advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | LY4052031 | NECTIN4 | Topoisomerase I inhibitor | Known or suspected uncontrolled CNS disease excluded | Recruiting | |
| Study BT8009-100 in Subjects with Nectin-4 Expressing Advanced Malignancies | Phase I/II | NCT04561362 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | BT8009 | NECTIN4 | Auristatin microtubule inhibitor | Active and untreated CNS and LMD excluded | Recruiting | |
| CAB-ROR2-ADC Safety and Efficacy Study in Patients with TNBC or Head & Neck Cancer (Ph1) and NSCLC or Melanoma (Ph2) | Phase I/II | NCT03504488 | Locally advanced, unresectable or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | Ozuriftamab vedotin | ROR2 | Auristatin microtubule inhibitor | Uncontrolled CNS disease excluded | Recruiting | |
| Study of XB002 in Subjects with Solid Tumors (JEWEL-101) | Phase I | NCT04925284 | Locally advanced, unresectable, or metastatic solid tumors, including metastatic NSCLC, that is refractory or not amenable to the standard of care therapy | XB002 | TF | Auristatin microtubule inhibitor | Treated BM included; untreated and active BM or cranial epidural disease excluded | Active, not recruiting | |
| A Study to Evaluate TROP2 ADC LCB84 Single Agent and in Combination with an Anti-PD-1 Ab in Advanced Solid Tumors | Phase I/II | NCT05941507 | Advanced solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | LCB84 | TROP2 | Auristatin microtubule inhibitor | Stable and treated BM included; active and progressing CNS disease or LMD excluded | Recruiting | |
| A Phase 1/2 Study of OBI-992 in Subjects with Advanced Solid Tumors | Phase I/II | NCT06480240 | Advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | OBI-992 | TROP2 | Topoisomerase I inhibitor | Treated and stable BM included; untreated CNS disease excluded | Recruiting | |
| Phase I-II, FIH, TROP2 ADC, Advanced Unresectable/Metastatic Solid Tumors, Refractory to Standard Therapies (A264) | Phase I/II | NCT04152499 | Locally advanced or metastatic solid tumors, including NSCLC, that is refractory or not amenable to the standard of care therapy | Sacituzumab tirumotecan | TROP2 | Topoisomerase I inhibitor | Symptomatic and active BM, history of LMD, brainstem metastasis, and spinal cord metastasis excluded | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, D.J.H.; Cohen, S.F.; Lazaratos, A.-M.; Bouganim, N.; Dankner, M. Antibody–Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases. Curr. Oncol. 2024, 31, 6314-6342. https://doi.org/10.3390/curroncol31100471
Bian DJH, Cohen SF, Lazaratos A-M, Bouganim N, Dankner M. Antibody–Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases. Current Oncology. 2024; 31(10):6314-6342. https://doi.org/10.3390/curroncol31100471
Chicago/Turabian StyleBian, David J. H., Sara F. Cohen, Anna-Maria Lazaratos, Nathaniel Bouganim, and Matthew Dankner. 2024. "Antibody–Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases" Current Oncology 31, no. 10: 6314-6342. https://doi.org/10.3390/curroncol31100471
APA StyleBian, D. J. H., Cohen, S. F., Lazaratos, A.-M., Bouganim, N., & Dankner, M. (2024). Antibody–Drug Conjugates for the Treatment of Non-Small Cell Lung Cancer with Central Nervous System Metastases. Current Oncology, 31(10), 6314-6342. https://doi.org/10.3390/curroncol31100471





